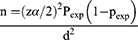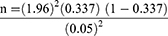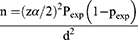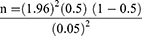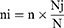Back to Journals » Infection and Drug Resistance » Volume 15
Schistosoma mansoni Epidemiology Among Snails, Rodents and Children: A One Health Approach
Authors Alehegne KD, Mitiku BA
Received 23 June 2022
Accepted for publication 22 August 2022
Published 24 September 2022 Volume 2022:15 Pages 5629—5643
DOI https://doi.org/10.2147/IDR.S363953
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Kenaw Dessie Alehegne, Birhan Agmas Mitiku
Department of Veterinary Science, College of Agriculture and Environmental Sciences, Bahir Dar University, Bahir Dar, Ethiopia
Correspondence: Birhan Agmas Mitiku, Email [email protected]
Background: Schistosoma is one of the prevalent parasitic infection in humans and animals. Schistosomiasis in children is particularly serious and results in liver and spleen enlargement, anemia, stunting, reduced ability to learn and death. The aim of this study was to measure the prevalence and distribution of schistosomiasis in children, rodents and snail populations in Aleffa and Takusa districts, north-west Ethiopia.
Methods: Disease status and exposure were simultaneously measured from December 2020 to December 2021. School children’s stool specimens were collected for schistosomiasis examination by Kato Katz and formal-ether techniques. Live rodents and snails were trapped and collected in search of adult schistosoma, eggs, and cercariae, respectively. Multiple logistic regression analytic technique by using SPSS version 20 was conducted.
Results: Of 460 stool specimens examined, 116 (25.22) were found positive for S. mansoni infection. In the present finding, the possible determinants for the occurrence of intestinal schistosomiasis in school children were female sex (AOR = 1.09, 95% CI: 1.37– 2.96); working with bare foot (AOR = 1.21, 95% CI: 1.08– 1.52); skin cut/abrasion history (AOR = 3.6, 95% CI: 1.08– 7.43) and swimming habit (AOR = 1.58, 95% CI: 1.33– 1.99). The overall (n = 108) rodent prevalence of S. mansoniwas 23 (21.3%). Only 6 snails were shedding the infective stage of schistosoma cercariae.
Conclusion: The study revealed that there is a moderate prevalence of schistosomiasis in different hosts. Thus, multi-host intervention is crucial to achieving the goal of interrupting transmission of schistosomiasis in the study area. Further research to better understand and exploit the broader environmental, ecological context and encompassing dynamic interactions between all hosts over time will be crucial for building predictive models beyond the known fact of having or not having reservoirs/hybridization of schistosoma in our study area.
Keywords: distribution, occurrence, rodents, schistosomiasis, school children, snail
Background
Schistosoma is one of the most recurrent water-contact parasitic pathogens that affects humans and animals.1 Schistosomiasis is one of the most common in areas where sanitation is inadequate and water supplies are unsafe. The disease burden caused by schistosomiasis alone is estimated at 4.5 million disability-adjusted life years.2,3 According to the WHO more than 220 million people from low-income countries are at risk of schistosomiasis. It is the most killer parasitic infection next to malaria in the African region alone.4,5
In Ethiopia, schistosomiasis is one of the major ongoing public health problems reported in the different parts of the country. Relatively, S. mansoni species was reported in most parts of the country and S. haematobium was reported in some low altitude areas of the country.6 The increasing human and livestock population leads to increased agricultural production through expansion in the natural ecosystem and intensification by using irrigation that increases contact between humans and animals. Increasing the exchange in animal–human ecosystem interfaces increases the transmission of infections like schistosomiasis.7
Schistosoma is a waterborne infection that can pose a serious health consequence for all living things in general and humans in particular.8 Depending on different factors; it causes schistosomiasis ranging from mild to severe life-threatening infections in humans. It affects primarily women and school-aged children. Schistosomiasis in children is particularly serious and results in liver and spleen enlargement, anemia, stunting, reduced ability to learn and can result in death.8,9
Intestinal schistosomiasis also infects rodents.8,10,11 Different studies show that the high prevalence and parasitic burden of S. mansoni are confirmed in rodents.13 Rodents preferred areas of dense herbaceous vegetation near the ground, as well as courses and waterbodies.12 High S. mansoni infection of rodents was recorded among those found in human sewage contamination around home ranges, in high local snail abundance areas and a high movement pattern of rodents between transmission sites. The level of S. mansoni infection in rodents increased with proximity to human habitations, which is also related to the level of infection in humans.13,14 Thus, rodents might be an important reservoir of schistosoma and a source of infections for other groups of animals and humans. New phenomena concerning that the spillover of hybrids can generate from rodents a higher risk of infection for humans.15–20 Hybridization of different animal schistosomes causes the emergence of virulent species for human infection.20
In the context of intense anthropogenic environmental change that causes disease under evolutionary pressure. Thus, recognising the multi-host/different host aspects of disease systems is of crucial importance to achieving the WHO targets for interrupting transmission of schistosomiasis in sub-Saharan Africa.16 Effective schistosomiasis prevention and control is no longer a matter of heavy application of molluscicides and mass drug administration blindly; rather, scientific information about the disease distribution/burden, presence of intermediate host and the burden in other possible reservoir hosts (like rodents) are paramount important. Knowing the epidemiology of snails and cercariae is an indispensable tool for intervention measures against schistosomiasis. The species of schistosomiasis varies with the type of snail intermediate host.3,21,22
As depicted above, schistosomiasis occurrence is highly variable and is contingent on the ecology of snail intermediate hosts and other factors.23 There might be the highest risk of infection in our study area because our study area (districts) is a geographical location near Lake Tana, which might be suitable habitation for an intermediate host/snail. In addition, most of the community uses the lake water for their day-to-day activities such as fishing, swimming, washing clothes, irrigation and other agricultural activities. The presence of large marshy areas, vegetation and rice farming might also be contributing factors.
