Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Schisandrin Inhibits NLRP1 Inflammasome-Mediated Neuronal Pyroptosis in Mouse Models of Alzheimer’s Disease
Authors Li Q, Wang Q, Guan H, Zhou Y, Liu L
Received 27 August 2020
Accepted for publication 24 December 2020
Published 29 January 2021 Volume 2021:17 Pages 261—268
DOI https://doi.org/10.2147/NDT.S279147
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Quan Li,1 Qi Wang,2 Huibo Guan,3 Yanyan Zhou,2 Li Liu4
1Department of Organs, First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin 150040, Heilongjiang, People’s Republic of China; 2Teaching and Research Department of Basic Theory of Traditional Chinese Medicine, Heilongjiang University of Chinese Medicine, Harbin 150040, Heilongjiang, People’s Republic of China; 3Teaching and Research Department of Diagnostics of Traditional Chinese Medicine, Heilongjiang University of Chinese Medicine, Harbin 150040, Heilongjiang, People’s Republic of China; 4Department of Cardiovascular Diseases, First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin 150040, Heilongjiang, People’s Republic of China
Correspondence: Li Liu
Department of Cardiovascular Diseases, First Affiliated Hospital, Heilongjiang University of Chinese Medicine, No. 26 Heping Road, Xiangfang District, Harbin 150040, Heilongjiang, People’s Republic of China
Tel +86 451-82111401 Ext 6975
Email [email protected]
Background: In recent years, schisandrin (SCH) was proved to improve Alzheimer’s Disease (AD). The aim of our study is to explore the effect of SCH on neuronal pyroptosis in the disease.
Methods: A Morris water maze test was performed to evaluate the spatial learning and memory retention of AD mouse. ELISA was fulfilled to examine the concentration of Aβ, IL-1β, and IL-18. Western blot was performed to detect the expression of apoptosis- and pyroptosis-related proteins. Besides, the neuronal apoptosis rate was examined using TUNEL assay. Immunohistochemistry was utilized to detect the activation of NLRP1 inflammasome.
Results: Here, AD mice have serious cognitive impairment. Meantime, Aβ was highly expressed in the brains of AD mice. SCH could effectively rescue the cognitive impairment in AD mice and impede the production of Aβ. Subsequently, we further demonstrated that SCH repressed neuronal apoptosis, pyroptosis-related proteins expression, and the activation of NLRP1 inflammasome in the hippocampus of AD mice. We also proved that Aβ induced neuronal apoptosis and pyroptosis in vitro. However, the effects of Aβ on neuronal apoptosis and pyroptosis were partly reversed by SCH treatment.
Conclusion: Overall, our data indicated that SCH improved cognitive impairment in AD mice through inhibition of NLRP1 inflammasome-mediated neuronal pyroptosis and neuronal apoptosis. Our works provided new evidence to support SCH acting as a potential treatment method in AD.
Keywords: Alzheimer’s disease, pyroptosis, schisandrin, apoptosis, traditional Chinese medicine
Introduction
Alzheimer’s disease (AD) is an irreversible neurodegenerative disease associated with human aging. Healthcare cost of the patients with AD has continuously increased, resulted in a significant economic burden.1 Worryingly, there have been no new drugs approved by the US Food and Drug Administration for AD treatment since 2003. Hence, relieving symptoms and slowing symptom progression are still the major goals of existing AD treatments.2 The clinical features of AD patients included progressive decline of learning and memory function and subsequent loss in cognitive function, caused by the excessive accumulation of amyloid-β (Aβ) peptide and tau protein.3 Moreover, obvious loss of neuron and neuropathologic lesions were found in multiple brain regions of patients with AD.4,5 It was proved that interleukin 1β (IL-1β)-mediated inflammation response plays an important role in the pathogenesis of AD.6 However, the clearly molecular mechanism of neuronal lose in AD is still unknown.
