Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Scutellaria barbata D. Don Polysaccharides Inhibit High Glucose-Induced Proliferation and Angiogenesis of Retinal Vascular Endothelial Cells
Received 9 December 2020
Accepted for publication 13 April 2021
Published 31 May 2021 Volume 2021:14 Pages 2431—2440
DOI https://doi.org/10.2147/DMSO.S296164
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Wenjun Li,1 Hongxia Xiao2
1Department of Ophthalmology, NHC Key Laboratory of Hormones and Development, Tianjin Key Laboratory of Metabolic Diseases, Chu Hsien-I Memorial Hospital & Tianjin Institute of Endocrinology, Tianjin Medical University, Tianjin, 300134, People’s Republic of China; 2Department of Ophthalmology, Jingmen NO.2 People’s Hospital, Jingmen, 448000, People’s Republic of China
Correspondence: Hongxia Xiao Email [email protected]
Purpose: The traditional Chinese medicine Scutellaria barbata D. Don (S. barbata) has been reported to exhibit anti-cancer and anti-inflammation activities. The ethanol extract of S. barbata has been confirmed to attenuate diabetic retinopathy (DR). This study aimed to investigate the effects and underlying mechanisms of the polysaccharides isolated from S. barbata (PSB) on the proliferation and angiogenesis of retinal vascular endothelial cells (RVECs) in DR.
Methods: Human RVECs (HRVECs) were cultured in normal glucose (NG, 5.5 mM), mannitol (MA, 30 mM), high glucose (HG, 30 mM) and HG plus 40 μg/mL PSB, respectively. Then, cell proliferation, migration and angiogenesis were evaluated. The cell proliferation was also estimated in the presence of SLIGKV, which was used to induce the phosphorylation of ERK (p-ERK).
Results: PSB reduced normal and HG-induced HRVECs cell viability in a concentration-dependent manner. The protein expression of proliferating cell nuclear antigen (PCNA) and proliferating antigen KI67 (Ki67), the migration rate and tube formation ability, which were increased by HG treatment, were significantly decreased by PSB. PSB also inhibited the phosphorylation of Raf, MEK and ERK in HG-stimulated HRVECs. Moreover, the application of SLIGKV recovered cell viability and the expression of p-ERK, PCNA and Ki67, in HG plus PSB-treated cells. Finally, the HG-enhanced expression of VE-cadherin, Frizzed, β-catenin, MMP-2 and MMP-9 was all reversed by PSB.
Conclusion: PSB could inhibit HG-induced HRVECs proliferation, migration and neovascularization, and these effects might work through blocking the activation of MEK/ERK pathway and VEGF/VE-cadherin axis.
Keywords: Scutellaria barbata, polysaccharides, retinal vascular endothelial cells, diabetic retinopathy
Introduction
Diabetic retinopathy (DR) is caused by abnormal insulin metabolism in diabetic patients, which leads to changes in ocular tissues, nerves and blood vessels and microcirculation, resulting in poor ocular nutrition and damage of visual function.1 It is one of the common complications of diabetes and the primary cause of blindness.2 Many different biological processes are involved in the pathological changes of DR, such as oxidative stress, mitochondrial dysfunction, retinal cell proliferation and angiogenesis, but the specific pathogenesis of DR remains to be elucidated.3–5 At present, the main therapeutic approaches for DR are anti-vascular endothelial growth factor (VEGF) drug therapy (such as oral angiotensin-converting enzyme inhibitors), cold therapy, photocoagulation therapy, and vitrectomy. However, these therapies have not achieved satisfactory therapeutic effects.6 Therefore, it is urgent to develop novel and effective drugs for the prevention and treatment of DR.