Despite a high variability of schistosomiasis occurrences in different places and our study area is ideal for endemicity of the disease in humans, there has been scanty epidemiological information for intervention measures of schistosomiasis. Therefore, this study was designed to assess the burden of schistosomiasis in schoolchildren, rodents and snail intermediate host populations of our study area.
Methods
Study Location
Takussa and Alefa districts, in the surroundings of Lake Tana in the Amhara region of Ethiopia, were areas of the study (Figure 1). Takussa District is located about 831 km northwest of Addis Ababa, the capital city of Ethiopia. The district is situated at 36°50ʹE and 12° 20’ N. The altitude is 1840 meters above sea level. The mean annual temperature of the location is 24°C. The district has 179,208 human populations.24
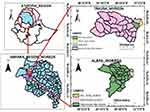 |
Figure 1 Geographical map of the study area (GIS Software, 2021). |
Alefa district is located about 882 km northwest of Addis Ababa, the capital city of Ethiopia. The district is located at 36°50’ E and 11° 35ʹN. The altitude is 2190 meters above sea level, and the mean annual temperature of the location is 22.5°C. The annual rainfall ranges from 1300 to 1500 mm. The district has a population of 237,917 people.25
In both districts, agriculture plays a very important livelihood and social role. The farms in the district are small-scale mixed farms based on crop and livestock production. For economic support and immediate food, fishing activity is also conducted in the nearby Lake Tana. The dominant soil type in both districts is black soil, which is suitable for both crop and animal production, and more than 95% of people rely on mixed farming.1
StudyPopulation
A school-child infection survey was used as a key for measuring public infection occurrence.10 All school children found in Alefa and Takussa districts were the source population of this study. All six primary schools (Delgie, ChachAlwa, Chemera, Esydebir, Degelber, and Juanitequa) students that attended during the study period were our study population. Our study units were those primary school children selected randomly and voluntarily for informed consent thus included in our sample.
Another study population was rodents; those were sampled at the six study villages (with respected primary school location villages), namely Delgie, ChachAlwa, Chemera, Esydebir, Degelber and Juanitequa. In the six villages, rodent traps caught 108 rodents. In addition, the snail samples were collected for malacological surveys at the site in open water sources used by the communities and their animals.
Study Design
A cross-sectional study design was conducted from December 2020 to December 2021 to estimate the occurrence and distribution of schistosomiasis in schoolchildren, rodents and snail intermediate host.
Sample Size
The sample size was calculated based on the following formula to estimate the prevalence of S. mansoni among the schoolchildren.26
where n = sample size, pexp= estimated prevalence of S. mansoni among in school of children (33.7%) from a study by Mathewos et al27 d2= margin of error (0.052), and Zα/2=the value of standard normal distribution corresponding to a significant level of alpha (1.962).
n=343
Therefore, the calculated sample size of the prevalence of S. mansoniamong school children was 343. To improve the precision of the study (by considering non-response rate and for pretest), 117 additional samples were added. Thus, 460 schoolchildren were included in this study.
The minimum number of rodents in the selected districts/study area/was estimated by using the minimum sample size determination technique,
where “n” is a minimum expected sample size; “Z” is the standard value (at 95% CI, Z = 1.96); “P” is the minimum expected disease occurrence and “d” is a marginal error (5%). Since no report was yet recorded for S. mansoniin rodents, the P-value was considered 50%.
n =384
where n is the sample size,
1.96 is the value of Z at the 95% confidence level.
p = expected minimum prevalence
d = absolute precision (5%)
Due to logistical and time constraints, as well as the nature of the sampling, only 108 live rodents were sampled.
Sampling Methods
To reach the sample sources or respondents, probability and non-probability sampling techniques were employed. First, Takussa and Alefa districts were selected purposively due to their high potential; their relatively large marsh area and their geographical location near Lake Tan, which is the main source of an intermediate host of schistosoma snails than other districts at zonal level and ease of data collection. In the second stage, six primary schools were chosen by a simple random sampling method. In the third stage, representative study subjects were selected from six primary schools using a simple random sampling technique based on a class roster (Figure 2).
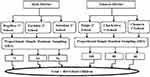 |
Figure 2 Diagrammatic presentation of study participant selection. |
The total number of students enrolled in the six schools was 8965. The sample size of each school was determined using a proportional allocation formula:
Where ni is the sample size of each school, n is the total sample size, Nj is the total number of students in each school, and N is the total number of students in all study areas. Of the total students, about 460 children were sampled.
In addition, 108 rodents were taken as the minimum sample size. The villages where primary schools are located were selected for rodent sampling too. Accordingly, 18 rodents were trapped in each village, namely, Delgi, ChachAlwa, Chemera, Esydebir, Degelber and Jantekua.
