Back to Journals » Journal of Multidisciplinary Healthcare » Volume 13
Screening for Bone Mineral Density and Assessment Knowledge Level of Low Peak Bone Risk Factors and Preventive Practices Among Kuwaiti Future Mothers
Authors Al-Ayyadhi N , Refaat LAER, Ibrahim MM, Abd ElGalil HM
Received 9 September 2020
Accepted for publication 19 November 2020
Published 18 December 2020 Volume 2020:13 Pages 1983—1991
DOI https://doi.org/10.2147/JMDH.S280261
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Najla Al-Ayyadhi,1 Laila Abd ElRaheem Refaat,2 Mahasen Mohamed Ibrahim,2 Heba Mohamed Abd ElGalil2
1Public Health Department, Ministry of Health, Kuwait City, Kuwait; 2Community and Occupational Medicine Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
Correspondence: Heba Mohamed Abd ElGalil
Community and Occupational Medicine Department, Faculty of Medicine, Al-Azhar University, Yousef Abbas Street, Cairo 11754, Egypt
Email [email protected]
Background: Attaining greater peak bone mass (PBM) prior to the onset of bone loss is getting increasing public health attention as healthy strong bones are essential to maintain our life. Females are more susceptible to bone loss. Knowledge and awareness about low peak bone mass and its related risk factors are important contributors to its preventive behavior.
Objective: To screen apparently healthy young Kuwaiti future mothers for low bone mineral density (BMD) and to assess their knowledge level about determinants of PBM and preventive practices.
Methods: A cross-sectional comparative study on 445 eligible women aged 18– 35 years (either students or employees from Kuwait University) was performed. Data on socio-demographic and lifestyle variables were obtained by a semi-structured questionnaire. Their knowledge was assessed using the modified Osteoporosis Knowledge Assessment Tool (OKAT). Bone mineral Density (BMD) was measured using Quantitative Ultrasonography (QUS).
Results: More than half (59.3%) of females had poor knowledge. A statistically significant relation was noted between the overall knowledge score and age, college, occupation, and socioeconomic class (P< 0.05 for each). Only 13.9% have low Z-score by QUS. By logistic regression, less carbonated beverages and coffee consumption positively affect BMD, while inadequate exercises level, indoor exposure to sunlight, and less frequency of brisk walking negatively affect BMD.
Conclusion: Unacceptable knowledge score significantly associated with BMD Z-score status. More attention should be devoted to education programs targeting adolescents and young females to promote knowledge about PBM and practice towards accrual and maintenance of bone health.
Keywords: PBM, BMD, Kuwaiti, knowledge
Introduction
Peak bone mass (PBM) is defined as the amount of bony tissue present at the end of the skeletal maturation. Approximately 40–60% of adult bone mass is accrued during the adolescent years,1 and PBM is achieved toward the end of the second decade of life, although there may still be some net bone deposition into the third decade.1
Optimizing PBM early in life and stabilizing it during young adulthood is receiving public health attention because it is considered a mean of attenuating the effects of postmenopausal bone loss among women.2
PBM is largely influenced by genetics and can be modified by many factors including ethnicity, gender, lifestyle behaviors (such as physical activity, smoking, caffeine, tea, carbonated beverage and alcohol consumption), diets (calcium and protein intake), endocrine status such as sex hormones, growth hormone, and insulin-like growth factor.3
Vitamin D, high socioeconomic level, and early menarche are thought to have positive effects on bone mineral density (BMD), while inadequate sun exposure and low body mass index (BMI) have negative effects.4,5
Women tend to experience minimal change in total bone mass between age 30 and menopause and are more prone to bone loss as a result of having smaller skeletons compared to males, and the estrogen patterns that accompany menarche and menopause.6
Previous studies on women revealed that bone loss may begin as early as late adolescence or as late as age 39 years in the femoral neck and age 49 years in the lumbar spine.7
BMD is an indirect measure of bone strength; a decrease of 1 SD in BMD significantly increases the risk of fractures by 50%, irrespective of the site where the measurement was taken.8
Therefore, identification of bone mass quality among young females at the peak of their bone mass can help in early detection and prevention of osteoporosis, consequently reducing the fracture-related morbidity and mortality as well as the overall socio-economic burden.9
Misunderstanding and lack of preventive action for low BMD are still common; making a primary prevention at an early age, which is widely understudied, is the preferable intervention.10
In order to plan for effective preventive programs, with necessary life changes, one requires sufficient information about people’s health knowledge, practices, and their cultural and socio-economical features.11
Thus, this research aimed to 1) assess the level of knowledge about determinants of PBM in apparently healthy young Kuwaiti females; 2) identify their bone health related behaviors; 3) screen them for low BMD, and, finally, 4) explore probable factors that could be associated with low BMD, hence a proper strategy could be initiated to reduce it later in life.