In the late stage of DR, new blood vessels grow along the retinal interface, which in turn causes complications such as vitreous hemorrhage, traction retinal detachment, and neovascular glaucoma. Angiogenesis is a complex process involving the migration, proliferation and formation of endothelial cell tubes.7 Increased VEGF content in the vitreous is the key to retinal neovascularization. VEGF can induce the proliferation and migration of retinal vascular endothelial cells (RVECs), the formation of vascular lumen and the formation of abnormal new blood vessels.8 It has been suggested that VEGF could activate MEK/ERK pathway and VEGF/vascular endothelial (VE)-cadherin axis to regulate RVECs proliferation and angiogenesis.9 Although research is ongoing in the field of angiogenesis, the underlying molecular mechanism remains to be totally clarified.
Scutellaria barbata D. Don (S. barbata), also known as “Ban Zhi Lian” in China, is an important species belonging to the Lamiaceae family and has been applied in traditional Chinese medicine (TCM). S. barbata has long been utilized for heat-clearing and detoxification in the TCM system.10 Modern pharmacological studies have shown that S. barbata exhibits a wide range of biological activities, including anti-cancer, anti-inflammation, and anti-microbial.11,12 S. barbata contains complex chemical composition including flavonoids, lactones and polysaccharide, among them, each specific component has been reported to possess different degrees of anti-inflammatory and anti-tumor properties. The polysaccharides isolated from S. barbata (PSB) was illustrated to inhibit tumor angiogenesis in lung cancer.13 In a previous study, the ethanol extract of S. barbata has been confirmed to attenuate DR by preventing retinal inflammation and downregulating tight junction protein expression,14 whereas no attention was devoted to investigate whether PSB could relieve the proliferation, migration and angiogenesis of DR.
In the present study, we aimed to explore the effects and underlying mechanisms of PSB on high glucose (HG)-induced human retinal vascular endothelial cells (HRVECs) proliferation and angiogenesis. First, the effect of different concentrations of PSB on normal and HG-induced HRVECs viability was evaluated. Second, cell proliferation, cell migration, tubular network formation and the expression of VEGF, MEK/ERK pathway and VEGF/VE-cadherin axis-related proteins were investigated in the presence of normal glucose (NG, 5.5 mM), mannitol (MA, 30 mM), HG (30 mM)15 and HG plus 40 μg/mL PSB. Finally, the effect of phosphorylated (p)-ERK agonist-SLIGKV on the functions of PSB in HG-stimulated HRVECs was determined. The results showed that PSB could inhibit HG-induced HRVECs proliferation, migration and tube formation through blocking the activation of MEK/ERK pathway and VEGF/VE-cadherin axis. The findings presented in this work may contribute to promising new approaches for DR therapy.
Methods
Cell Culture and Treatment
Human retinal vascular endothelial cells (HRVECs; Procell Life Science&Technology Co., Ltd., Wuhan, China; Cat NO. CP-H130) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, USA) with 10% fetal bovine serum (FBS) (Gibco, USA), 100 U/mL penicillin and streptomycin (Sigma-Aldrich, USA) in a humidified atmosphere with 5% CO2 at 37°C.
The cells in normal glucose (NG), mannitol (MA), high glucose (HG) groups were cultured with 5.5 mmol/L glucose, 30 mM MA and 30 mM glucose (Sigma-Aldrich) for 48 h, respectively. For PSB (Nanjing Ze Lang Pharmaceutical Technology, Ltd., cat. no. ZL20131124BD; purity>95%; dissolved in dimethyl sulphoxide) treatment, cells were treated with different concentrations of PSB for 24h. For HG+PSB group, cells were cultured in HG medium for 48 h and then treated with PSB for 24 h. SLIGKV (100 μM, Sigma-Aldrich) was used to stimulate cells for 24 h to induce the phosphorylation of ERK1/2.16
Cell Counting Kit-8 (CCK-8)
HRVECs were seeded in 96-well plates with density of 2×104 cells per well, then subsequently maintained in indicated medium for 48 h, followed by treated with or without different concentrations of PSB for 48 h. Thereafter, 10 μL CCK-8 (Beyotime Biotechnology Co., LTD, China) solution was added to each well and incubated at 37°C for 2 h, and OD values were measured at 450 nm through a microplate reader (Bio-Rad Model 550, USA).