Identification of Schistosoma
Data Collection and Laboratory Procedure
School children who were randomly selected and volunteered to participate in the study were given orientation on how to handle and submit their stool samples. Thereafter, children were given a plastic sheet and an applicator stick to bring their fresh stool samples. The Kato Katz and formal-ether techniques were used to prepare stool smears on slides for microscopic examination of eggs of S. mansoni.28
Fecal samples were preserved in 10% formalin until processed. Four grams of stool were put in 10mL of 10% formalin and mixed thoroughly for fixation to take place. The mixture was strained through the gauze one size into a 15mL centrifuge tube and 0.85% sodium chloride (NaCl) solution was added and centrifuged for 10 minutes at 5000 rpm; the supernatant fluid was decanted, and the sediment was re-suspended in normal saline or 10% formalin; 4–5 mL of ethyl acetate was added to extract the debris from fecal material. The mixture was shaken vigorously for about 30 seconds and centrifuged for 10 minutes at 5000 rpm. The supernatant was discarded, leaving the sediment. A small amount of the sediment was added to a slide, a drop of iodine solution was placed on the slide to stain the fecal sample, covered with a coverslip and examined under the low power magnification of a research microscope.29,30
Collection of Rodents and Parasites
Rodents were collected along with water sources and flooded areas by live traps set at an interval of 13 m between traps. The age of rodents was estimated according to the growth curve obtained from the laboratory, and three age groups were categorized as described by juveniles with a body mass less than 110 g in both sexes, young adults with a body mass more than 110 g and less than 190 g in males and less than 215 g in females and adults with a body mass more than 190 g in males and 215 g in females.31
Rodent abundance was estimated as the number of animals captured under a constant trapping regime (total number of traps per trapping period). Rodents were humanely euthanized (euthanized by injection of formalin). Worms were collected manually from intestinal and peritoneal cavities (on blood vessels around the mesentery). Worms were removed and placed in petri dishes with saline 0.9% solution and were examined for adalatschistosoma with the help of a hand lens. In addition to the worm, the intestinal content was subjected to microscopic examination in search of the egg. Schistosome parasites were confirmed based on worm and egg morphology.14
DataCollection Tools and Procedures to Identify Risk Factors
A pretested semi-structured questionnaire consisting of closed-ended questions was used to identify determinants of schistosomiasis. The data was collected via interview. The standard English version of the questionnaire was first developed and then translated into their mother tongue language (Amharic language) for suitability and easiness in approaching the study participants.
Method of Snail Intermediate Hosts Collected and Their Associated Schistosoma Cercariae Identification
Six sampling sites (1 m2) were marked, and a 100 m interval was kept between them. Snail sampling was done using a cooping net.32 The collected snails were transported to the Bahari Dar Regional laboratory in plastic buckets containing water and vegetation for proof of identity and determination of infection. Intermediate nails were recognized to genus level morphologically by using the keys of Mandahl-Barth et al.33 Collected snail samples were inserted into vials containing water individually and exposed to electrical light for about one hour for the shed of cercariae. Shaded cercariae were identified up to genus level based on the morphology of the tail.
Study Variables
Dependent Variable
The dependent variable was a dichotomous variable of S. mansoni’s positive or negative status.
Independent Variables
Independent variables are potential risk factors for schistosomiasis variability in schoolchildren. Residence district, sex, age, residence Kebele, working with bare foot, history of previous flooding in the community, skin cut abrasion, presence of flood pooled water, improved access to water and sanitation, use of toilets or latrines, presence of vegetation, knowledge about schistosomiasis, migration from an endemic area, presence of construction dam, residence of the study area and water in puddle nearby home were among those assessed in the study.
Ethical Clearance
The ethical clearance committee at Bahir Dar University provided the required approval of this research proposal (Ref No. 2/2210/1-1-3) and obtained permission from the Amhara Regional Public Health Institute and from the study district education office to conduct the study. The study was complied with the Declaration of Helsinki ethical principles for medical research involving human subjects.34 Informed consent was obtained from each study participant and his or her parents/guardians. Bahir Dar University's research ethics committee approved this consent process. The committee and Amhara Regional Public Health Institute followed this study throughout the study period. The final report was presented and submitted in hard copy to the committee containing a summary of the study’s findings and conclusions. The students who were found positive for schistosomiasis were treated with praziquantel. Live animal research ethics were obtained from the ethical review committee of the College of Agriculture and Environmental Science, Bahir Dar University.
Data Management and Analysis
SPSS version 20 statistics were used to analyze the data. Data cleaning was performed to check for accuracy, consistencies, missed values and variables. To explain the study population in relation to relevant variables, descriptive statistics (frequencies, mean, percentage) were used. Both bivariate and multivariate logistic regression was used to assess the association between schistosoma infection occurrence and risk factor variables. In the bi-variate logistic regression analysis, each explanatory variable with an outcome variable (S. mansoni positivity) was assessed for its association. Those variables which give P-value <0.025 were considered as significant and ported to multiple logistic regression for further analysis. Finally, multiple logistic regression analysis models were used to identify the determinant factors of schistosoma infection. The adjusted odds ratio at a 95% confidence interval value not including one was considered statistically significant.
