Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Sclerotherapy in Lymphatic Malformations with Intralesional Hemorrhage: A Retrospective Comparison with Non-Hemorrhagic Lymphatic Malformations
Authors Wang W , Liu B , Long J , Bi J , Huo R
Received 19 August 2022
Accepted for publication 19 October 2022
Published 26 October 2022 Volume 2022:15 Pages 2275—2284
DOI https://doi.org/10.2147/CCID.S386813
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Wenjing Wang,1 Boce Liu,1 Junsong Long,1 Jianhai Bi,2 Ran Huo1,2
1Department of Plastic Surgery, Shandong Provincial Hospital, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Plastic Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China
Correspondence: Ran Huo, Department of Plastic Surgery, Shandong Provincial Hospital, Shandong University, Jinan, Shandong, People’s Republic of China, Tel +86-15168889001, Fax +86 531-68778153, Email [email protected]
Purpose: To compare the outcomes of sclerotherapy with bleomycin and lauromacrogol between lymphatic malformations (LM) with and without intralesional hemorrhage and identify the factors affecting the outcomes of LM with hemorrhage.
Patients and Methods: This retrospective study examined patients with LM who underwent sclerotherapy with bleomycin and lauromacrogol between January 2019 and December 2021. Regression models were used to analyze the factors associated with the outcomes of LM with hemorrhage.
Results: 52 patients were included in the study, including 26 with intralesional hemorrhage. Masses with bluish skin (p = 0.026) and pain (p = 0.001) were more common in LM with hemorrhage. With similar outcomes, the average number of sessions was 2.9 in LM with hemorrhage and 2 in LM without hemorrhage (p = 0.028). The efficacies of the macrocystic and mixed types (93.3% and 83.3%, respectively) were higher than that of the microcystic type (40%) (p = 0.036). As the number of sessions increased, the relapse rate decreased (p = 0.018).
Conclusion: Sclerotherapy with bleomycin and lauromacrogol is effective and safe for LM with hemorrhage. An increased number of injections for patients with hemorrhage was associated with similar efficacy for those without hemorrhage and similar relapse rates.
Keywords: lymphatic malformations, hemorrhage, sclerotherapy, bleomycin, lauromacrogol
Corrigendum for this paper has been published.
Introduction
Lymphatic malformations (LM) are rare low-flow vascular malformations that consist of dilated lymphatic vessels that arise from embryological disturbances in the development of the lymphatic system.1–3 LM is formerly known as lymphangiomas, which are congenital vascular malformations secondary to venous malformations.4 The incidence in children is 1/4000–1/2000, accounting for approximately 5% of benign tumors in children.5 Additionally, the incidence is approximately 1:1 between men and women, with no racial differences.6 LM are more common in lymphatic-rich areas, such as the head and neck. In these areas, the incidence is approximately 66%.7 Around 90% of patients with LM have the disease before the age of 2 years, while 50% of patients with LM have the disease at birth.2,8 Depending on the size of lymphatic cysts, LM can be divided into macrocystic, microcysttic, and mixed types. A maximum diameter of more than 1 cm with the number of cysts exceeding 50% is macrocystic; a maximum diameter less than 50% is mixed; and a maximum diameter of less than 1 cm is microcystic.9 Several researchers classified cysts according to their volume.10 The macrocystic type contains one or more cysts of ≥ 2 cm3 in volume; the microcystic type consists of multiple < 2 cm3 cysts; and the mixed type has both.
LM presents as painless masses under normal skin in most cases. Some LM enlarge rapidly due to intralesional hemorrhage after trauma or other causes.7 If the lesions where bleeding occurs are located in special parts, such as the head, neck, and joints, it may lead to disfigurement, deformity, compression of vital organs, long-term sequelae, and even life-threatening complications.8 Hence, patients suffering from LM with intralesional hemorrhage should undergo prompt intervention. Sclerotherapy has emerged as the first-line management for LM, with an efficacy rate of 71%-97%.11 Although there are a few studies on LM with hemorrhage, the choice of sclerosing agents, number of injections, and efficacy remain controversial.