Wound Healing Assay
HRVECs (2 × 105 cells/well) were seeded in 6-well plates and being starved for 24 h, after which, 10 μL tip was used to scratch the cells. Subsequently, cells were washed with PBS and treated with NG, MA, HG and HG + 40 μg/mL PSB at 37°C for 24 h. Images of the scratches were captured at 0 h and 24 h to observe cell confluence. The average widths of the scratch wounds were analyzed using Image J software. The migrated distances were calculated as the width of the scratch at 24 h minus the width of the scratch at 0 h. The relative migration distance was calculated by normalizing to NG group.
Transwell Assay
Transwell assays were performed using 24-well culture plates inserted with 8-mm pore size (Transwell; Falcon, BD Biosciences). The lower chamber was filled with 600 µL DMEM containing 10% FBS. Cells (1×105 cell/well) were seeded into the upper chamber. After 24 h of incubation, the migrated cells were fixed with 5% glutaraldehyde and then stained with crystal violet, and the number of cells in the bottom well was counted by counting chamber.
Tube Formation Assay
Cells (3×104 cells/well) were seeded into the 96-well plate, which was precoated with 30 μL matrigel and then immediately placed at 37°C for 2 h for solidification. The plate was kept at 37°C for 24 h. The pictures of cells were captured using a microscope (Olympus, Japan; magnification, x40) and the number of formed capillaries was counted.
Immunofluorescence Staining
HRVECs were fixed with 4% paraformaldehyde, blocked with 3% FBS albumin (Sigma-Aldrich), and next incubated with polyclonal rabbit anti-proliferating antigen KI67 (Ki67) (Santa Cruz Biotechnology, CA, USA) primary antibody (1:200) overnight at 4°C. Secondary antibody Alexa Fluor 488 donkey anti-rabbit (1:200) was employed for 1 h at 37°C. Nuclei were visualized by staining with 4′, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). An Olympus FV1000 confocal laser scanning microscope (Olympus, Japan; magnification, x200) was used to acquire images.
Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate (dUTP)-Biotin Nick End Labeling (TUNEL) Apoptosis Staining
Apoptotic cells were examined by an apoptosis detection kit (Roche, Basel, Switzerland) according to standard protocol. Briefly, cells were stained by a TUNEL kit, followed by the counterstaining with DAPI for the nuclei. The staining was observed under a fluorescence microscope (Olympus, Japan; magnification, x200). Cell apoptosis rate was captured under a fluorescence microscope (Olympus Corporation, magnification, x200).
Western Blot Analysis
The HRVECs were lysed with ice-cold RIPA buffer and the concentration of protein was determined using a BCA protein assay reagent kit (Beyotime, China). The proteins (15 μg) were separated by dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene fluoride (PVDF) membrane. Then, the membrane was blocked with 5% skimmed milk for 2 h at room temperature. The PVDF membranes were then incubated overnight at 4°C with the following primary antibodies to: proliferating cell nuclear antigen (PCNA; Cell Signaling Technology, Boston, MA, USA), Ki67 (Santa Cruz Biotechnology, CA, USA), (p-) Raf (Cell Signaling Technology, Boston, MA, USA), (p-) MEK (Cell Signaling Technology, Boston, MA, USA), (p-) ERK (Cell Signaling Technology, Boston, MA, USA), VE-cadherin (Cell Signaling Technology, Boston, MA, USA), Frizzed (Abcam Company, Cambridge, UK), β-catenin (Cell Signaling Technology, Boston, MA, USA), MMP-2 (Abcam Company, Cambridge, UK), MMP-9 (Abcam Company, Cambridge, UK) and GAPDH (Cell Signaling Technology, Boston, MA, USA). Followed by being incubated with a horseradish peroxidase (HRP)-labeled secondary antibody (Abcam, Cambridge, MA, USA). The results were visualized with an enhanced chemiluminescence (ECL) detection kit. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to quantify the density of the bands.