Materials and Methods
Study Design
A cross-sectional comparative study was carried out during the period from March, 2018 to March 2019.
Study Setting
Subjects were recruited from Kuwait University, Al-Khaldeya campus, which is comprised of three faculties, using multi-stage random sampling with proportionate allocation from five campuses.
Study Population and Sampling
Systematic random selection of 445 apparently healthy adult females during the academic year of 2018–2019 aged 18–35 years old was made by obtaining two different lists, one for the students and another for employees from the students and employees affairs departments from each of the selected faculties.
Ever married or females with a history of chronic illness (diabetes, HTN, rheumatoid, SLE, other autoimmune diseases) or current use of drugs that might affect bone mass and Vitamin D supplements for at least 3 months before participation in the current study were excluded.
Sample Size
Sample size was calculated using EPI info version 7, according to the assumption that the prevalence of low BMD was 50%, as the prevalence in postmenopausal women in Kuwait12 where the prevalence was unknown for younger ones using a 5% margin of error, confidence level of 95%, and response distribution of 80%, with oversampling to overcome any shortage in completing the study questionnaire and investigation.
Informed consent was obtained from those willing to participate during the academic year of 2017–2018. The study protocol was approved by the local Ethics Committee at the Faculty of Medicine for girls, Al-Azhar University, and ethics committee for scientific research at Kuwait Ministry of Health. All procedures were in accordance with the Declaration of Helsinki.
Study Tools
Eligible females were asked to complete a face-to-face interview semi-structured questionnaire contained the following:
- Detailed socio-demographics data (age, governorates, faculty, and socioeconomic class (SES)); history of age at menarche which was categorized into: ideal if age at menarche 11–14 years, early if age at menarche <11 years, and late if age at menarche >14 years, and family history of osteoporosis.
- Assessment of knowledge about age at maximum bone mineral density accrual and risk factors affecting bone health using Osteoporosis Attitude knowledge Test (OAKT) validated questionnaires.13,14 From which 13 questions concerning knowledge were chosen. The items were summed for a possible range of 0–13, with higher scores reflecting greater knowledge; according to the mean score the participants with a score ≥7 were considered to have good knowledge and those with a score <7 were considered to have poor knowledge.
- Lifestyle of the participants, including coffee and carbonated beverages consumption, habitual sun exposure during the last 3 months, and physical activity according to the World Health Organization (WHO) guidelines.15
- Anthropometric measurements were done using a scale to the nearest 0.2 kg and standing height was measured using a stadiometer to the nearest 0.1 cm wearing light clothes and no shoes. BMI was calculated as weight divided by height squared (kg/m2) and categorized into three groups: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (≥25−29.9 kg/m2), and obesity (≥30 kg/m2), according to WHO criteria.16
- Quantitative Ultrasound (QUS):
Dual-energy X-ray absorptiometry (DXA) and scans of axial skeletal sites is the standard assessment tool to diagnose low BMD, but its use is limited due to the high cost of scan, lack of portability, and deleterious effects of radiation.17 While QUS is relatively cheaper and portable, in addition it represents a surrogate marker of bone quality as for calcaneal site; also phalangeal QUS has been reported previously to be a reliable tool for bone health screening and may even predict osteoporotic fractures.18
Bone mass quality was assessed by QUS measurements using the Lunar Achilles Ultrasonometer In Sight (GE Healthcare, Chicago, IL, USA), with a temperature-controlled water-based system used as a coupling medium for wave propagation from the left calcaneus. The modified cutoff criteria at −1.5 as provided in previously published studies using QUS in young adults conducted in Saudi Arabia was used.19,20 Z-score ≤−1.5 is indicative of low BMD (Group I) and Z-score >-1.5 is indicative of a normal BMD (Group II).
