Back to Journals » Infection and Drug Resistance » Volume 15
Scedosporium apiospermum and Lichtheimia corymbifera Co-Infection Due to Inhalation of Biogas in Immunocompetent Patients: A Case Series
Authors Song Y, Zhou M, Gong Q, Guo J
Received 31 August 2022
Accepted for publication 27 October 2022
Published 2 November 2022 Volume 2022:15 Pages 6423—6430
DOI https://doi.org/10.2147/IDR.S388166
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yu Song,1 Mi Zhou,2 Qingmei Gong,1 Jinlin Guo3
1Department of Intensive Care Unit, Shanxi Provincial People’s Hospital, Taiyuan, Shanxi, 030012, People’s Republic of China; 2Department of Pharmacy, Children’s Hospital of Soochow University, Suzhou, Jiangsu, 215000, People’s Republic of China; 3Department of Pharmacy, Shanxi Provincial People’s Hospital, Taiyuan, Shanxi, 030012, People’s Republic of China
Correspondence: Qingmei Gong, Intensive Care Unit, Shanxi Provincial People’s Hospital, Shuangtasi Street 59#, Taiyuan, Shanxi, 030012, People’s Republic of China, Tel +86-13934647196, Email [email protected] Jinlin Guo, Department of Pharmacy, Shanxi Provincial People’s Hospital, Shuangtasi Street 59#, Taiyuan, Shanxi, 030012, People’s Republic of China, Tel +86-18335136581, Email [email protected]
Abstract: This is the first report describing co-infection of Scedosporium apiospermum and Lichtheimia corymbifera caused by biogas inhalation in two people without underlying medical conditions. Two patients fell into the same pig manure pit at the same time while rescuing another patient (this person died in a few hours) and inhaled biogas. Both patients were diagnosed with pulmonary fungal disease and developed acute liver failure around Day 52. Their results were negative for the 1,3-β-
Keywords: Scedosporium apiospermum, pulmonary mucormycosis, acute liver failure, Lichtheimia corymbifera, immunocompetent patients, inhalation of biogas
Plain Language Summary
- This is the first report to describe co-infection of Scedosporium apiospermum and Lichtheimia corymbifera induced by biogas inhalation in people without underlying medical conditions.
- Both patients showed negative results in the 1,3-β-D-glucan test and weakly positive results in the galactomannan test; this may be a characteristic of a co-infection of Lichtheimia. spp and Scedosporium apiospermum in the lungs.
- For patients with mucormycosis confined to the lungs who cannot tolerate intravenous drip amphotericin B, increasing the dose of nebulised administration maybe a salvage regimen.
Introduction
Every individual inhales a few hundred fungal spores on average per day; however, some immunocompetent patients develop invasive mycosis due to the inhalation of fungal spores.1 Pulmonary mucormycosis is a rare but fatal opportunistic pulmonary fungal infection caused by order of the Mucorales that include the Rhizopus species, Mucor, Lichtheimia, Cunninghamella, Rhizomucor, Apophysomyces, and Saksenaea. It often occurs secondary to underlying conditions such as diabetes, haematological malignancies, and solid organ transplantation.2–4 Scedosporium apiospermum infection mostly occurs in immunodeficient patients, such as cases of haematological tumours, acquired immunodeficiency syndrome, organ transplantation, and high-dose glucocorticoids or immunosuppressants administration. It is challenging for physicians to diagnose the co-infection of Scedosporium apiospermum and Lichtheimia corymbifera and select the appropriate antifungal agents. We report two uncommon cases of pulmonary mucormycosis pneumonia presenting with biogas-induced co-infection of Scedosporium apiospermum in immunocompetent critically ill patients.