Reverse Transcription-Quantitative PCR (RT-qPCR)
Total RNA was isolated from HRVECs using TRIzol reagent (Invitrogen), and then reverse-transcribed (RT) into cDNA using the PrimeScript™ RT reagent kit (TaKaRa). qPCR was performed using TB Green Fast qPCR Mix (TaKaRa) according to instructions. The PCR reaction procedure was as follows: 5 min at 95°C, with 40 cycles of 30 sec at 95°C and 45 sec at 65°C. The relative quantification of mRNA levels was determined using the 2−ΔΔCt method. The real-time PCR primers were as followed: VEGF, forward 5ʹ-ATGCGGATCAAACCTCACCA-3ʹ, reverse 5ʹ-CACCAACGTACACGCTCCAG-3ʹ; VEGF-receptor 2 (R2), forward 5ʹ-GCAGGAAGTAGCCGCATTTG-3ʹ, reverse 5ʹ- ACTCGAAGAACACGCAACCT-3ʹ. GAPDH, forward 5ʹ-GCAACCGGGAAGGAAATGAATG-3ʹ, reverse 5ʹ-CCCAATACGACCAAATCAGAGA-3ʹ. GAPDH was used as a reference gene.
Statistical Analysis
All experiments were repeated three times. All data are represented as mean ± standard deviation (SD) from 3 independent experiments. One-way ANOVA followed by Bonferroni’s test was utilized to analyze the difference among multiple groups by GraphPad Prism 5 software. A value of P < 0.05 was considered statistically significant.
Results
PSB Inhibits HG-Induced HRVECs Proliferation
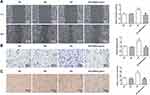
Firstly, different concentrations (0, 10, 20, 40 and 80 μg/mL) PSB was used to treat cells for 24 h to observe the effect of PSB on HRVECs viability. Results showed that PSB decreased HRVECs viability in a concentration-dependent manner, and 80 μg/mL PSB led to more than 50% decrease in cell viability (Figure 1A). Additionally, 10, 20 and 40 μg/mL PSB had no effects on apoptosis of HRVECs, but 80 μg/mL PSB caused cell apoptosis as comparison to the untreated group (Figure 1B and C). Therefore, 10, 20 and 40 μg/mL PSB were used to perform the subsequent experiments. Then, cells were treated with NG, MA, HG and HG plus PSB (10, 20 and 40 μg/mL). As shown in Figure 1D, compared with NG and MA group, HG resulted in a markedly increase in cell viability. However, the presence of PSB gradually canceled this elevation in cell viability and the cell viability of HRVECs in HG + 40 μg/mL PSB group was lower than NG group. Therefore, 40 μg/mL PSB was chosen for the following experiments.
HRVECs were subjected to NG, MA, HG and HG + 40 μg/mL PSB, then the expression of proteins involved in cell proliferation was assessed. We found that HG significantly enhanced the protein expression of PCNA and Ki67, which as reversely reduced by additional treatment of 40 μg/mL PSB (Figure 1E). The same results are observed in Figure 1F, which presented the immunofluorescence staining for Ki67. The above data demonstrated the inhibitory effect of PSB on HG-caused HRVECs proliferation enhancement.
PSB Suppresses HG-Stimulated HRVECs Migration and Angiogenesis
Next, we performed wound healing and transwell assay to investigate cell migration. Figure 2A reveals that HG-induced enhancement in migration distance was remarkably reversed by 40 μg/mL PSB. Similarly, PSB canceled the HG-increased number of migrated cells in transwell assay (Figure 2B).
The ability of capillary formation in HRVECs was also estimated. As shown in Figure 2C, compared with NG and MA group, the number of capillary-like structure was considerably boosted in HG group. However, the addition of PSB also obviously resulted in a decrease in the number of capillary-like structure. These data implicated that PSB could block HG-induced HRVECs migration and angiogenesis.