The investigators pre-tested the questionnaire on 10% of the study population to ensure clarity and understanding. Modifications were made accordingly and those women were excluded from the study.
Statistical Design
Quantitative data were expressed in the form of mean±(SD), student’s T-test as well as analysis of variance (ANOVA) were used to study the difference in total scores for different groups of continuous variables. Qualitative data were expressed in number and percentage. Chi square-test was used for comparison between groups. Stepwise multivariate regression analysis was used to explore the effect of different variables on the Z-score. Significance level was taken at a P-value≤0.05. SPSS, version 25 (SPSS Inc, Chicago, IL, USA) was used for data analysis.
Results
Tables 1 and 2 summarize the participant’s general characteristics. The mean age of 445 healthy young females was 21.8 (±3.6) years, most (84.5%) of them ranged between 18–24 years old (85.2%), 52.4% were students and scientific faculties attaches, respectively. Nearly half (51.5%) were of middle socioeconomic class. Mostly (78.0%) of ideal menarche age and (47.6%) were overweight or obese. Low BMD Z-score at −1.5 by QUS was detected in 13.9% of females.
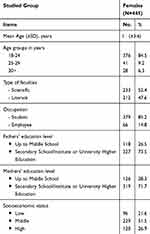 |
Table 1 Socio-Demographic Profile of the Studied Females |
 |
Table 2 General Profile of the Studied Females |
Figure 1A and B show the results of OKAT, only 1.6% answered correctly age of PBM accrual. The majority of females were aware that inadequate Vitamin D or sun exposure, advanced age, excess intake of carbonated beverages, lack of physical activity, and low body weight (83.4%, 81.8%, 81.3%, 76.2%, 72.6%, and 64.5%, respectively) are risk factors for low BMD. Although 57.2% correctly identified that women are at more risk than men, the majority (87.9% and 82.9%) was unaware that late and early menarche, respectively, were risk factors. Furthermore, nearly half (52.6% and 51.0%) were unaware that early menopause as well as excess intake of caffeine, respectively, affect BMD. The overall mean knowledge score was 6.7±(2.4) accordingly, with 59.3% having poor knowledge about bone health.
 |
Figure 1 (A) Females’ Knowledge about age of PBM accrual and determinants of peak bone mass. (B) Level of knowledge about bone health among studied females. |
Socio-demographic factors affecting knowledge toward low PBM disease were tested (Table 3), which revealed statistically significant differences when it came to: age group, occupation, type of college, socioeconomic level and positive family history of osteoporosis; while being insignificant to the residing area and parents ‘education.
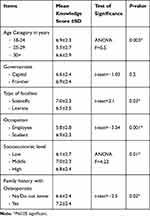 |
Table 3 Overall Knowledge Score According to Socio-Demographic Profile and Family History of Osteoporosis |
Regarding lifestyle, more females (63.0%) with a good knowledge score engaged in exercise than those with poor knowledge. Accordingly, the former reported an insignificantly higher physical activity level (28.7%) than the latter (20.1%).
A greater proportion (39.2%) of those with good knowledge are presently or have in the past had exposure outdoors, for 15–30 minutes (28.2%) and exposed more body parts, such as arms, face, hands, and legs (38.7%), with comparative figures (33.7%, 22.0%, 27.6%), respectively, among females with poor knowledge. Nearly every group applies sunscreen (72.7%, 77.9%, respectively) either sometimes or regularly (P>0.05).