Therefore, this retrospective study aimed to compare the outcomes of sclerotherapy with bleomycin and lauromacrogol between LM with and without intralesional hemorrhage and to identify the factors affecting the outcomes of LM with hemorrhage.
Materials and Methods
Study Population
We consecutively included patients with LM with intralesional hemorrhage who underwent percutaneous sclerotherapy at our center between January 2019 and December 2021 in group A. Patients with LM without intralesional hemorrhage treated with sclerotherapy during the same period at our center were also reviewed. The diagnosis of intralesional hemorrhage was based on cystic puncture fluid and cytology. If the fluid was bloody and the cytology shows lymphocytes and red blood cells with macrophages or not, the diagnosis would be made. Considering the excessive number of children with non-hemorrhagic LM, only some were included in group B. To ensure homogeneity between the included patients and the whole population, the R-3.6.2 software was used to randomly select patients diagnosed with non-hemorrhagic LM for inclusion into the group.12 We entered patients’ information into the software digitally to make sure the choice of patients was random. The inclusion criteria were as follows: (1) patients diagnosed with LM by two plastic surgeons based on the criteria of the International Society for the Study of Vascular Anomalies (ISSVA) classification;13 (2) patients who completed the standard sclerotherapy with a complete medical record; and (3) patients in group A with intralesional hemorrhage and patients in group B without intralesional hemorrhage. The exclusion criteria were as follows: (1) patients who received other treatments; (2) patients with lung disease (eg asthma and pulmonary fibrosis); (3) patients with abnormal liver and kidney function; (4) patients allergic to sclerosing agents; (5) patients with complex syndromes with other vascular malformations (eg Gorham-Stout syndrome, congenital lipomatous (fatty) overgrowth, epidermal nevi and scoliosis/skeletal/spinal anomalies (CLOVES syndrome)); and (6) patients who participated in other clinical trials.
The medical records of patients with LM who underwent sclerotherapy, including age at onset and treatment initiation, sex, type, location affected by LM, complications of the lesion, volume before and after treatment, number of sessions, improvement scale of symptoms, relapse, and adverse events, were collected. The types of LM were classified as macrocystic, microcystic, or mixed based on the size of the cysts.14 The macrocystic type contained lesions with cysts >1 cm. The microcystic type contained lesions with cysts <1 cm. Mixed lesions contained both macrocystic and microcystic components.
Treatment and Follow-Up
Preprocedural imaging included ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI), and the choice of imaging examination was based on the patients’ or parents’ opinions and the characteristics of LM. All sclerotherapy procedures were performed under general anesthesia.
The sclerosants used were bleomycin and lauromacrogol. Bleomycin (15 mg, 15U) was diluted with physiological saline (15 mL to a concentration of 1 mg/mL). After adjusting the dosage according to the size of the lesion, the single dose did not exceed 2 mg, while the total course dose did not exceed 15 mg. In addition, a three-way valve connecting two injection syringes was used: one contained 1% lauromacrogol liquid (10 mL; 100 mg), while the other contained air. The syringes were then injected toward each other approximately 30 times via the eddy method to create a liquid-to-air ratio of 1:4.15 The dosage of each course was no more than 2mL. The procedures were performed in an interventional radiologic suite using sterile low-frequency ultrasound and a probe to monitor the needle path and tip position. Both lymphatic fluid extraction and drug injection into the cysts were performed under ultrasonic guidance. First, we extracted as much lymphatic fluid or hematocele as possible and conducted the cytological examination. The injection of bleomycin was followed by injection of lauromacrogol foam into the lesion. After sclerotherapy, the injection site was compressed with sterile gauze for 5 min for hemostasis, and erythromycin ointment was applied externally, all of which were pressed with an elastic sleeve for 48 h. Postoperative care included close observation of the patient’s vital signs, such as body temperature, and monitoring whether the patient had rapid swelling of the lesion and whether the blood circulation in the extremities had been compromised while the wound was pressurized and bandaged. If multiple procedures were required, they were spaced four weeks apart, which could be evaluated via ultrasound in the outpatient setting.