The Agonist of p-ERK, SLIGKV Recovers PSB-Inhibited HRVECs Proliferation
Subsequently, we observed that, consistent with previous studies,17,18 the mRNA level of VEGF and VEGF-Receptor 2 (R2) was significantly increased upon HG stimulation (Figure 3A and B). At the same time, HG treatment promoted the phosphorylation Raf, MEK and ERK proteins, which were the crucial components of MEK/ERK pathway. However, PSB also reduced the expression of p-Raf, p-MEK and p-ERK, indicating that the activation of MEK/ERK pathway might contribute to the effects of PSB on HG-treated HRVECs (Figure 3C).
Therefore, we utilized SLIGKV, which has been confirmed to result in significantly greater induction of ERK phosphorylation,16 and in order to stimulate HG + PSB-treated HRVECs. As illustrated in Figure 3D, the expression of p-ERK was indeed enhanced by SLIGKV, as compared to HG + PSB group. In addition, the decreased cell viability and blunted expression of PCNA and Ki67, caused by PSB treatment, were also partially rescued by the addition of SLIGKV (Figure 3E and F). The above results suggested that PSB may inhibit HRVECs proliferation through inactivating MEK/ERK pathway.
PSB Inactivates VEGF/VE-Cadherin Axis in HG-Induced HRVECs
Finally, the expression of proteins associated with VEGF/VE-cadherin axis was detected. As demonstrated in Figure 4, compared with NG and MA group, the expression of VE-cadherin, Frizzed, β-catenin, MMP-2 and MMP-9 were also remarkably increased upon HG stimulation. However, the addition of PSB in HG-induced HRVECs, led to a considerably decrease in the expression of these proteins expression, implicating the involvement of VEGF/VE-cadherin axis in the functions of PSB in HG-stimulated HRVECs.
Discussion
DR is the most common cause of diabetic blindness, and the pathological process of DR is retinal microvascular complications. New blood vessel formation is the main feature of DR. This study aims to investigate the beneficial effects of PSB on DR and reveal the underlying molecular mechanisms.
In the present study, the diabetic cell model was established by culturing HRVECs in medium containing high concentration of glucose (HG, 30 mM). HG is known to impair the physiology of normal human endothelial cells and to induce hyperosmolarity, which can promote the development of DR.19–21 Moreover, hypoxia induced by HG in retinal vascular tissues can stimulate angiogenesis by regulating the balance of pro-angiogenic and anti-angiogenic mediators, which results in retinal neovascularization, which is a hallmark of DR.22 To exclude the influence of osmotic pressure, MA group was also set up in this study. In accordance with previous studies, we observed that cell proliferation, migration and angiogenesis were remarkably enhanced in response to HG.21,23,24 Furthermore, the mRNA level of VEGF and VEGF-R2 was also increased by HG. VEGF is recognized as one of the most effective angiogenesis factors and is closely related to DR.25 Under normal physical circumstances, VEGF level in retinal tissues is low, thus playing a key role in the formation and stability of retinal blood vessels. While in a state of hyperglycemia, VEGF level is increased and participates in the progression of DR. VEGF-R2 is one of the high-affinity receptors of VEGF in retinal endothelial cells. Previous studies have shown that induction of the VEGF expression and its receptors can promote the proliferation of endothelial cells, stimulate angiogenesis, increase the permeability of small blood vessels, and thereby exacerbate DR.26 Our results verified the successful establishment of DR cell model induced by HG, and further confirmed the effects HG on DR.