Regular coffee consumption was slightly insignificantly higher among those of poor knowledge (89.8%) as well as those reported drinking ≥1 cup per day (66.7%); while, more carbonated beverages (76.9%) with a significant ≥1 can/bottle per day (47.0%) vs those of good knowledge (86.7%, 59.1%, 72.4%, 37.0% respectively). See Tables 4 and 5-and Supplementary Table S1
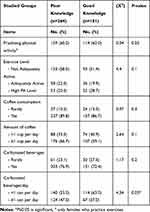 |
Table 4 Lifestyle Preventive Practices Among Studied Females According to Knowledge Level |
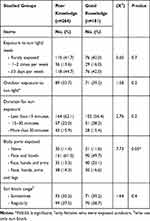 |
Table 5 Sun Exposure Among Studied Females According to Knowledge Level |
Fewer females (19.4%) with low BMD (Group I) have significantly good knowledge, compared to 44.1% of those with normal BMD (Group II) (Figure 2).
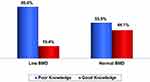 |
Figure 2 Knowledge level according to BMD status. |
The majority of women attained menarche between the ages of 11–14 years, an ideal age, in both groups. However, significantly more females (21.0%) of group I had late menarche, vs 10.7% in group II. Only 27.4% of group I were physically active, either adequately (12.9%) or highly active (14.5%); while in group II, nearly half (47.6%) were almost equally distributed adequately active (22.5%) or high physical activity level (25.1%). Nevertheless, overweight/obesity was higher among them (49.9%), P<0.05. The risk of having low BMD was significantly less than half for milk consumption, doubled for those with a history of indoor exposure (OR=2.5; 1.2–4.9), 4-times among coffee consumers (OR=4.40; 1.3–18.7), and tripled among those who consumed ≥1 can per day of carbonated beverages (OR=3.96; 1.77–8.86) (Table 6).
 |
Table 6 Risk Factors for Low BMD Experienced by Studied Females |
Regression analysis showed that BMD was positively significantly associated with good knowledge score about bone health, and less carbonated beverage or coffee consumption, while inadequate exercises level and indoor exposure to sunlight negatively affect it (P<0.05) (Supplementary Table S2).
Discussion
Low level of BMD, either osteopenia or osteoporosis, is generally asymptomatic and patients usually become aware of their condition when they develop a fracture. Thus, empowering women with knowledge about behaviors helping good bone health and encouraging them to take on those behaviors is important.21
This study clarifies the potential impact of adequate knowledge in optimizing bone mass and preventing bone loss in young Kuwaiti females below 35 years, using OKAT. It showed that more than half of females had insufficient information about determinants of PBM; where most of the women correctly recognized aging, inadequate sun exposure, as well as Vitamin D, lack of exercise, excessive carbonated beverages, and low body weight as risk factors for low BMD. However, more were not aware about other risk factors such as female gender, late and early menarche, early menopauses, and excessive intake of coffee. Similarly, Saudi Arabian young college women were unable to identify significant risk factors for low BMD.22
Also, inconsistant with other studies conducted in different regions which indicated that the adolescents or young adults had poor knowledge about bone health.13,22,23 On the contrary, Puttapitakpong et al24 reported a high knowledge score (85.2%) among Pakistani women. This difference may be attributed to variation in cutoff knowledge score.
Our findings revealed a significant relation of age, educational level, type of college, and socioeconomic level on one hand and the overall knowledge score on the other. In younger generations,13,25 however, some researchers found knowledge was independent of age.26,27 This discrepancy may be attributed to differences in the perceived risk of developing the disease with various age groups.
Furthermore, students had slightly more knowledge about bone health than employees; also scientific faculties’ attaches had a higher score than literate ones. These results in line with other studies that highlighted a difference based on type of education or study discipline where allied health colleges’ students showed a higher knowledge score than other colleges’ students.25,26 Moreover, women belonging to middle or high social class had higher knowledge score. That was compatible with other studies.22,27
In the current study, there was a discrepancy between knowledge and practice; although, many participants identified lack of physical activity as a risk factor for low BMD, however, the adequate or high physical activity were slightly more among those with good knowledge level. This can be explained by previous studies, which stated that young female generations were less active physically.27,28
Also, most females knew the importance of Vitamin D and sun exposure, but obviously slightly more females with good knowledge level insignificantly exposed outdoors for a longer period (≥15 minutes) and exposed more body parts (such as arms and legs) compared to females with poor knowledge who clearly avoid sun exposure. This was supported by researches27,28 that pointed to hot climate and dust storm as main contributors for sun exposure avoidance.