The efficacy evaluation criteria were based on the volume change of lesions before and after treatment. A volume reduction greater than 75% was given 3 points. A volume reduction ranging from 50 to 75% corresponded to 2 points. A volume reduction ranging from 25 to 50% corresponded to 1 point. Lastly, a volume reduction less than 25% corresponded to 0 points. The satisfaction scale included appearance, family, study or work, and society, with three points for satisfaction in four aspects, two points for satisfaction in three aspects, one point for satisfaction in two aspects, and zero point for the rest.16 The response or satisfactory rate was defined as the proportion of patients with 3 or 2 points in each group: effective rate = number of patients (3 points+2 points) / all patients in the group (3 points+2 points+1 point+0 points). Two experienced physicians, who were not involved in the treatment, in-dependently assessed the efficacy and reached a consensus. If they disagreed, conflict was resolved by a professional radiologist. Adverse events during the treatment were also recorded. During the follow-up period, if the lesion was enlarged by more than 50% of the volume after the last session, it was considered a relapse.
Statistical Analyses
The general characteristics and outcomes of sclerotherapy were compared between the two groups. The general characteristics included the age at treatment initiation and onset, sex, type, location, complications of LM, and lesion volume before treatment. The latter included the number of sessions, grade of efficacy and satisfaction, improvement of symptoms, relapse rate, and adverse events. It should be noted that the two groups of children were matched, so that their treatment outcomes were comparable. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 26.0 (IBM, Armonk, NY, USA). Data with a skewed distribution are expressed as mean values. Categorical data are presented as percentiles. The data were analyzed using the Pearson’s chi-square test, Yates corrected chi-squared test, Mann–Whitney U-test, Kruskal–Wallis H-test, or Fisher’s exact test depending on the sample volume. A p value of < 0.05 was considered statistically significant.
Results
Patient Demographics and Patient Characteristics
A total of 26 patients with LM with intralesional hemorrhage were classified as group A. On the other hand, 26 patients with non-hemorrhagic LM were randomly selected and recorded as group B (Figure 1).
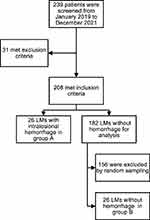 |
Figure 1 Screening of the participants. |
The two groups were similar in six aspects, including age at treatment initiation and onset, sex, and type, location, and volume of the lesion before treatment. Their differences had no statistical significance. However, the clinical manifestations of the two groups, such as pain and skin color, were statistically significant. The average age at initial treatment in group A was 35.7 months, while that in group B was 35.9 months. The mean age of onset was 8.3 months, while that in Group B was 9.6 months. Most patients had an abnormal mass before 1 year. The ratio of the incidence between men and women was 1.36:1 in Group A and 1.17:1 in Group B. The main types of lesions were macrocystic, of which 61.5% were in group A, and 53.8% in group B. Locations included the head and neck, trunk, limbs, and axilla, with 11, 4, 9, and 2 cases in group A and 8, 6, 9, and 3 cases in group B, respectively. Images before treatment showed that the average lesion volume in group A was 40.4 cm3, while that in group B was 24.7. In terms of clinical manifestations, 61.5% of patients in group A had a mass with bluish skin, whereas 69.2% of patients in group B manifested a mass with normal skin (p = 0.026). Based on subjective pain evaluation or the children’s facial expression pain scale, 18 patients in group A reported pain, compared to only 5 in group B (p = 0.001). The mean follow-up was 23.7 months (range: 3.7–39.2 months). The general characteristics of the patients in both the groups are shown in Table 1.
 |
Table 1 The General Characteristics of Patients in Two Groups |
Outcomes of Sclerotherapy
There was a statistically significant difference in the number of sessions between the two groups (p = 0.028), whereas there were no statistical differences in the changes in lesion volume, patient and family satisfaction, incidence of treatment-related adverse effects, and recurrence rate. The number of sessions was one to seven in Group A, with an average of 2.9 (range: 1–7 sessions), while the mean number of sessions in Group B was two (range: 1–4 sessions). The effective rates of lesion volume change in groups A and B were 80.8% and 76.9%, respectively, whereas the satisfaction rates were 80.8% and 69.3%, respectively. Among the patients presenting with pain, 11 (61.1%) in group A and three (60%) in group B completely recovered from pain after sclerotherapy. During the treatment course, five patients in group A had treatment-related adverse effects, including three cases of pigmentation at the injection site and two cases of fever. Two patients in group B had pigmentation, all of which improved without intervention. During the follow-up period, six patients in group A had lesion enlargement. They were thus diagnosed with relapse, with a mean recurrence time of 23.5 months (range 12.4–32.3 months). A total of four patients was given sclerotherapy again, while two patients were given sclerotherapy combined with surgery. There were two cases of relapse in group B, with a recurrence time of 17.9 months and 24.1 months, and both patients underwent sclerotherapy again. A comparison of the outcomes between the two groups is presented in Table 2.