In China, TCM has been widely used to prevent and relieve a great diversity of diseases for more than 1000 years, and certain agents utilized to treat cancer have been derived from TCM. However, the exact anti-disease mechanisms of TCM remain to be fully clarified, which hinders the application of TCM in international clinical treatment. PSB is the polysaccharides extracted from a Chinese medicine S. barbata. Herein, we demonstrated that PSB effectively inhibited HRVECs proliferation, migration and angiogenesis upon HG stimulation, indicating the potential role of PSB in alleviating DR. Moreover, we found that the HG-increased mRNA level of VEGF and VEGF-R2 was significantly reduced by PSB treatment, suggesting that PSB may exert its beneficial role in DR via lowering VEGF and VEGF-R2 expression. Similarly, the anti-angiogenic effect of S. barbata through inhibiting VEGF expression has been proposed by previous studies, although these studies were mainly focused on the anti-tumor effect of S. barbata.27–29 Our study for the first time shed light on the anti-angiogenic effect of S. barbata in retina.
The MEK/ERK pathway has been reported to function as an important regulator in proliferation, differentiation and survival of numerous different cells. It has been demonstrated that Raf/MEK/ERK pathway could be activated in the retina and its endothelial cells in diabetes, and this cascade might promote angiogenesis in the pathogenesis of diabetic retinopathy.30,31 In addition, VEGF has been confirmed to activate downstream MEK/ERK pathway thereby contribute to retinopathy.9,32 In the current study, we also detected the activation of Raf/MEK/ERK pathway in response to HG, which was markedly prevented by the application of PSB. Inactivation of MEK/ERK has been implicated to restrain the proliferation, migration, invasion and angiogenesis of retinoblastoma cells.33 Therefore, we speculated that PSB inhibited HG-induced HRVECs proliferation via inactivating MEK/ERK pathway. Subsequently, the agonist of p-ERK, SLIGKV was applied to treat HRVECs in the presence of HG plus PSB. Results showed that SLIGKV re-activated the expression of p-ERK and also blunted the inhibitory effect of PSB on cell proliferation in HG-induced HRVECs. We inferred that PSB inhibited HG-induced HRVECs proliferation via inactivating MEK/ERK pathway.
VE-Cadherin is a type II endothelial-restricted cadherin, which is the main transmembrane adhesion molecule of endothelial adhesion connection.34 The evidence indicated that silence of the VE-cadherin gene led to severe angiogenesis defects, causing abnormal VEGF signaling. VEGF has recently been recognized as an important angiogenic factor in DR. In addition, angiogenic factors include MMP2 and MMP9, which are downstream of and activated by VEGF.35 In this study, we found that HG stimulation also increased the protein expression of VEGF/VE-cadherin axis including VE-cadherin, Frizzed and β-catenin, together with the expression of MMP2 and MMP9. However, PSB treatment cancelled the enhancement of these proteins induced by HG, indicating that PSB may inhibit HRVECs angiogenesis through blocking VEGF/VE-cadherin and VEGF/MMP axis.
Conclusion
Collectively, we have demonstrated for the first time that PSB restrained DR by suppressing retinal cells proliferation through inactivating MEK/ERK pathway, inhibiting angiogenesis via inhibition of VEGF/VE-cadherin axis and MMP expressions in HG-stimulated HRVECs. Although specific functions of PSB in DR remain to be verified by performing in vivo experiments, the molecular mechanisms of PSB on DR need to be further studied. Our results suggested the potential value of PSB in treating DR.
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
Jingmen Science and Technology Research and Development Plan Project (grant number 2019YFYB018).
Disclosure
The authors declare that they have no competing interests.
References
1. Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61(1):29–38. doi:10.1007/s00125-017-4435-8
2. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi:10.1016/S0140-6736(09)62124-3
3. Heng LZ, Comyn O, Peto T, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabet Med. 2013;30(6):640–650. doi:10.1111/dme.12089
4. Kowluru RA, Shan Y. Role of oxidative stress in epigenetic modification of MMP-9 promoter in the development of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):955–962. doi:10.1007/s00417-017-3594-0
5. Mishra M, Lillvis J, Seyoum B, Kowluru RA. Peripheral blood mitochondrial DNA damage as a potential noninvasive biomarker of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57(10):4035–4044.