Inconsistent to other studies,29,30 coffee drinking and carbonated beverages are quite prevalent among studied females, although they knew them as risk factors, where they consumed them more frequently either in those with good or poor knowledge.
In the current study, the prevalence of low BMD at PBM age was low (13.9%), which is similar to other countries in the gulf region19,20 but higher than what was reported from the other parts of the world.31,32 The reason for this might include differences in diet and lifestyle or could be the tendency to avoid direct sunshine by our people and the preference of air-conditioned rooms. These findings highlight the need for using local cutoff values.
The striking features of the current study support an epidemiological study33 which revealed that delayed puberty and late menarche age may have a strong association with poor peak bone mass; as more females with low BMD reported significantly late menarche than those with normal BMD.
Our findings in relation to a positive significant association of BMI and BMD are in agreement to the results of Wilson et al,34 who revealed that a higher BMI is associated with a higher BMD and this is observed in premenopausal and even in younger women. In addition, Njeze et al35 observed that obesity is traditionally viewed to be beneficial to bone health because there is an established positive effect of mechanical loading as a result of the weight of the body on formation of bone. Some studies showed conflicting evidence, a higher body mass has a significant risk for fragility fracture, especially for fractures occurring at sites other than the hip.36
Concerning lifestyle behaviors; the practice of physical activity was associated with higher BMD in accordance with previous reports that emphasized the positive effect of physical activity on bone mass and preventing bone loss.37 A possible explanation of hindering the attainment of PBM includes low exercise activity among females due to social factors.19
Females with lower BMD had a higher caffeine intake, which is in line with Antonioli and Haskó,38 who stated that high intakes of caffeine are associated with bone loss. On the contrary other reports found no evidence contraindicating the consumption of the equivalent of three-to-four cups of coffee per day in healthy adults.39 Thus, the effects of coffee on bone metabolism remain controversial because they are associated with nutrition, exercise, alcohol intake, smoking and lifestyle factors, as well as the amount of caffeine contained in the coffee.4
In the present study, consuming ≥1 can per day of carbonated beverages was associated with low BMD. As reported by Tucker et al,40 who found an inverse association between carbonated soft drink consumption, particularly cola beverages, and BMD in adolescent girls that can be explained by Olsen et al,41 who revealed that consumption of carbonated beverages among adolescents usually occur at the expense of milk consumption as one fifth of low BMD rarely consumed milk in our study.
In accordance with a previous study,24 exposure to sunrays behaviors was an important predictor of low BMD, as females in the normal BMD group were exposed to sunrays outdoors more often than in the low group.
In fact, stepwise multivariate analysis clarified that having a good knowledge score about bone health, less carbonated beverages, and coffee consumption was associated with normal BMD, while lack of exercise and sun exposure were associated with lower BMD. These results came in consistent with other studies.24,37 However, these findings should be interpreted with caution because the study was cross-sectional and does not show a cause and effect relation. Further longitudinal studies are therefore required to support such claims.
Conclusion and Recommendations
There was a lack of specific knowledge about low BMD risk factors. An unacceptable knowledge score was significantly associated with BMD Z-score status. Also, the participants’ preventive behaviors were found to be low. The sample in the current study consisted of women in the age group of 18–35 years, so the results of low BMD are significant, suggesting potential use of screening measures for osteopenia and osteoporosis in early/middle aged women. Initiatives should be planned with a view to translate a high knowledge level to healthy behaviors that contribute to better bone health and improve quality-of-life.
Disclosure
Najla Al-Ayyadhi reports grants from Kuwait Foundation for advanced Science (KFAS) Project ID: PR18-13MM, during the conduct of the study. Mahasen Mohamed Ibrahim reports grants and non-financial support from KMOH, during the conduct of the study; grants and non-financial support from Kuwait Ministry of Health, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Sasson H, Carpenter CL. Achievement of peak bone mass in women is critically dependent on adolescent calcium intake. OA Sports Medicine. 2013;1(2):16. doi:10.13172/2053-2040-1-2-908
2. Al-Mendalawi M. Low bone mineral density and its risk factors in an urban adult population of South India. Int J Health Allied Sci. 2019;8(4):288. doi:10.4103/ijhas.IJHAS_8_19
3. Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281–1386.