Pyroptosis, a programmed cell death pathway discovered in recent years, is an inflammatory form of cell death triggered by activated inflammasomes, including the NOD-like receptor (NLR) family (NLRP3, NLRP1, NLRC4).7 Pyroptosis involves two mainly pathways: the classical caspase-1-dependent pathway and non-classical caspase-4- and caspase-5-dependent pathways. For the canonical caspase-1-dependent pathway, activated inflammasomes can bind to adaptor protein apoptosis-associated speck like proteins (ASC) and activate caspase-1. Then cell pyroptosis was induced by pro-IL-1β and pro-IL-18 processing, and cleavage of gasdermin D (GSDMD).8 It was reported that pyroptosis is closely associated with the pathogenesis of many human disorders, such as cardiovascular diseases and sepsis.9 Recently, Tan et al10 found that the expression of NLRP1 is increased in cerebral of AD transgenic mice, Aβ1-42 induces caspase-1-dependent pyroptosis of cortical neuron via activation of NLRP1. In addition, Zhao et al11 revealed that amentoflavone could effectively reduce Aβ1-42-induced neurological dysfunction of an AD animal model via suppression of neuronal pyroptosis by targeting AMPK/GSK3β signaling. These results prompt that Aβ-induced neuronal pyroptosis is a potential pathogen of AD.
As a traditional Chinese medicine (TCM), Schisandra chinensis (Turcz.) Bail. (S. chinensis), native chiefly to northeastern China, Korea, eastern Russia, and Japan, and was utilized against multiple disorders like autoimmune disease, cardiovascular disease, acne, as well as neurological disease.12 Currently, the chemical composition of S. chinensis is still not fully known. Dibenzo[a,c]cyclooctadiene lignans, also named as schisandra lignans, are the most important components of S. chinensis berries.13 Growing evidence has indicated that S. chinensis has anti-cancer, anti-inflammatory, hepatoprotective, anti-bacterial, and multiple other activities.14 Schisandrin (SCH) and schizandrol are the representative lignans of S. chinensis. The anti-inflammatory effect of them has already been reported in a number of studies.15 It was reported that SCH notably suppresses propionibacterium acnes-induced pyroptosis of THP-1 cells through obstructing the activation of NLRP3 inflammation and secretion of IL-1β.16 In addition, it was revealed that SCH plays a protective role in AD.17 This present studyproved that SCH obviously improved the learning and memory functions in an AD mouse model. Mechanistically, SCH repressed Aβ-induced NLRP-1-mediated neuronal pyroptosis and subsequent neuronal apoptosis, thus improving AD.
Materials and Methods
Animals and Groups
APP/SP1 double transgenic mice (B6.Cg-Tg(APPswe,PSEN1dE9)85Dbo/Mmjax) expressing chimeric mouse/human APP with Swedish familial mutations and mutant human Presenilin-1, and their age- and background-matched C57BL/6 wide-type (WT) mice were obtained from the Model Animal Research Center of Nanjing University (Nanjing, China). All mice were housed in a controlled environment with enough water and food. The ethical approval for this study was obtained from the First Affiliated Hospital, Heilongjiang University of Chinese Medicine (No20200212c0230120 [101]). All animal care and experimental procedures conformed to the Standard Medical Laboratory Animal Care and Use Protocols (Ministry of Health PR China, 1998) and the Laboratory Animal Ethical Standards of First Affiliated Hospital of Heilongjiang University of Chinese Medicine.
Twelve-month-old mice were randomly divided into three groups (n=6 per each group): WT group, AD group, and AD+SCH group. C57BL/6 WT mice served as blank control. The mice in the AD+SCH group were administrated SCH (2 mg/kg/day, Weikeqi Biological Technology Company, Ltd., Sichuan, China) by means of intragastric administration for 2 weeks. The mice in the AD group were given the same volume of distilled water for 2 weeks.