 |
Table 2 Comparisons of the Differences of Reduction in Size, No. of Sessions, Satisfaction, Incidence of Adverse Events and Relapse Between the Two Groups |
Factors Associated with Sclerotherapy Outcomes for LM with Hemorrhage
The interval from rapid mass enlargement to treatment, age of onset, sex, type and location of lesions, and number of sessions were included as potential influencing factors associated with the efficacy, adverse effects, and relapse for LM with intralesional hemorrhage. It was found that the efficacy of different types was statistically significant (p = 0.036), and the efficacy of macrocystic and mixed types was higher than that of the microcystic type, with efficacies of 93.3%, 83.3%, and 40%, respectively. A female patient with macrocystic LM with hemorrhage in the chest wall was sent to our department because the suddenly enlarged mass (Figure 2A–C). After three times of sclerotherapy, her clinical manifestation significantly improved (Figure 2D–H). Treatment-related adverse effects included pigmentation and postoperative fever, and the analysis of each factor did not differ significantly. However, as the number of treatments increased, the recurrence rate decreased (p = 0.018). Among patients with less than or equal to three sessions, five patients (50%) had relapsed, compared with one (6.3%) in those with more than three sessions. The analysis of factors associated with the outcomes is shown in Table 3.
 |
Table 3 Univariate Analysis Associated with Efficacy, Adverse Effects and Relapse on LM with Intralesional Hemorrhage |
Discussion
In this study, 52 patients with LM were included, with an incidence rate of 1.26:1 between men and women. The ratio of male to female patients with hemorrhage was 1.36:1, considering that the incidence of trauma in boys was higher than that in girls, and the intralesional hemorrhage of LM is mostly related to the collision of lesions.17 Depending on the size of lymphatic cysts, LM can be divided into macrocystic, microcystic, and mixed types.
Intralesional hemorrhage occurred mostly in macrocystic LM. A total of 15 cases (57.7%) of LM with hemorrhage in this study were macrocystic, possibly because macrocystic LM were larger and more susceptible to external trauma.18,19 Additionally, they had fewer peripheral tissue connections. On the other hand, macrocystic LM can easily extract lymph, and the diagnostic criterion for hemorrhage is bloody lymphatic fluid, thus improving the diagnostic rate. LM can occur in various parts of the lymphatic network distribution, including the head, neck, armpits, mediastinum, and limbs. Most appear as painless skin-colored masses.3,6 In this study, LM with hemorrhage was mainly located in the head and neck (42.3%) and limbs (34.6%), whereas 18 (69.2%) children showed various degrees of pain, which might be related to the rapid increase in the volume of the cyst and the inflammatory response after intracystic bleeding. Sixteen cases (61.5%) manifested masses with bluish skin. Imaging examination found that these lesions were mostly located in the superficial subcutaneous fascia layer, while the masses with normal skin were mostly in the deep fascial layer or muscle layer, where bleeding was difficult to observe. Thus, physicians should be alerted when LM patients who experienced trauma at the lesion presented with painful bruised lesions because these lesions could possibly be LM with intralesional bleeding. Thus, timely treatment was needed to alleviate the symptoms of compression, thereby avoiding unnecessary damage caused by further development of the hemorrhage.
Cystic puncture fluid is usually yellowish and clear. When it is combined with intralesional bleeding, it becomes bloody. In this study, lymphatic fluid from 26 patients with LM with intralesional hemorrhage was sent for cytological examination, and the results showed mature lymphocytes, red blood cells, and phagocytes, of which the morphology and number of phagocytes varied greatly. This might be related to the time and location of bleeding. Further research is needed.