Results
Prevalence and Intensity of S. mansoni Infection
Out of 460 schoolchildren, 116 (25.2%) were found positive for S. mansoni infection. The lowest prevalence of infection was in Alefa district (22.4%), while the highest prevalence was in Takussa district 66 (27.8%). The frequency of S. mansoni infection with respect to different factors is depicted in Table 1.
 |
Table 1 Socio-Demographic and Behavioral Characteristics of School Children in Takusa and Aleffa Districts |
Possible Risk Factors Associated with Schistosomiasis in Children
The bi-variable logistic regression analysis showed that difference in district, sex, working with bare foot, previous flooding in the community, skin cut/abrasion history, presence of flood pooled water, improved access to potable and clean water, presence of vegetation, knowledge about schistosomiasis and swimming habit were identified to be significantly associated with schistosomiasis in school children. The present finding by using bi-variable analysis indicates that the remaining variables did not show any statistically significant association with the occurrence of intestinal schistosomiasis in the school children (Table 2).
 |
Table 2 Possible Risk Factors Associated with Schistosomiasisin Children (n = 460) |
Among all the covariances which were imported into multivariable logistic regression analysis, the only independent variables which remained significantly associated with the occurrence of intestinal schistosomiasis included sex, working with bare foot, skin cut/abrasion history and swimming habit.
The odds of female children were 1.09 times more likely to have schistosomiasis than male’s (AOR = 1.09; 95% CI: 1.37–2.96); those working with bare foot were 1.21 times more likely to have S. mansoni infection than working with boot and shoe wear foot (AOR = 1.21; 95% CI: 1.08–1.52). Similarly, respondents whose skin cut/abrasion history was 3.6 times more likely to have S. mansoni infection than those with no skin cut/abrasion history (AOR = 3.6; 95% CI: 1.08–7.43). The odds of a swimming habit were 1.58 times more likely to have S. mansoni infection than having no swimming habit (AOR = 1.58; 95% CI: 1.33–1.99) (Table 2).
Burden of Schistosoma Among Rodents
A total of 108 rodents were captured of which 23 (21.3%) were S. mansoni positive. The infection prevalence of S. mansoni in rodents of Takussa district was 16 (29.6%) and 7(13%) was recorded in Alefa. Table 3 depicts the occurrence with respect to fixed determinants such as rodent sex, rodent age and kebele trapped.
 |
Table 3 Prevalence of S. mansoni among Rodents in Takusa and Aleffa Districts |
Type of Snail Intermediate Hosts Collected and Their Associated Schistosoma Cercariae Released
In this study, a total of 140 snails were collected throughout the study period. Biomphalaria (56), Lymnaea (31), Bulinus (53) genus were detected from six snail collection sites in Delgi, Chachalwa, Chimera, Eseyidebir, Dengeliber and Janitekua villages around Lake Tana. High numbers of Biomphalaria genus were found in Delgi, Eseyidebir, Dengeliber, Chachalwa, Chemera and Janitekua sampling villages. The average number of snails in six sampling points (1 m2 area) at 100 m intervals was 24, 13, 12, 6, 4, and 7 per m2, respectively. However, only 6(10.71%) Biomphalaria snails were shedding the infective stage of schistosoma cercariae.
Discussion
Up to our knowledge, this is the first study attempting to assess the burden of schistosomiasis in schoolchildren, rodents and snail intermediate host populations of Aleffa and Takusa districts in Ethiopia. Even within a small geographic location, schistosomiasis occurrences varied significantly in human and animal species. A total of 116 (25.22%) schistosomiasis prevalence was detected at significantly higher levels in school children, of which 50 (22.4%) from Aleffa and 66 (27.8%) from Takusa. This shows that the levels of schistosomiasis prevalence in school children in a small geographic area variation might be due to the distance from Lake Tana, the swampy nature difference and the availability of clean water.
This was an average one fourth of school children affected by S. mansoni infection in our study area in line with the findings of a cross-sectional survey conducted by different scholars in other parts of the country. For instance, with 25.6% in Amhara Regional State, Ethiopia,35 25.8% in Southern Ethiopia,36 29.9% in Zegie,37 24% in Manna district/Southwest Ethiopia38 and 29% in Methara/Eastern Ethiopia.39
Dissimilarity; in the present study, S. mansoniinfection observed was relatively lower than a study conducted in the Sanja area, North Western Ethiopia 82.8%,40 Bushulo village, Southern Ethiopia 73.7%41 and Yachi areas, Southwest Ethiopia 42.9%,42 37.9% in Zarima town, northwest Ethiopia43 and 33.7% S. mansoniinfection among school children in two primary schools in North Gondar.27
In addition, the present study was relatively higher than 10.1% in Gorgora, Northwest Ethiopia,44 Amibera 0.8%,45 Gondar 4%,46 Gorgora 20.6%,47 Jimma 27.6%,48 11.4% in Wondo district, Ethiopia49 and Gilgel Gibe area50 and it is also higher than studies conducted from other places outside Ethiopia, such as Ghana 19.8%,51 Yemen 9.3%52 and Sudan 2.95%.53 The observed high infection prevalence (in our study one fourth of school children had schistosomiasis) might be due to socioeconomic status, exposure to environmental, occupational, and recreational risk factors. Such as the presence of several rivers that are tributaries to Lake Tana, having two main landing sites (Delgie and Essay Debere), movement of infected people from other parts of the lake, swimming and playing in water, washing their clothes in open waterbodies, irrigation practice and the presence of high swampy areas where those rearing of cattle, participating in fishing activities and all day-to-day activities in contact with water and wet soil might be major exposure activities in Aleffa and Takusa districts. However, our statistical analysis identifies sex, working with bare feet, skin cut/abrasion history and swimming habits as the only possible determinant factors for the occurrence of intestinal schistosomiasis. In addition, the altitude and availability of a comfortable ecosystem for intermediate hosts, the population and water contact behavior of the schoolchildren, a subsistence economy and labor-intensive agricultural work that has high water contact activity may contribute.