Morris Water Maze Test
The Morris Water Maze (MWM) behavioral test was performed to examine the spatial learning and memory abilities of mice. At 2 weeks later for SCH treatment, all mice were administered the MWM test for 6 consecutive days, as previously described.18 The MWM test includes a navigation test and a probe trial test. The apparatus consisted of an opaque circular water tank (90 cm in diameter × 50 cm in height, Beijing Sunny Instruments Co. Ltd., Beijing, China) with an escape platform (4.5 cm in diameter) placed in the midpoint of IV quadrant (target quadrant) and submerged 1 cm below the water surface. From day 1 to day 5, the escape platform was placed under the water for the navigation test. Each mouse were subjected to four trials every day. The escape latency, the time between a mouse being put into the water and climbing onto the platform, and path length for each mouse were recorded. Each mouse was left to find the platform for 60 seconds. If it failed, the mouse would be placed on the platform for 60 seconds. On day 6, the platform was removed, and the mouse was put into the water from the opposite side of the target quadrant. Each mouse were allowed to swim freely for 60 seconds before the experiment. The percentage of time that each mouse spent in the target quadrant and the number of times each mouse crossed the center of the target quadrant were recorded.
ELISA Assay
The concentration of Aβ1-42 and Aβ1-40 in brain tissues, IL-1β and IL-18 in hippocampus tissues, and IL-1β and IL-18 in cell culture medium were examined by using ELISA assay. The Beta Amyloid x-40 and x-42 ELISA kits were purchased from Covance. IL-1β and IL-18 ELISA kits were from R&D Systems (Minneapolis, MN, USA). All experiments were carried out in accordance with the manufacturer’s instructions.
TUNEL Assay
The apoptosis rate of hippocampus neurons in each mouse and SH-SY5Y cells were measured by using TUNEL assay (In Situ Cell Death Detection Kit, POD; Roche). For the tissue samples, tissues were fixed with 4% paraformaldehyde at 4°C overnight. Next, paraffin-embedded tissues were sliced into 4 μm-thick sections, which were then treated with dewaxing and hydration. After that, the sections were maintained with proteinase-K (20 μg/mL) for 15 minutes at room temperature. Then, the sections were incubated with 2% H2O2 for 5 minutes, TUNEL working solution for 1 hour at 37°C in the dark, stop solution for 30 minutes at 37°C, peroxidase-streptavidin conjugate solution for 30 minutes at room temperature, and fresh DAB solution for 5 minutes. Lastly, the sections were stained with hematoxylin staining solution. For the cell samples, cells were fixed with 4% paraformaldehyde for 30 minutes at 4°C, and washed with 0.01 M PBS twice. Next, the cells were maintained with 50 μL TUNEL solution for 1 hour at 37°C, and incubated with DAPI staining solution for 5 minutes at room temperature. Finally, a fluorescence microscope (Olympus, BX51) was used to examine the number of TUNEL-positive cells.
Western Blotting Assay
RIPA lysis buffer was utilized to isolate the total protein from hippocampus tissues and cells. After detection of the protein concentration, equal amounts of protein (20 μg) were separated on 12% SDS-PAGE gel, and then were transferred onto PVDF membranes. Next, membranes were blocked with 5% non-fat milk for 1 hour at room temperature and subsequently incubated with primary antibodies against NLRP1 (1:2,000, Santa Cruz, CA), ACS (1:1,000, Santa Cruz), cleaved caspase-1 (1:1,000, Abcam, Cambridge, UK), IL-1β (1:2,000, Abcam), and IL-18 (1:2,000, Abcam) at 4°C overnight. Then, membranes were incubated with HRP-linked secondary antibody (1:4,000, Abcam) for 1 hour at room temperature. The protein bands were displayed by using ECL kit (Beyotime Haimen, Jiangsu, China). Relative expression of the proteins was normalized to β-actin.