Case Presentation
Case 1
The patient was a 56-year-old man with no previous underlying disease working on a pig farm. Both patients described in this case report fell into the same pig manure pit while rescuing another patient, who died in a few hours. Case 1 inhaled biogas and developed nausea and vomiting about half an hour later and gradually fell into a coma. The patient was immediately taken to a local hospital, where blood gas analysis indicated severe respiratory failure, necessitating tracheotomy and mechanical ventilation. On Day 3 after the accident, the patient was transferred to the ICU of our hospital (Figure 1). On admission, the patient showed the following findings: normal body temperature; white blood cell count, 12.88×109/L; neutrophil count, 12.10×109/L; procalcitonin, 100.53 ng/mL; C-reactive protein, 220 mg/L; 1,3-β-
 |
Figure 2 Characterisation of the two fungi. Abbreviation: BALF, bronchoalveolar lavage fluid. |
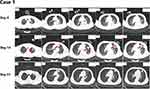 |
Figure 3 Lung CT scans obtained of case 1. |
Case 2
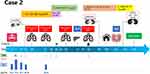
Identical to the medical history of Case 1, the patient was a 34-year-old man with no underlying diseases or injuries working on a pig farm. The patient lost consciousness due to the inhalation of biogas. The patient underwent a tracheotomy and mechanical ventilation on the day of the accident and was transferred to the ICU of our hospital on Day 3 (Figure 4). At admission, the patient’s findings were as follows: body temperature, normal; white blood cell count, 13.69×109/L; neutrophil count, 12.41×109/L; procalcitonin, >200 ng/mL; C-reactive protein, 325.43 mg/L. On day 7, the G-test was 70.23 pg/mL, and the GM-test showed weakly positive findings. Since both cases showed the same medical history and Case 1 had shown a positive BALF culture for Scedosporium apiospermum on Day 10, we also treated patient 2 with voriconazole on Day 10. This patient showed massive haemoptysis from Day 20 and underwent bronchial artery embolisation several times; the CT scan on Day 23 showed cavity lesions in the right upper lobe, right middle lobe and left upper lobe in the lungs (Figure 5). On Day 33, mucormycosis was suspected, so posaconazole suspension (200 mg, every 6 hours) was administered for treatment. On Day 36, the BALF culture showed Lichtheimia corymbifera (Figure 2), so pulmonary mucormycosis was diagnosed and D-AMB therapy was initiated. Posaconazole was discontinued due to budgetary reasons on Day 42; however, a gastrointestinal bleeding event occurred on the same day. After an acute liver failure occurred on Day 52, all medications were suspended and artificial extracorporeal liver support was initiated. The abdominal CT showed multiple cysts in the liver, the same as before. On Day 54, microscopic findings and MALDI-TOF of the BALF culture showed Scedosporium apiospermum, and CT scans showed early absorption of the lesion. On Day 54, the patient experienced massive haemoptysis again, and an intravenous drip combined with aerosol inhalation of D-AMB was resumed, and the patient’s liver function subsequently improved. Lichtheimia spp. hyphae were found several times in the patient’s sputum smears during the treatment, and the lung CT scans showed an improvement on Day 99 (Figure 5). After discharge on D104, D-AMB nebulisation with intravenous drip treatment was continued. On Day 217, the patient’s CT scans improved, but Lichtheimia spp. hyphae still could be found in the sputum smear. On Day 251, the patient experienced acute renal failure (serum creatinine 353 umol/L). Intravenous D-AMB was discontinued, while nebulised inhalation therapy was continued at a dose of 10 mg, 6 times per day. On Day 316, the patient’s chest CT showed no obvious lesions, so D-AMB was stopped after one month, and the patient recovered fully.
 |
Figure 5 Lung CT scans obtained of case 2. |
Discussion
On searching PubMed, Web of Science, and Embase databases, we believe that this is the first report describing co-infection with Scedosporium apiospermum and Lichtheimia corymbifera induced by biogas inhalation in a person without underlying medical conditions.