6. Horton MB, Silva PS, Cavallerano JD, Aiello LP. Clinical components of telemedicine programs for diabetic retinopathy. Curr Diab Rep. 2016;16(12):129. doi:10.1007/s11892-016-0813-8
7. Capitão M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem. 2016;117(11):2443–2453. doi:10.1002/jcb.25575
8. Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19(6).
9. Jin J, Yuan F, Shen MQ, Feng YF, He QL. Vascular endothelial growth factor regulates primate choroid-retinal endothelial cell proliferation and tube formation through PI3K/Akt and MEK/ERK dependent signaling. Mol Cell Biochem. 2013;381(1–2):267–272. doi:10.1007/s11010-013-1710-y
10. Jiang W, Zhao B, Li Y, Qi D, Wang D. Modification of the 8th American Joint Committee on Cancer staging system for gallbladder carcinoma to improve prognostic precision. BMC Cancer. 2020;20(1):1129. doi:10.1186/s12885-020-07578-7
11. Zhang L, Fang Y, Feng JY, et al. Chloroform fraction of Scutellaria barbata D. Don inhibits the growth of colorectal cancer cells by activating miR-34a. Oncol Rep. 2017;37(6):3695–3701.
12. Zhang H, Jin B, Bu J, et al. Transcriptomic insight into terpenoid biosynthesis and functional characterization of three diterpene synthases in Scutellaria barbata. Molecules (Basel, Switzerland). 2018;23(11):2952. doi:10.3390/molecules23112952
13. Yang J, Yang G, Hou G, et al. Scutellaria barbata D. Don polysaccharides inhibit the growth of Calu-3 xenograft tumors via suppression of the HER2 pathway and angiogenesis. Oncol Lett. 2015;9(6):2721–2725. doi:10.3892/ol.2015.3127
14. Mei XY, Zhou LY, Zhang TY, Lu B, Ji LL. Scutellaria barbata attenuates diabetic retinopathy by preventing retinal inflammation and the decreased expression of tight junction protein. Int J Ophthalmol. 2017;10(6):870–877. doi:10.18240/ijo.2017.06.07
15. Chen Y, Wang Y, Jiang Y, Zhang X, Sheng M. High-glucose treatment regulates biological functions of human umbilical vein endothelial cells via Sirt1/FOXO3 pathway. Ann Transl Med. 2019;7(9):199. doi:10.21037/atm.2019.04.29
16. Svensson KJ, Kucharzewska P, Christianson HC, et al. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci U S A. 2011;108(32):13147–13152. doi:10.1073/pnas.1104261108
17. Liu P, Jia SB, Shi JM, et al. LncRNA-MALAT1 promotes neovascularization in diabetic retinopathy through regulating miR-125b/VE-cadherin axis. Biosci Rep. 2019;39(5).