4. Khwanchuea R. Determinants of Bone Mass. Merit Res J. 2014;2(2):29–40.
5. Gordon CM, Zemel BS, Wren TA, et al. The Determinants of Peak Bone Mass. J Pediatr. 2017;180:261–269.
6. Lee N, Kim J. A review of the effect of swim training and nutrition on bone mineral density in female athletes. J Exe Nutrition Biochem. 2015;19(4):273.
7. Bainbridge K, Sowers M, Crutchfield M, Lin X, Jannausch M, Harlow S. Natural history of bone loss over 6 years among premenopausal and early postmenopausal women. Am J Epidemiol. 2002;156(5):410–417.
8. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733.
9. Multani N, Kaur H, Chahal A. Impact of sporting activities on bone mineral density. J Exe Sci Physiotherapy. 2011;7(2):103.
10. Organization WH. Scientific Group on the Prevention and Management of Osteoporosis. Report. Geneva: WHO; 2003.
11. Des Bordes J, Prasad S, Pratt G, Suarez-Almazor ME, Lopez-Olivo MA. Knowledge, beliefs, and concerns about bone health from a systematic review and metasynthesis of qualitative studies. PLoS One. 2020;15(1):e0227765. doi:10.1371/journal.pone.0227765
12. Al-Shoumer KAS, Nair V. Prevalence of low bone mass in postmenopausal Kuwaiti women residents in the largest province of Kuwait. Arch Osteoporos. 2012;7(1–2):147–153. doi:10.1007/s11657-012-0092-1
13. Hatta NNKNM, Nurumal MS, Isa MLM, et al. Knowledge and Attitudes of Maintaining Bone Health among Post-Menopausal Women in Malaysia. Central Asian J Global Health. 2019;8(1):1. doi:10.5195/cajgh.2019.348
14. Sayed-Hassan RM, Bashour HN. The reliability of the Arabic version of osteoporosis knowledge assessment tool (OKAT) and the osteoporosis health belief scale (OHBS). BMC Res Notes. 2013;6(1):138. doi:10.1186/1756-0500-6-138
15. Gerovasili V, Agaku IT, Vardavas CI, Filippidis FT. Levels of physical activity among adults 18–64 years old in 28 European countries. Prev Med. 2015;81:87–91. doi:10.1016/j.ypmed.2015.08.005
16. World Health Organization W. WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Level; 2007.
17. Rudani S. Prevalence of osteopenia and osteoporosis among women in Bhuj, Gujarat, India–A cross‑sectional study. ParipexIndian J Res. 2016;5:83–84.
18. Catalano A, Morabito N, Basile G, et al. Fracture risk assessment in postmenopausal women referred to an Italian center for osteoporosis: a single day experience in Messina. Clin Cases Mineral Bone Metabo. 2013;10(3):191.
19. Al-Habdan IM, Sadat-Ali M, Al-Muhanna FA, Al-Elq AH, Al-Mulhim AA. Bone mass measurement using quantitative ultrasound in healthy Saudi women. A cross-sectional screening. Saudi Med J. 2009;30(11):1426–1431.