Immunohistochemistry Analysis
The sections of hippocampus tissues from each mouse were orderly incubated with 5% BSA blocking buffer for 30 minutes at 37°C, rabbit polyclonal antibody against NeuN (1:500, Abcam) and mouse monoclonal antibody against NLRP1 (1:500, Abcam) at 4°C overnight, and FITC-labeled anti-mouse IgG (1:200, Invitrogen) and TRITC-labeled anti-rabbit IgG (1:200, Invitrogen) for 1 hour at 37°C in the dark. Finally, the images were obtained using a Leica confocal microscope (Leica Microsystems, Wetzlar, Germany).
Cell Culture and Treatment
The human neuroblastoma cell line SH-SY5Y was purchased from American Type Culture Collection (ATCC). All cells were cultured in DMEM (Invitrogen, Grand Island, NY) supplemented with 10% FBS (Invitrogen), 1% penicillin, and 1% streptomycin at 37°C in an incubator with 5% CO2. The cells were divided into three groups: control group, Aβ1-42 group, and Aβ1-42+SCH group. The cells in the Aβ1-42 group were treated with 10 μmol/L Aβ1-42 (Sigma Company, Ltd, Beijing, China) for 24 hours. The cells in the Aβ1-42+SCH group were co-treated with 10 μmol/L Aβ1-42 and 10 μmol/L SCH for 24 hours.
Statistical Analysis
All statistical analyses were implemented by using SPSS17.0 statistics software (SPSS, Inc., Chicago, IL, USA), and the data are shown as mean±SD. The values of P lower than 0.05 were recorded as a significant difference. Comparisons between groups were analyzed by one-way analysis of variance (ANOVA).
Results
SCH Attenuated the Learning and Memory Impairments in AD Mice
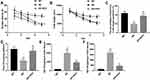
Here, all mice were accepted MWM test at 2 weeks later for SCH treatment. Our data shows that AD mice spent more time in escape latency compared with normal mice. Nevertheless, SCH treatment could obviously shorten the escape latency time in AD mice (Figure 1A). Compared with normal mice, AD mice move a longer path length to find the escape platform, while the length can be obviously declined by SCH treatment (Figure 1B). Compared with normal mice, AD mice spent less time in the target quadrant, while SCH treatment could increase the residence time (Figure 1C). Furthermore, the time of AD mice crossing the platform was significantly less than normal mice. SCH treatment also significantly increased the time of crossing the platform in AD mice (Figure 1D). According to the results of MWM test, SCH effectively improved the cognitive impairment in AD mice. Furthermore, we found that the production of Aβ1-42 and Aβ1-40 in the brains of AD mice were promoted, while SCH treatment could markedly reduce the production of them (Figure 1E and F).
SCH Repressed Neuronal Apoptosis and Pyroptosis in AD Mice
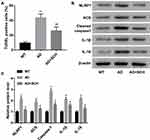
Subsequently, to explore the effect of SCH on neuronal death in AD mice, the following experiments were implemented. Firstly, a higher apoptosis rate of neurons was found in the hippocampus of AD mice compared with normal mice, but the rate can be significantly downregulated by SCH treatment (Supplementary Figure 1 and Figure 2A). Next, we proved that the expression of pyroptosis-related proteins, including NLRP1, ACS, cleaved caspase-1, IL-1β, and IL-18, were upregulated in the hippocampus of AD mice, while SCH treatment could effectively reduce the expression of these factors (Figure 2B and C). Moreover, the results of immunohistochemistry indicated that NLRP1 inflammasomes were obviously activated in the hippocampus neuron of AD mice, and SCH treatment could inactivate the NLRP1 inflammasome (Supplementary Figure 2 and Figure 3A). In addition, the production of IL-1β and IL-18 was enhanced in AD mice, while it was downregulated by SCH treatment (Figure 3B and C). In summary, AD-induced neuronal apoptosis and pyroptosis were improved by SCH treatment.