LM progresses slowly and rarely resolves naturally.3 However, it can enlarge suddenly due to intracystic hemorrhage after trauma or inappropriate invasive treatment. In particular, lesions located in the face, neck, arms, and legs can cause disfigurement, deformity, or compression symptoms, such as respiratory obstruction or motor function limitation, causing a serious impact on patients and even endangering life. Therefore, patients with hemorrhage should be treated appropriately and in a timely manner. Surgery was the main treatment in the past, but it was gradually replaced by sclerotherapy due to increased perioperative bleeding and easy damage to important tissues.9 Studies found that the efficacy of sclerotherapy was good due to its associated minimal invasion, simplicity, and resulting fewer scars.11,13 The sclerosants included bleomycin, absolute ethanol, OK-432 (picibanil), ethanolamine oleate, polidocanol, doxycycline, and sodium tetradecyl sulfate (STS).20–22 Notably, absolute ethanol and bleomycin are more efficacious than other sclerosants. However, absolute ethanol is highly corrosive, thus easily causing tissue necrosis.23 Therefore, bleomycin was used as the primary sclerosant in this study. By binding to the DNA of lymphatic endothelial cells (LECs), bleomycin inhibited DNA synthesis, cut DNA strands, damaged endothelial cells, thickened walls, narrowed the lumen, and eventually caused focal atrophy. However, bleomycin has dose-dependent pulmonary toxicity, and there is a risk of pulmonary fibrosis when the cumulative dose exceeds 160 mg.24 Therefore, in this study, the cumulative dose of bleomycin did not exceed 15 mg. As a result, there was no pulmonary toxicity-related side effects. Simultaneously, lauromacrogol, a secondary sclerosing agent, was combined with bleomycin to destroy the cell membrane of LECs, cause organ fibrosis, prolong the action time of bleomycin in the lesion, reduce the amount of bleomycin, and accelerate luminal occlusion and lesion atrophy.
In this study, no statistical differences in efficacy, satisfaction, side effects, and recurrence were found between the two groups by comparing the outcomes of sclerotherapy for LM with intralesional hemorrhage versus those without hemorrhage. However, patients with bleeding required more injections (p = 0.028). There might be two reasons. For the patients with old intralesional hemorrhage, hemorrhage could cause inflammatory response and the medication was not easy to penetrate into the endothelial tissues. For those patients with fresh hemorrhage, more sessions of sclerotherapy were given to compression hemostasis. The efficacy of percutaneous sclerotherapy with bleomycin and lauromacrogol in the treatment of LM with hemorrhage was 80.8%, of which the efficacy of the macrocystic type was 93.3%, while that of the mixed type was 83.3%, which was significantly higher than that of the microcystic type (40%) (p = 0.036). This is probably because the macrocystic lesions can easily extract lymph, and bleomycin and lauromacrogol can be in full contact with the cyst wall to take effect. However, the microcystic type has smaller cysts, which are difficult to extract completely, thereby resulting in reduced efficacy. Thus, during the operation, the lymphatic fluid in the cysts should be extracted as much as possible so that the sclerosant is in full contact with the LECs. This study showed that the symptom improvement rate in patients with LM with hemorrhage was 61.1%, and 11 of the 18 children with pain were effectively relieved after sclerotherapy. A total of 21 children and their families was satisfied with the treatment, with a satisfaction rate of 80.8%. Sclerotherapy for LM with bleeding can effectively alleviate the symptoms and improve the physical and mental health of children. During the treatment period in this study, a total of five (19.2%) patients experienced mild treatment-related side effects, including pigmentation of injection sites and fever, which improved without any intervention. Notably, no pulmonary toxic side effects, such as pulmonary fibrosis, were observed. During the follow-up period, a total of six (23.1%) patients was diagnosed with relapse, with an average recurrence time of 23.5 months. The influencing factors related to relapse were analyzed, and the number of treatments was related to the relapse rate (p = 0.018). There was one (6.3%) patient with more than three treatments compared to five (50%) patients with sessions ≤3. As the number of sessions increases, more sclerosants are injected into lymphatic cysts and LECs to reduce the possibility of recanalization or neocysts probably. Treatment after recurrence included sclerotherapy alone combined with surgical resection. The prognosis of patients who underwent this kind of treatment was good. Among the patients who relapsed, four cases showed that the lesion volume increased rapidly after trauma, and the lymph fluid was bloody. By reviewing the patients’ previous imaging data, it was found that the cystic cavity edge showed a blood flow signal of the venule or arteriole, considering whether the rebleeding was related to the small blood vessels, and embolizing these vessels around the cysts might reduce the occurrence of rebleeding, which needed to be confirmed by further clinical studies.