A review of different studies measuring the prevalence of schistosomiasis in a wide variety of geographical locations in humans revealed that it varied spatially and temporally depending on different factors. The present study focused on the main known and recently prioritized observed environmental, occupational and recreational risk factors that possibly predispose and spread schistosomiasis. In our study, multivariable logistic regression analysis screened out only independent variables which remained significantly associated with the prevalence of schistosomiasis including sex, working with bare foot, skin cut/abrasion history and swimming habit. Females were more likely to be infected by S. mansoni in schoolchildren as compared with males (AOR = 1.09; 95% CI: 1.37–2.96). This could be because females are more responsible for fetching water, washing clothes and crossing waterbodies, so they come into contact with water more frequently.54 In addition, females engage in wood and dung collection for fuel which makes their skin abraded and most females have no shoes other than male children. The snail transmitted a cercaria-preferred ecosystem of freshwater which is mostly utilized for day-to-day activities of human beings.
Humans who come into contact with water are afflicted by free-swimming cercariae that are released from just an intermediate host snail.55 Our multivariable analysis showed that the prevalence of S. mansoni infection was significantly higher among children working with bare foot, skin cut abrasion history and swimming habits. This finding was similar to a study conducted at Zarima town, northwest Ethiopia, where S. mansoni infection had a statistically significant association with the swimming habits of school children, similar to the study by Alemu et al.43 The similarity may be because both study areas are near to waterbodies in which swimming is a common habit in both areas. These water contact activities are a potential risk factor for children’s exposure to free-swimming cercariae and subsequent infection. This is consistent with the findings of earlier studies that reported a significant correlation between S. mansoni infection rate and contact with freshwater bodies.40,56
In the present study, the occurrence of schistosoma in wild rodents was 23 (21.3%). Similar to our findings, a study conducted by Catalano et al14 founds 9% to 28.6% schistosoma in wild rodents in areas of Richard Toll and Lake Guiers, Senegal. Recently, some schistosoma epidemic studies have linked a real risk of wild rodents as schistosoma species zoonotic reservoirs and/or hosts of hybrid occurrence in tropical zones.15,17–19 For a long time, rodents were not recognized as a reservoir of schistosoma and a potential source of infection. However, they are usually infected by schistosomiasis. The prevalence of schistosoma infections among rodents has been previously reported many times in various countries.11–15 How ever, up to our knowledge; this is the first study that currently estimates S. mansoni prevalence in wild rodents in Ethiopia. The role of rodents as local reservoirs of schistosoma locally serves as either a spillover or disease emergence of virulent schistosomes or will be used as key hosts in the transmission maintenance of schistosomiasis. An essential understanding of the occurrence and distribution of S. mansoni in rodents and humans in Aleffa and Takusa districts will provide an essential foundation for successful mitigation strategies. However, there are still considerable gaps about the complex mechanisms that lead to the emergence of hybrid schistosoma and the interaction that takes place between rodents and human hosts, enabling the transfer of S. mansoni which was beyond the aim of this study.
The presence of Schistosoma intermediate host snail of the geniuses Biomphalaria, Lymnea, and Bulinus, were detected in our study area. However, our study indicates that the Biomphalaria genus of snails is frequently seen and that only 6 (10.71%) of this snail genus shed schistosome cercariae. Our finding has a similar prevalence of S. mansoni cercariae shedding of Biomphalaria in Gombe ecosystem of western Tanzania (12%).57 Relatively lower prevalence S. mansoni cercariae than our finding was recorded in Senegal, 1.8–7.1% in Biomphalaria.14 Our finding was lower prevalence than Hailegebriel et al58 reported 15.9% in other parts of Ethiopia. The cercariae carrier state of snail still remains a basic factor for the possible survival and spread of schistosomiasis. The average number of snails in six sampling points (1 m2 area) at 100 m intervals was 66 this shows the high number of intermediate hosts in Aleffa and Takusa districts.
Visual observation of Aleffa and Takusa district habitat characteristics during the snail survey showed that the flow of tributaries of Lake Tana was slow, the lake water was slightly turbid, vegetation was abundant and there was grass growth in and around Lake Tana. Such habitat characteristics are suitable for snails’ fauna59. The presence of several rivers and tributaries to Lake Tana and irrigation canals also supports snail inhabitants.
Limitations
This multi-host study has the following limitations: it does not use the PCR method to envisage the interaction of S. mansoni in different hosts (humans, rodents and snail populations) in one health epidemiological context. Our study was cross-sectoral in that the seasonal variability of the disease intensity was not addressed. Possible risk factors were assessed through a pretested questionnaire interview of children; there might be response bias due to socially acceptable behavior and failure to recall.