Lichtheimia spp. enters susceptible hosts primarily by inhalation, broken skin, or the ingestion of contaminated food. These routes lead to rhino-orbito-cerebral, lung, gastrointestinal, or skin infections, but the hosts are usually immunosuppressed, diabetic, or malignant.5,6 Scedosporium apiospermum infection is more common in patients with immunodeficiency, such as patients with haematological malignancies or AIDS and those receiving solid organ transplantation and/or large-dose glucocorticoid and immunosuppressant treatments, and is relatively rare in immunocompetent patients.7–12 Scedosporium apiospermum is the most common fungus causing invasive infections after drowning and can cause a clinical syndrome of lung and central nervous system infections in immunocompetent hosts.13 In Case 1, the pulmonary lesions were distinctly absorbed after voriconazole treatment (Figure 3); therefore, the diagnosis of Scedosporium apiospermum pulmonary infection in this patient was clear. The patient’s condition worsened again and CT scan showed new lesions after a BALF culture revealed the presence of Lichtheimia corymbifera, and improved after treatment with amphotericin B. AMB is naturally resistant for Scedosporium apiospermum and effective for mucor. Therefore, we believe that this patient was co-infected with Scedosporium apiospermum and Lichtheimia corymbifera. Scedosporium apiospermum was not detected in Case 2 in the early stage of treatment. However, because the two patients had the same medical history, we presumed that this patient also had Scedosporium apiospermum lung infection. The patient was administered with voriconazole and posaconazole, but his symptoms did not improve. Although Scedosporium apiospermum was detected from the patient’s BALF culture in D68, while Scedosporium apiospermum was naturally resistant to amphotericin B,14 he had improved significantly under amphotericin B treatment, so we considered this a case of Scedosporium apiospermum colonization. There was one report that described a co-existing mucormycosis and Scedosporium apiospermum infection on the face of a car accident victim, but the patient had taken high doses of glucocorticoids before the fungus was detected.15
In the first two weeks of admission, the two patients were diagnosed with Escherichia coli bloodstream infection and pneumonia, and were intubated and treated with multiple antibiotics that were consistent with the risk factors of the fungal infections. However, both patients had a history of exposure to biogas, and the same fungi were found in the BALF culture of both patients, which was difficult to explain with ventilator-associated pneumonia. In addition, ventilator-associated pneumonia caused by Scedosporium spp. or mucor was very rare in our hospital. Therefore, we believe that the fungal infection of the patients was related to the inhalation of biogas.
Humans inhale a few hundred fungal spores on average.1 However, the vast majority of immunocompetent patients are rarely infected with fungi. Invasive pulmonary mycosis is usually seen in patients with underlying conditions and risk factors such as inherited immunodeficiency, severe prolonged neutropenia, steroid dependency, use of immunosuppressive medications, transplant patients, and AIDS.4 Human defense against the inhaled spores begins with the mucous layer and the ciliary action located in the respiratory tract. Macrophages normally react upon recognizing fungi key cell wall components such as beta-D-glucan, secreting inflammatory mediators that ultimately attract neutrophils to initiate cellular immunity. Many kinds of fungi like Aspergillus produce toxic metabolites (aflatoxins, mycotoxins 3-nitropropionic acid, and ochratoxin A) which inhibit the action of macrophage and neutrophil phagocytosis and lead to infection.1,16 For both patients, we assume that the patients inhaled large amounts of mold. The large amount of toxins produced by the mold inhibits the phagocytosis of macrophages and neutrophils, which ultimately leads to infection in patients.
The clinical manifestations of Lichtheimia spp. and Scedosporium apiospermum lung infections are nonspecific. Common symptoms include cough, shortness of breath, blood in the sputum, and even massive haemoptysis.7,17 Both patients in this case series developed haemoptysis, and Case 2 underwent repeated bronchial artery embolisation (Figure 4). Mucormycosis usually shows negative results in G- and GM-tests,18 while Scedosporium apiospermum may show positive and negative results in the G- and GM-tests, respectively.19 The diagnosis of both illnesses relies on histopathological and culture findings. Here, the two cases were diagnosed using BALF culture combined with imaging and clinical symptoms of the patient, but they both showed negative results for the G-test and weakly positive results for the GM-test; this may be a characteristic of co-infection of Lichtheimia. spp and Scedosporium apiospermum in the lungs.
Both patients developed acute liver failure around Day 52 and we ruled out drug-induced liver failure. Imaging studies of the two patients did not indicate significant liver lesions; however, our patients did not undergo biopsies. The liver function of two patients improved after symptomatic treatment and amphotericin B treatment. Case 2 had gastrointestinal bleeding on Day 42, and both patients had constipation, similar to the clinical presentation of gastrointestinal mucormycosis previously reported.20 However, liver failure secondary to mucormycosis has not been reported previously. The levels of procalcitonin and C-reactive protein for both patients were very high; therefore, we speculate that liver failure could have been an inflammatory response caused by a serious infection.