The limitation of this study is the small number of patients. Only 52 patients were included, consisting of 26 patients with hemorrhage and 26 patients without hemorrhage. In addition, the longest follow-up period in our cohort was 39 months, which failed to outline the long-term efficacy and adverse events of sclerotherapy. Furthermore, there were inevitable biases in this retrospective study, such as withdraw bias and selection bias. Nevertheless, we took these measures to minimize bias: (1) only patients with complete medical records and follow-up were included; (2) patients in group B were randomly selected to make sure the homogeneity between the included patients and the respective study population; (3) general characteristics were compared between the two groups to ensure the comparability of treatment outcomes. At last, this study used non-validated scales to measure volume reduction and patient satisfaction. Although some objective and subjective scales have been applied in other papers, there is no unified standard in how to evaluate the curative effect of sclerotherapy. A validated scale is needed.
Conclusions
After the occurrence of intralesional hemorrhage in LM, the volume of the lesion can suddenly increase, resulting in symptoms of compression, deformity, disfigurement, dysfunction, and even death, in which case instant treatment was essential. Moreover, ultrasound-guided percutaneous sclerotherapy with bleomycin and lauromacrogol for LM with bleeding is an effective and safe treatment and an increased number of treatment sessions for patients with hemorrhage was associated with similar efficacy for those without hemorrhage and similar relapse rates. An increase in the number of sessions does not increase additional adverse effects. However, these findings need to be confirmed in prospective multicenter studies with larger sample sizes.
Abbreviations
LM, lymphatic malformations; CT, computed tomography; MRI, magnetic resonance imaging; STS, sodium tetradecyl sulfate; LECs, lymphatic endothelial cells.
Ethics Approval and Informed Consent
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Shandong Provincial Hospital (SWYX: NO.2022-235). Informed consent was obtained from all individual participants’ parents included in the study. The authors affirm that human research participants provided informed consent for publication of the images in Figure 2.
Acknowledgments
We would like to acknowledge all the patients and their parents who participated in the test and we are grateful for their kind contributions.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This research was funded by the National Nature Science Foundation of China, NO. 82172227 and the Clinical Medical Science Innovation Program of Jinan, 202019076.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kulungowski AM, Patel M. Lymphatic malformations. Semin Pediatr Surg. 2020;29:150971. doi:10.1016/j.sempedsurg.2020.150971
2. Mäkinen T, Boon LM, Vikkula M, Alitalo K. Lymphatic malformations: genetics, mechanisms and therapeutic strategies. Circ Res. 2021;129:136–154. doi:10.1161/CIRCRESAHA.121.318142
3. Oliver G, Kipnis J, Randolph GJ, Harvey NL. The lymphatic vasculature in the 21st Century: novel functional roles in homeostasis and disease. Cell. 2020;182:270–296. doi:10.1016/j.cell.2020.06.039
4. Bertino F, Trofimova AV, Gilyard SN, Hawkins CM. Vascular anomalies of the head and neck: diagnosis and treatment. Pediatr Radiol. 2021;51:1162–1184. doi:10.1007/s00247-021-04968-2
5. Colbert SD, Seager L, Haider F, Evans BT, Anand R, Brennan PA. Lymphatic malformations of the head and neck-current concepts in management. Br J Oral Maxillofac Surg. 2013;51:98–102. doi:10.1016/j.bjoms.2011.12.016
6. De Leacy R, Bageac DV, Manna S, et al. A radiologic grading system for assessing the radiographic outcome of treatment in lymphatic and lymphatic-venous malformations of the head and neck. AJNR Am J Neuroradiol. 2021;42:1859–1864. doi:10.3174/ajnr.A7260
7. Furue A, Mochizuki J, Onishi Y, et al. Ultrasonic findings of fetal axillary lymphangioma with intralesional hemorrhage. J Med Ultrason. 2016;43:285–289. doi:10.1007/s10396-015-0695-4
8. Patel KC, Kalantzis G, El-Hindy N, Chang BY. Sclerotherapy for orbital lymphangioma - case series and literature review. Vivo. 2017;31:263–266. doi:10.21873/invivo.11055
9. Anggreyni G, Agustriani N, Agustriani N, Gunadi G. Comparison of different therapeutic approaches for children with common lymphatic malformation. Med J Malaysia. 2020;75:32–36.