20. Zeidan ZA, Sultan IE, Guraya SS, Al-Zalabani AH, Khoshhal KI. Low bone mineral density among young healthy adult Saudi women. Prevalence and associated factors in the age group of 20 to 36 years. Saudi Med J. 2016;37(11):1225–1233. doi:10.15537/smj.2016.11.16248
21. Al-Muraikhi H, Chehab MA, Said H, Selim N. Assessing health beliefs about osteoporosis among women attending primary health care centres in Qatar. J Taibah Univ Med Sci. 2017;12(4):349–355. doi:10.1016/j.jtumed.2016.11.003
22. Alshareef SH, Alwehaibi A, Alzahrani A, Fagihi A, Alkenani AA. Knowledge and Awareness about Risk Factors of Osteoporosis among Young College Women at a University in Riyadh, KSA. Journal of Bone Research. 2018;6(02):2. doi:10.4172/2572-4916.1000194
23. Ngozi RN, Ikechukwu O, Miriam A, Olanike A-U, Ulugo DA, Nneze CN. Awareness of osteoporosis in a polytechnic in Enugu, South East Nigeria. Arch Osteoporos. 2017;12(1):51. doi:10.1007/s11657-017-0342-3
24. Puttapitakpong P, Chaikittisilpa S, Panyakhamlerd K, Nimnuan C, Jaisamrarn U, Taechakraichana N. Inter-correlation of knowledge, attitude, and osteoporosis preventive behaviors in women around the age of peak bone mass. BMC Women’s Health. 2014;14(1):35. doi:10.1186/1472-6874-14-35
25. Khan YH, Sarriff A, Khan AH, Mallhi TH. Knowledge, attitude and practice (KAP) survey of osteoporosis among students of a tertiary institution in Malaysia. Tropical J Pharm Res. 2014;13(1):155–162. doi:10.4314/tjpr.v13i1.22
26. Elnaem MH, Jamshed SQ, Elkalmi RM, et al. Osteoporosis Knowledge among future healthcare practitioners: findings from a Malaysian public university. J Pharm Bioallied Sci. 2017;9(2):115.
27. Barzanji AT, Alamri FA, Mohamed AG. Osteoporosis: a study of knowledge, attitude and practice among adults in Riyadh, Saudi Arabia. J Community Health. 2013;38(6):1098–1105. doi:10.1007/s10900-013-9719-4
28. Al-Raddadi R, Bahijri S, Borai A, AlRaddadi Z. Prevalence of lifestyle practices that might affect bone health in relation to vitamin D status among female Saudi adolescents. Nutrition. 2018;45:108–113. doi:10.1016/j.nut.2017.07.015
29. Rastgoo F, Vasli P, Rohani C, Amini A. Predictors of osteoporosis preventive behaviors among adolescent.. Biomed Res. 2019;30(2):280–287. doi:10.35841/biomedicalresearch.30-19-050
30. Khani Jeihooni A, Kashfi SM, Khiyali Z, Jamshidi H, Kashfi SH. The effect of education based on based health belief model on osteoporosis and bone mineral density among women. J Res Health. 2019;9(1):11–20. doi:10.29252/jrh.9.1.11
31. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. doi:10.1002/jbmr.2269
32. Compston J. NOGG and NICE: new guidelines and quality standards for osteoporosis. Maturitas. 2017;106:97–98. doi:10.1016/j.maturitas.2017.08.002
33. Bharathi R. Influence of age at menarche on bone mineral density of rural women. Indian J Appl Res. 2015;5:149–151.
34. Wilson S, Sharp CA, Davie MWJ. Health-related quality of life in women referred for bone density assessment: relationships with bone mineral density, fracture and co-morbidity. Quality Life Res. 2015;24(5):1235–1243. doi:10.1007/s11136-014-0851-0
35. Njeze NR, Agwu-Umahi O, Ezeofor SN, et al. Reduced bone mineral density in nigerian women: A Prevalence Study. Nigerian J Orthopaedics Trauma. 2019;18(1):9. doi:10.4103/njot.njot_32_18
36. Compston JE, Watts NB, Chapurlat R, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124(11):1043–1050. doi:10.1016/j.amjmed.2011.06.013
37. Lombardi G, Ziemann E, Banfi G. Physical activity and bone health: what is the role of immune system? A narrative review of the third way. Front Endocrinol. 2019;10.
38. Antonioli L, Haskó G. Caffeine and Bones: If Less is Good, More May Not Be Better. publishers 140 Huguenot Street, 3rd Floor New: Mary Ann Liebert, Inc; 2019.
39. Bolignano D, Coppolino G, Barillà A, et al. Caffeine and the kidney: what evidence right now? J Renal Nutrition. 2007;17(4):225–234.
40. Tucker KL, Morita K, Qiao N, Hannan MT, Cupples LA, Kiel DP. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: the Framingham Osteoporosis Study. Am J Clin Nutr. 2006;84(4):936–942.
41. Olson ML, Maalouf NM, Oden JD, White PC, Hutchison MR. Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J Clin Endocrinol Metab. 2012;97(1):279–285.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