Conclusion and Recommendations
The present study revealed that a moderate prevalence of S. mansoni infection was observed among schoolchildren and rodents. This study highlighted behavioral practices those lead to schistosomiasis risk from their day-to-day activities, which are an important contributing factor to this neglected tropical infection. Multiple logistic regression analysis showed that sex, working with bare foot, skin cut/abrasion history and swimming habits were possible factors of schistosomiasis addressed by the respondents. It is likely that rodents and humans have a significant role in the maintenance of schistosomiasis parasites locally.
Therefore, we summarize interventional recommendations for health stockholders and our suggestions for further research for researchers. Improve protection for vulnerable and marginalized children, particularly those working with bare feet, a history of skin cut/abrasion and swimming habits. Better protection for areas that rely on farm lands with swampy soils and are close to lakes should strengthen and coordinate policy responses to the schistosomiasis-endemic impact on human and animal health. Moreover, support resilient provision of a safe and adequate water supply support more resilient school-aged children’s health education and behavioral change, periodic deworming and snail control. The development of an early routine screening and treatment program in children is crucial for preventing severe infections and reducing the risk of impairment in teenagers. Further research in the broader environmental-ecological context and encompassing dynamic interactions between all components of the environment and hosts over time will be crucial for building predictive models for reservoirs of schistosoma and hybrid possibilities. Such enhanced understanding could lead to innovative interventions to prevent and manage schistosomiasis of human and animal health disease states.
Data Sharing Statement
All data and materials are available upon reasonable request to the corresponding author.
Acknowledgment
We would like to extend our thanks to Amhara Regional Veterinary Laboratory and Delgi Primary Hospital workers for their basic support in idea and technical support and for the giving of materials, chemicals and reagents. We are especially grateful to the schoolchildren and their families who volunteered and participated in this study by answering questions and submitting stool for laboratory testing. We would like to thank Dr. Shewatateke Melaku, Mr. Endayehu Mulat, Mr. Getachew Worku, Mr. Woldie Getnet, Mr. Alamirew Tigab and Mr. AbiyotWorkelul for their invaluable and genuine professional assistance, guidance and material support during our research work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Alfa Agricultural and livestock development office and Bahir Dar University; College of Agriculture and Environmental Science; (ref. No. 17.2/0409/2019).
Disclosure
The authors declare that they have no competing interests.
References
1. Mekuria W, Mekonnen K. Determinants of crop–livestock diversification in the mixed farming systems: evidence from central highlands of Ethiopia. Agric Food Secur. 2018;7:60. doi:10.1186/s40066-018-0212-2
2. GBD. DALYs and Hale Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1736–1788. doi:10.1016/S0140-6736(18)32203-7
3. McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primer. 2018;4:13. doi:10.1038/s41572-018-0013-8
4. World Health Organization. Schistosomiasis: Progress Report 2001–2011, Strategic Plan 2012–2020. Geneva: World Health Organization; 2013: 1–72.
5. Chala B, Torben W. An epidemiological trend of urogenital schistosomiasis in Ethiopia. Front Public Heal. 2018;6:1–9. doi:10.3389/fpubh.2018.00060
6. Bekana T, Hu W, Liang S, Erko B. Transmission of Schistosoma mansoni in Yachi areas. Southwestern Ethiopia. 2019;5:1–8.
7. Rohr JR, Barrett CB, Civitello DJ, et al. Emerging human infectious diseases and the links to global food production. Nature Sustainability. 2019;2(6):445–456. doi:10.1038/s41893-019-0293-3
8. World Health Organization. Schistosomiasis; 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis.
9. Sacolo H, Chimbari M, Kalinda C. Knowledge, attitudes and practices on schistosomiasis in sub-Saharan Africa: a systematic review. BMC Infect Dis. 2018;18:46. doi:10.1186/s12879-017-2923-6
10. Theron A, Sire C, Rognon A, Prugnolle F, Durand P. Molecular ecology of Schistosoma mansoni transmission inferred from the genetic composition of larval and adult infrapopulations within intermediate and definitive hosts. Parasitology. 2004;129:571–585. PMID: 15552402. doi:10.1017/S0031182004005943
11. Oleaga A, Rey O, Polack B, et al. Epidemiological surveillance of schistosomiasis outbreak in Corsica (France): are animal reservoir hosts implicated in local transmission. PLoS Negl Trop Dis. 2019;13(6):e0007543. doi:10.1371/journal.pntd.0007543
12. Silva-Souza N, Vasconcelos SD. Histopathology of Holochilus brasiliensis (Rodentia: Cricetidae) infected with Schistosoma mansoni (Schistosomatida: Schistosomatidae). Rev Patol Trop. 2005;34(2):145–150.
13. Maldonado A, Gentile R, Andrea PSD, D’Andrea PS, Lanfredi RM, Rey L. Helminth communities of Nectomys squamipes naturally infected by the exotic trematode Schistosoma mansoni in southeastern Brazil Braz. J Helminthol. 2006;80:369–375. doi:10.1017/JOH2006366
14. Catalano S, Sène M, Diouf ND, et al. Rodents as natural hosts of zoonotic Schistosoma species and hybrids: an epidemiological and evolutionary perspective from West Africa. J Infect Dis. 2018;218:429–433. doi:10.1093/infdis/jiy029
15. Léger E, Borlase A, Fall CB, et al. Prevalence and distribution of schistosomiasis in human, livestock, and snail populations in northern Senegal: a One Health epidemiological study of a multi-host system. Lancet Planet Health. 2020;4:330–342. doi:10.1016/S2542-5196(20)30129-7
16. Standley CJ, Dobson AP, Stothard JR. Out of animals and back again: schistosomiasis as a zoonosis in Africa. In: Rokni MB, editor. Schistosomiasis. InTech; 2012: 209–230.