10. Bouwman FCM, Kooijman SS, Verhoeven BH, et al. Lymphatic malformations in children: treatment outcomes of sclerotherapy in a large cohort. Eur J Pediatr. 2021;180:959–966. doi:10.1007/s00431-020-03811-4
11. De Maria L, De Sanctis P, Balakrishnan K, Tollefson M, Brinjikji W. Sclerotherapy for lymphatic malformations of head and neck: systematic review and meta-analysis. J Vasc Surg Venous Lymphat Disord. 2020;8:154–164. doi:10.1016/j.jvsv.2019.09.007
12. Wang L, Li S, Gao Q, et al. Oral propranolol therapy in parotid hemangiomas: a retrospective comparison with other infantile hemangiomas. Head Neck. 2021;43(5):1553–1562. doi:10.1002/hed.26625
13. ISSVA Classification for vascular anomalies. Available from: http://wwwissvaorg/UserFiles/file/ISSVA-Classification-2018.
14. Berenstein A, Bazil MJ, Sorscher M, et al. Percutaneous sclerotherapy of microcystic lymphatic malformations: the use of an innovative gravity-dependent technique. J Neurointerv Surg. 2022;28. doi:10.1136/neurintsurg-2021-018526
15. Chai Y, Zhou Z, Song J, et al. Safety of intralesional injection of lauromacrogol combined with triamcinolone for infantile hemangiomas. J Dermatol. 2019;46(9):770–776. doi:10.1111/1346-8138.14992
16. Nevesny F, Chevallier O, Falvo N, et al. Bleomycin for percutaneous sclerotherapy of venous and lymphatic malformations: a retrospective study of safety, efficacy and mid-term outcomes in 26 patients. J Clin Med. 2021;10(6):1302. doi:10.3390/jcm10061302
17. Theurer WM, Bhavsar AK. Prevention of unintentional childhood injury. Am Fam Physician. 2013;87:502–509.
18. Vermersch C, Boccara O, Chiaverini C, et al. Health care transition for patients with vascular malformations: a French multicenter cross-sectional study. Orphanet J Rare Dis. 2021;16:352. doi:10.1186/s13023-021-01970-7
19. Dubois J, Thomas-Chaussé F, Soulez G. Common (cystic) lymphatic malformations: current knowledge and management. Tech Vasc Interv Radiol. 2019;22:100631. doi:10.1016/j.tvir.2019.100631
20. Trenor CC, Chaudry G. Complex lymphatic anomalies. Semin Pediatr Surg. 2014;23:186–190. doi:10.1053/j.sempedsurg.2014.07.006
21. Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23:178–185. doi:10.1053/j.sempedsurg.2014.07.002
22. Kato M, Watanabe S, Iida T, Watanabe A. Flow pattern classification in lymphatic malformations by indocyanine green lymphography. Plast Reconstr Surg. 2019;143:558e–564e. doi:10.1097/PRS.0000000000005362
23. Al-Faky YH, Alkatan HM. Histopathological changes of lymphatic malformation after bleomycin injection. Arq Bras Oftalmol. 2022;21:S0004–27492022005005214. doi:10.5935/0004-2749.20230048
24. Cho AL, Kiang SC, Lodenkamp J, Tritch WTH, Tomihama RT. Fatal lung toxicity after intralesional bleomycin sclero-therapy of a vascular malformation. Cardiovasc Intervent Radiol. 2020;43:648–651. doi:10.1007/s00270-020-02420-w
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.