SCH Inhibited Aβ1-42-Induced Neuronal Pyroptosis and Apoptosis
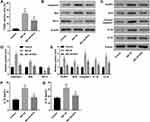
Next, we further verified the role of SCH in neuronal pyroptosis in vitro. Our data shows that Aβ1-42-induced neuronal apoptosis were reversed by SCH treatment (Supplementary Figure 3 and Figure 4A). Furthermore, the expression of pro-apoptosis factors, caspase-3 and Bax, were increased in Aβ1-42-stimulated neuron, and anti-apoptosis factor, Bcl-2, was decreased. SCH treatment could obviously repress the expression of caspase-3 and Bax, and facilitate Bcl-2 expression (Figure 4B and D). In addition, highly expressed pyroptosis-related factors including NLRP1, ACS, cleaved caspase-1, IL-1β, and IL-18, in Aβ1-42-stimulated SH-SY5Y cells were reduced by SCH treatment (Figure 4C and E). IL-1β and IL-18 production levels in the cell culture medium of Aβ1-42-treated SH-SY5Y cells were increased, while then they were declined by SCH treatment (Figure 4F and G). Overall, our data revealed that Aβ1-42-induced neuronal pyroptosis and subsequent apoptosis were repressed by SCH.
Discussion
APP/PS1 double transgenic mice express both APP and PS1, which are two genes closely associated with AD. Mutation of APP can result in excessive production of Aβ in mice brain, and mutation of PS1 can accelerate the formation of amyloid plaques.19 It was demonstrated that obviously amyloid plaques can be found in the brains of APP/PS1 double transgenic mice when 4-months-old, and cognitive impairment occurred in 12-month-old mice.20 Hence, APP/PS1 double transgenic mice is widely regarded as an AD mouse model. In this present study, APP/PS1 double transgenic mice were used for the animal experiments.
It was evaluated that about 50 million people affected by AD globally, and the prevalence may be up to 100 million by 2050.21 Currently, the pharmacotherapy of AD focuses mainly on cholinergic and glutamatergic systems impairment. Donepezil, rivastigmine, and galantamine are the important drugs for AD treatment. However, existing drugs are only symptomatic but do not modify the disease.22 In recent years, the excellent therapeutic effect of TCM in many disorders was gradually accepted by the world, such as salvia miltiorrhizae was used against acute myocardial infarction, guanxinjing capsule was used toagainst coronary heart disease, and Danggui Shaoyao San was used against AD.23–25 Here, we clarified the regulatory mechanism of SCH in AD. SCH is a mainly active constituent of S. chinensis. The protective effect of SCH on multiple disorders has been reported in many studies.17 Wei et al18 indicated that SCH could obviously improve the cognitive impairment in APP/PS1 double transgenic mice, and reduce the deposition of Aβ. Moreover, Zhao et al26 revealed that SCH protects against Aβ1-42-induced downregulation of cell viability in SH-SY5Y cells through activation of PI3K/AKT signaling. Song et al27 indicated that SCH could effectively attenuate the neuroinflammation and cognitive deficits in an streptozotocin-induced AD rat model via inactivation of NF-κB signaling. However, the action mechanism of SCH in AD development remains not fully clear. Here, we also found that SCH notably improved the learning and memory functions in AD mice. SCH declined the production of Aβ in brains of AD mice. In addition, we further indicated that SCH repressed the neuronal apoptosis in brains of AD mice as well as Aβ-induced SH-SY5Y cells.
Loss of neurons resulting from neuronal apoptosis is a crucial pathological feature of AD, related to a complex molecular mechanism. In recent studies, overwhelming numbers of studies have proved that pyroptosis, an inflammatory form cell death program, plays an important role in the development of AD.28 Pyroptosis mainly consists of NLRP1, ACS, IL-1β, and IL-18. It was proved that Aβ-induced activation of NLRP3 inflammasomes could activate caspase 1, and then induce the production of IL-1β and IL-18, finally leading to neuronal apoptosis in AD.29 Tan et al10 found that the expression of cerebral NLRP1 is increased in APP/PS1 double transgenic mice. NLRP1-mediated caspase-1-dependent neuronal pyroptosis was obviously impeded by knockdown of NLRP1 and caspase-1. In our work, we found that SCH could notably repress the activation of NLRP1 inflammasome and neuronal pyroptosis both in AD mice and Aβ-induced SH-SY5Y cells.