17. Han BA, Schmidt JP, Bowden SE, Drake JM. Rodent reser voirs of future zoonotic diseases. Proc Natl Acad Sci USA. 2015;112:7039–7044. doi:10.1073/pnas.1501598112
18. Webster JP, Gower CM, Knowles SC, Molyneux DH, Fenton A. One Health – an ecological and evolutionary framework for tackling neglected zoonotic diseases. Evol Appl. 2016;9:313–333. doi:10.1111/eva.12341
19. Stothard JR, Sekeleghe AK, Mohammad HA, Janelisa M, Bonnie LW. Future schistosome hybridizations: will all Schistosoma haematobium hybrids please stand-up. PLoS Negl Trop Dis. 2020;14(7):e0008201. doi:10.1371/journal.pntd.0008201
20. Huyse T, Webster BL, Geldof S, et al. Bidirectional introgressive hybridization between a cattle and human Schistosome species. PLoSPathog. 2009;5:e1000571.
21. Adekiya TA, Aruleba RT, Oyinloye BE, Okosun KO, Kappo AP. The effect of climate change and the snail-schistosome cycle in transmission and bio-control of schistosomiasis in sub-Saharan Africa. Int J Environ Res Public Health. 2019;17:181. doi:10.3390/ijerph17010181
22. Rostron P, Pennance T, Bakar F, et al. Development of a recombinase polymerase amplification (RPA) fluorescence assay for the detection of Schistosoma haematobium. Parasit Vectors. 2019;12:514. doi:10.1186/s13071-019-3755-6
23. Aula OP, McManus DP, Jones MK, Gordon CA. Schistosomiasis with a focus on Africa. Trop Med Infect Dis. 2021;6:109. doi:10.3390/tropicalmed6030109
24. Takussa Agricultural and Livestock Development Office. Socio-Economic and Weather Condition Base Line Information Report. unpublish document; 2020: 1–87.
25. Alafa Agricultural and Livestock Development Office. Socio-Economic, Natural Resource and Weather Condition Base Line Information Report. unpublish document; 2019: 1–105.
26. Daneil WW. Biostatistics a Foundation for Analysis in the Health Science.
27. Mathewos B, Alemu A, Woldeyohannes D, et al. Current status of soil transmitted helminths and Schistosoma mansoni infection among children in two primary schools in North Gondar, Northwest Ethiopia: a cross sectional study. BMC Res Notes. 2014;7:1–7. doi:10.1186/1756-0500-7-88
28. Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14(6):397–400.
29. Wentworth BB. Diagnostic procedures for mycotic and parasitic infections. Am J Public Health. 1971;61(1):206. doi:10.2307/3760067
30. Ukaga PIK, Onyeka BN. Practical medical parasitology for biological and medical students; 2002: 341.
31. Gentile RS, Barreto MG, Goncalves MM. The role of wild rodents in the transmission of Schistosoma mansoni in Brazil. Schistosomiasis. 2012;231–254. doi:10.5772/25909
32. Mello DA. The comparative morphology of the genital system of some African species of Biomphalaria. Reveta Bras Biol. 1972;32:443–450.
33. Mandahl-Barth. Key to the Identification of East and Central African Freshwater Snails of Medical and Veterinary Importance. Bull. World Health Organinzation; 1962.
34. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053.
35. Bekana T, Berhe N, Eguale T, Aemero M, Medhin G, Tulu B. Prevalence and factors associated with intestinal schistosomiasis and human fascioliasis among school children in Amhara Regional State, Ethiopia. Trop Med Health. 2021;49:35. doi:10.1186/s41182-021-00326-y
36. Kabatende J, Mugisha M, Ntirenganya L, et al. Prevalence, intensity, and correlates of soil-transmitted helminth infections among school children after a decade of preventive chemotherapy in Western Rwanda. Pathogens. 2020;9:1–20. doi:10.3390/pathogens9121076
37. Merem A, Endalkachew N, Abaineh M. Prevalence of intestinal helminthic infections and malnutrition among schoolchildren of the Zegie Peninsula, northwestern Ethiopia. J Infect Public Health. 2018;10(1):84–92. doi:10.1016/j.jiph.2017.07.022
38. Bajiro M, Tesfaye S. Schistosoma mansoni infection prevalence and associated determinant factors among school children in Mana District, Jimma Zone, Oromia Region, South West Ethiopia. J Bacteriol Parasitol. 2017;8:5. doi:10.4172/2155-9597.1000329
39. Eaton SL, Roche SL, Llavero Hurtado M, et al. Total protein analysis as a reliable loading control for quantitative fluorescent Western Blotting. PLoS One. 2013;8(8):e72457. doi:10.1371/journal.pone.0072457
40. Alebie G, Erko B, Aemero M, Petros B. Epidemiological study on Schistosoma mansoni infection in Sanja area, Amhara region, Ethiopia. Parasit Vectors. 2014;7:15. doi:10.1186/1756-3305-7-15
41. Ashenafi T, Techalew S, Mulugeta M, Asrat H, Berhanu E. Schistosomiasis mansoni and soil-transmitted helminthiasis in Bushulo village, southern Ethiopia. Ethiop J Heal Dev. 2011;25:46–50.