18. Bolinger MT, Antonetti DA. Moving past anti-VEGF: novel therapies for treating diabetic retinopathy. Int J Mol Sci. 2016;17(9):1498. doi:10.3390/ijms17091498
19. Trudeau K, Molina AJ, Guo W, Roy S. High glucose disrupts mitochondrial morphology in retinal endothelial cells: implications for diabetic retinopathy. Am J Pathol. 2010;177(1):447–455. doi:10.2353/ajpath.2010.091029
20. Wang M, Hu J, Yan L, et al. High glucose-induced ubiquitination of G6PD leads to the injury of podocytes. FASEB J. 2019;33(5):6296–6310. doi:10.1096/fj.201801921R
21. Madonna R, Giovannelli G, Confalone P, Renna FV, Geng YJ, De Caterina R. High glucose-induced hyperosmolarity contributes to COX-2 expression and angiogenesis: implications for diabetic retinopathy. Cardiovasc Diabetol. 2016;15:18. doi:10.1186/s12933-016-0342-4
22. Fernando KHN, Yang HW, Jiang Y, Jeon YJ, Ryu B. Diphlorethohydroxycarmalol isolated from Ishige okamurae represses high glucose-induced angiogenesis in vitro and in vivo. Mar Drugs. 2018;16(10):375. doi:10.3390/md16100375
23. Farnoodian M, Halbach C, Slinger C, Pattnaik BR, Sorenson CM, Sheibani N. High glucose promotes the migration of retinal pigment epithelial cells through increased oxidative stress and PEDF expression. Am J Physiol Cell Physiol. 2016;311(3):C418–436. doi:10.1152/ajpcell.00001.2016
24. Xing Q, Zhang G, Kang L, et al. The suppression of Kallistatin on high-glucose-induced proliferation of retinal endothelial cells in diabetic retinopathy. Ophthalmic Res. 2017;57(3):141–149. doi:10.1159/000447776
25. Ray D, Mishra M, Ralph S, Read I, Davies R, Brenchley P. Association of the VEGF gene with proliferative diabetic retinopathy but not proteinuria in diabetes. Diabetes. 2004;53(3):861–864. doi:10.2337/diabetes.53.3.861
26. Mesquita J, Castro-de-sousa JP, Vaz-Pereira S, Neves A, Passarinha LA, Tomaz CT. Vascular endothelial growth factors and placenta growth factor in retinal vasculopathies: current research and future perspectives. Cytokine Growth Factor Rev. 2018;39:102–115. doi:10.1016/j.cytogfr.2017.11.005
27. Shiau AL, Shen YT, Hsieh JL, Wu CL, Lee CH. Scutellaria barbata inhibits angiogenesis through downregulation of HIF-1 α in lung tumor. Environ Toxicol. 2014;29(4):363–370. doi:10.1002/tox.21763
28. Dai ZJ, Lu WF, Gao J, et al. Anti-angiogenic effect of the total flavonoids in Scutellaria barbata D. Don. BMC Complement Altern Med. 2013;13:150. doi:10.1186/1472-6882-13-150
29. Wei L, Lin J, Xu W, et al. Scutellaria barbata D. Don inhibits tumor angiogenesis via suppression of Hedgehog pathway in a mouse model of colorectal cancer. Int J Mol Sci. 2012;13(8):9419–9430. doi:10.3390/ijms13089419
30. Tang F, Pacheco MTF, Chen P, Liang D, Li W. Secretogranin III promotes angiogenesis through MEK/ERK signaling pathway. Biochem Biophys Res Commun. 2018;495(1):781–786. doi:10.1016/j.bbrc.2017.11.080
31. Mohammad G, Kowluru RA. Diabetic retinopathy and signaling mechanism for activation of matrix metalloproteinase-9. J Cell Physiol. 2012;227(3):1052–1061. doi:10.1002/jcp.22822
32. Zhang YH, Wei W, Xu H, Wang YY, Wu WX. Inducing effects of hepatocyte growth factor on the expression of vascular endothelial growth factor in human colorectal carcinoma cells through MEK and PI3K signaling pathways. Chin Med J. 2007;120(9):743–748. doi:10.1097/00029330-200705010-00002
33. Zeng Z, Gao ZL, Zhang ZP, et al. Downregulation of CKS1B restrains the proliferation, migration, invasion and angiogenesis of retinoblastoma cells through the MEK/ERK signaling pathway. Int J Mol Med. 2019;44(1):103–114. doi:10.3892/ijmm.2019.4183
34. Muramatsu F, Kidoya H, Naito H, Hayashi Y, Iba T, Takakura N. Plakoglobin maintains the integrity of vascular endothelial cell junctions and regulates VEGF-induced phosphorylation of VE-cadherin. J Biochem. 2017;162(1):55–62.
35. Cao HJ, Zheng LZ, Wang N, et al. Src blockage by siRNA inhibits VEGF-induced vascular hyperpermeability and osteoclast activity - an in vitro mechanism study for preventing destructive repair of osteonecrosis. Bone. 2015;74:58–68.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.