Conclusion
Overall, our data demonstrated that SCH effectively improved the cognitive functions in AD mice through inhibition of NLRP1-mediated neuronal pyroptosis and neuronal apoptosis. Our data provided strong evidence to support SCH acting as a potential drug for AD treatment. Meanwhile, we indicated a novel protective mechanism of SCH in AD.
Acknowledgment
This work was supported by research grants from National Natural Science Foundations of China (81573863), Natural Science Foundation of Heilongjiang Province (LH2019H052), Postdoctoral Fund of Heilongjiang Province (LBH-Z15213).
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Black CM, Fillit H, Xie L, et al. Economic burden, mortality, and institutionalization in patients newly diagnosed with Alzheimer’s disease. J Alzheimers Dis. 2018;61(1):185–193. doi:10.3233/JAD-170518
2. Hampel H, Mesulam MM, Cuello AC, et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 2018;141(7):1917–1933. doi:10.1093/brain/awy132
3. Jevtic S, Sengar AS, Salter MW, McLaurin J. The role of the immune system in Alzheimer disease: etiology and treatment. Ageing Res Rev. 2017;40:84–94. doi:10.1016/j.arr.2017.08.005
4. DeKosky ST, Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302(5646):830–834. doi:10.1126/science.1090349
5. Mantzavinos V, Alexiou A. Biomarkers for Alzheimer’s disease diagnosis. Curr Alzheimer Res. 2017;14(11):1149–1154. doi:10.2174/1567205014666170203125942
6. White CS, Lawrence CB, Brough D, Rivers-Auty J. Inflammasomes as therapeutic targets for Alzheimer’s disease. Brain Pathol. 2017;27(2):223–234. doi:10.1111/bpa.12478
7. Fang Y, Tian S, Pan Y, et al. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121:109595. doi:10.1016/j.biopha.2019.109595
8. Ruan J, Wang S, Wang J. Mechanism and regulation of pyroptosis-mediated in cancer cell death. Chem Biol Interact. 2020;323:109052. doi:10.1016/j.cbi.2020.109052
9. Jia C, Chen H, Zhang J, et al. Role of pyroptosis in cardiovascular diseases. Int Immunopharmacol. 2019;67:311–318. doi:10.1016/j.intimp.2018.12.028
10. Tan MS, Tan L, Jiang T, et al. Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis. 2014;5(8):e1382. doi:10.1038/cddis.2014.348
11. Zhao N, Sun C, Zheng M, Liu S, Shi R. Amentoflavone suppresses amyloid β1-42 neurotoxicity in Alzheimer’s disease through the inhibition of pyroptosis. Life Sci. 2019;239:117043. doi:10.1016/j.lfs.2019.117043
12. Guo M, An F, Wei X, Hong M, Lu Y. Comparative effects of schisandrin A, B, and C on acne-related inflammation. Inflammation. 2017;40(6):2163–2172. doi:10.1007/s10753-017-0656-8
13. Szopa A, Ekiert R, Ekiert H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: a review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem Rev. 2017;16(2):195–218. doi:10.1007/s11101-016-9470-4
14. Sun K, Huang R, Yan L, et al. Schisandrin attenuates lipopolysaccharide-induced lung injury by regulating TLR-4 and Akt/FoxO1 signaling pathways. Front Physiol. 2018;9:1104. doi:10.3389/fphys.2018.01104
15. Ran J, Ma C, Xu K, et al. Schisandrin B ameliorated chondrocytes inflammation and osteoarthritis via suppression of NF-κB and MAPK signal pathways. Drug Des Devel Ther. 2018;12:1195–1204. doi:10.2147/DDDT.S162014