42. Gebrezgabiher G, Mekonnen Z, Yewhalaw D, Hailu A. Reaching the last mile: main challenges relating to and recommendations to accelerate onchocerciasis elimination in Africa. Infect Dis Poverty. 2019;8(1):60. doi:10.1186/s40249-019-0567
43. Alemu A, Atnafu A, Addis Z, et al. Soil transmitted helminths and Schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia. BMC Infect Dis. 2011;11:189. doi:10.1186/1471-2334-11-189
44. Tekeste Z, Belyhun Y, Gebrehiwot A, Moges B, Workineh M, Ayalew G. Epidemiology of intestinal schistosomiasis and soil transmitted helminthiasis among primary school children in Gorgora, Northwest Ethiopia. Asian Pac J Trop Dis. 2013;3:61–64. doi:10.1016/S2222-1808(13)60013-4
45. Awoke W, Bedimo M, Tarekegn M. Prevalence of schistosomiasis and associated factors among students attending at elementary schools in Amibera District, Ethiopia. Open J Prev Med. 2013;3:199–204. doi:10.4236/ojpm.2013.32027
46. Gelaw A, Anagaw B, Nigussie B, et al. Prevalence of intestinal parasitic infections and risk factors among schoolchildren at the University of Gondar Community School, Northwest Ethiopia: a cross-sectional study. BMC Public Health. 2013;13:304. doi:10.1186/1471-2458-13-304
47. Essa T, Birhane Y, Endris M, Moges A, Moges F. Current status of Schistosoma mansoni infections and associated risk factors among students in Gorgora Town, Northwest Ethiopia. ISRN Infect Dis. 2013;636103:7. doi:10.5402/2013/636103
48. Bajiro M, Dana D, Ayana M, et al. Prevalence of Schistosoma mansoni infection and the therapeutic efficacy of praziquantel among school children in Manna District, Jimma Zone, southwest Ethiopia. Parasit Vectors. 2016;9:560. doi:10.1186/s13071-016-1833-6
49. Ansha MG, Kuti KA, Girma E. Prevalence of intestinal schistosomiasis and associated factors among school children in Wondo District, Ethiopia. J Trop Med. 2020;9813743:8. doi:10.1155/2020/9813743
50. Yami A, Mamo Y, Kebede S. Prevalence and predictors of intestinal helminthiasis among school children in Jimma zone; a cross-sectional study. Ethiop J Health Sci. 2011;21(3):167–174.
51. Anto F, Asoala V, Adjuik M, et al. Water contact activities and prevalence of schistosomiasis infection among school-age children in communities along an irrigation scheme in rural Northern Ghana. J Bacteriol Parasitol. 2013;4:177. doi:10.4172/2155-9597.1000177C
52. Sady H, Al-Mekhlafi HM, Mahdy MK, et al. Prevalence and associated factors of schistosomiasis among children in Yemen: implications for an effective control programme. PLoS Negl Trop Dis. 2013;7(8):e2377. doi:10.1371/journal.pntd.0002377
53. Hajissa K, Muhajir AE, Eshag HA, et al. Prevalence of schistosomiasis and associated risk factors among school children in Um-Asher Area, Khartoum, Sudan. BMC Res. 2018;11:779. doi:10.1186/s13104-018-3871-y
54. Angora EK, Boissier J, Menan H, et al. Prevalence and risk factors for schistosomiasis among schoolchildren in two Settings of Côte d’Ivoire. MDPI. Trop Med Infect Dis. 2019;4:110. doi:10.3390/tropicalmed4030110
55. Hairston NG. Human schistosomiasis. Am J Trop Med Hyg. 1971;20:164–165. doi:10.4269/ajtmh.1971.20.164
56. Alemayehu B, Tomass Z, Wadilo F, et al. Epidemiology of intestinal helminthiasis among school children with emphasis on Schistosoma mansoni infection in Wolaita zone, Southern Ethiopia. BMC Public Health. 2017;17:587. doi:10.1186/s12889-017-4499-x
57. Bakuza JS, Gillespie R, Nkwengulila G, et al. Assessing S. mansoni prevalence in Biomphalaria snails in the Gombe ecosystem of western Tanzania: the importance of DNA sequence data for clarifying species identification. Parasit Vectors. 2017;10(584). doi:10.1186/s13071-017-2525-6
58. Hailegebriel T, Nibret E, Munshea A. Prevalence of Schistosoma mansoni and associated risk factors in human and biomphalaria snails in Ethiopia: a systematic review and meta-analysis. Acta Parasitol. 2022;67(1):31–48. PMID: 34259986. doi:10.1007/s11686-021-00449-6
59. Lo CT, Redda A, Gemeda N. Malacological Studies of Human Schistosomiasis in Ethiopia. Proceedings of Symposium on Human Schistosomiasis in Ethiopia. Ayele T, Lo CT, Eds. Addis Ababa, Ethiopia: Institute of Pathobiology, Addis Ababa University; 1982.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.