16. Guo M, An F, Yu H, Wei X, Hong M, Lu Y. Comparative effects of schisandrin A, B, and C on propionibacterium acnes-induced, NLRP3 inflammasome activation-mediated IL-1β secretion and pyroptosis. Biomed Pharmacother. 2017;96:129–136. doi:10.1016/j.biopha.2017.09.097
17. Qi Y, Cheng X, Jing H, et al. Combination of schisandrin and nootkatone exerts neuroprotective effect in Alzheimer’s disease mice model. Metab Brain Dis. 2019;34(6):1689–1703. doi:10.1007/s11011-019-00475-4
18. Wei BB, Liu MY, Chen ZX, Wei MJ. Schisandrin ameliorates cognitive impairment and attenuates Aβ deposition in APP/PS1 transgenic mice: involvement of adjusting neurotransmitters and their metabolite changes in the brain. Acta Pharmacol Sin. 2018;39(4):616–625. doi:10.1038/aps.2017.135
19. Jankowsky JL, Fadale DJ, Anderson J, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13(2):159–170. doi:10.1093/hmg/ddh019
20. Xiong H, Callaghan D, Wodzinska J, et al. Biochemical and behavioral characterization of the double transgenic mouse model (APPswe/PS1dE9) of Alzheimer’s disease. Neurosci Bull. 2011;27(4):221–232. doi:10.1007/s12264-011-1015-7
21. Cummings JL, Tong G, Ballard C. Treatment combinations for Alzheimer’s disease: current and future pharmacotherapy options. J Alzheimers Dis. 2019;67(3):779–794. doi:10.3233/JAD-180766
22. Chu LW. Alzheimer’s disease: early diagnosis and treatment. Hong Kong Med J. 2012;18(3):228–237.
23. Spatz ES, Wang Y, Beckman AL, et al. Traditional Chinese medicine for acute myocardial infarction in western medicine hospitals in China. Circ Cardiovasc Qual Outcomes. 2018;11(3):e004190. doi:10.1161/CIRCOUTCOMES.117.004190
24. Zhang YQ, Guo QY, Li QY, et al. Main active constituent identification in Guanxinjing capsule, a traditional Chinese medicine, for the treatment of coronary heart disease complicated with depression. Acta Pharmacol Sin. 2018;39(6):975–987. doi:10.1038/aps.2017.117
25. You Y, Liu X, You Y, et al. Traditional Chinese medicine Danggui Shaoyao San for the treatment of Alzheimer’s disease: a protocol for systematic review. Medicine (Baltimore). 2020;99(15):e19669. doi:10.1097/MD.0000000000019669
26. Zhao ZY, Zhang YQ, Zhang YH, et al. The protective underlying mechanisms of schisandrin on SH-SY5Y cell model of Alzheimer’s disease. J Toxicol Environ Health A. 2019;82(19):1019–1026. doi:10.1080/15287394.2019.1684007
27. Song L, Piao Z, Yao L, Zhang L, Lu Y. Schisandrin ameliorates cognitive deficits, endoplasmic reticulum stress and neuroinflammation in streptozotocin (STZ)-induced Alzheimer’s disease rats. Exp Anim. 2020;69(3):363–373. doi:10.1538/expanim.19-0146
28. Yap JKY, Pickard BS, Chan EWL, Gan SY. The role of neuronal NLRP1 inflammasome in Alzheimer’s disease: bringing neurons into the neuroinflammation game. Mol Neurobiol. 2019;56(11):7741–7753. doi:10.1007/s12035-019-1638-7
29. Pirzada RH, Javaid N, Choi S. The roles of the NLRP3 inflammasome in neurodegenerative and metabolic diseases and in relevant advanced therapeutic interventions. Genes (Basel). 2020;11(2):11. doi:10.3390/genes11020131
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.