Surgical excision or debridement is the primary treatment option for mucormycosis.4 In these two cases, the extensive lung lesions and rapid disease progression before Day 70 led the surgeon and anaesthesiologist to rule out surgery since the potentially high fungal load of the lung lesions posed a high risk of fungemia. Although the patients’ condition stabilised and the lesions shrank after Day 70, the patients’ family members refused to provide consent for surgery.
The guidelines recommend against the use of D-AMB for mucormycosis due to the adverse effects of D-AMB and the long course of treatment required for mucormycosis.4 However, D-AMB remains the main treatment option for mucormycosis in Asia and low or middle-income countries due to the high cost of L-AMB and posaconazole. Molds have enhanced tropism to invade blood vessels and cause extensive tissue necrosis in a microenvironment of low-pH and low-oxygen levels, which does not favour drug penetration.21 Because aerosol inhalation of amphotericin B (AMB) can significantly increase the concentration of alveolar epithelial cell lining fluid,22 intravenous drip combined with aerosol inhalation of amphotericin B is used to treat critical pulmonary mucormycosis in our hospital. The guidelines recommend 25 mg/day aerosol inhalation of AMB for the prophylaxis of pulmonary mycosis in solid organ transplant patients and against the use aerosol inhalation of amphotericin B to treat fungal lung infection.23 The second patient developed acute renal injury at Day 251 and was forced to discontinue intravenous drip D-AMB. At that time, Lichtheimia spp. hyphae still could be found in the patient’s sputum smear, necessitating continuation of antifungal treatment. He could not afford other effective antifungal drugs for financial reasons. Therefore, we increased the aerosolised dose of D-AMB to 10 mg six times a day, and the patient did not experience obvious adverse reactions. On Day 316, the patient’s chest CT did not show obvious lesions, and D-AMB was stopped after one month.
Conclusion
In conclusion, this is first report describing a co-infection of Scedosporium apiospermum and Lichtheimia corymbifera caused by biogas inhalation in people without underlying medical conditions. Even in immunocompetent patients, transient inhalation of biogas carries a risk of mold infection. In patients with serious infection, liver function should be closely monitored to prevent liver failure. For patients with mucormycosis confined to the lungs who cannot tolerate intravenous drip amphotericin B, increasing the dose of nebulised administration maybe a salvage regimen.
Abbreviations
CT, computerised tomography; MALDI-TOF, matrix-assisted laser desorption ionization time of flight mass spectrometry; D-AMB, amphotericin B deoxycholate; G-test, 1,3-β-
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available since the patient’s medical records and data are private, but can be made available from the corresponding author on reasonable request under the consent from the patients.
Compliance with Ethics Guidelines
This study was conducted following the legal requirements and the Declaration of Helsinki and its subsequent amendments. Written informed consent for publication was obtained from the patients, including consent to publish accompanying images. According to the hospital protocol, no formal ethics approval was required for this study.
Acknowledgments
We would like to thank the patients for their approval of this paper.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Bandres MV, Modi P, Sharma S. Aspergillus fumigatus. In: StatPearls; 2022.
2. Feng J, Sun X. Characteristics of pulmonary mucormycosis and predictive risk factors for the outcome. Infection. 2018;46(4):503–512. doi:10.1007/s15010-018-1149-x
3. Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6(4):32.
4. Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the Mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi:10.1016/S1473-3099(19)30312-3
5. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi:10.1086/432579
6. Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S23–34. doi:10.1093/cid/cir866
7. Caston JJ, Linares MJ, Rivero A, Casal M, Torre-Cisneros J. Clinical differences between invasive pulmonary infection by Scedosporium apiospermum and invasive pulmonary aspergillosis. Mycoses. 2011;54(5):e468–73. doi:10.1111/j.1439-0507.2010.01952.x
8. Cortez KJ, Roilides E, Quiroz-Telles F, et al. Infections caused by Scedosporium spp. Clinical microbiology reviews. Clin Microbiol Rev. 2008;21(1):157–197. doi:10.1128/CMR.00039-07
9. Liu W, Feng RZ, Jiang HL. Scedosporium spp lung infection in immunocompetent patients: a systematic review and MOOSE-compliant meta-analysis. Medicine. 2019;98(41):e17535. doi:10.1097/MD.0000000000017535
10. Rodriguez-Tudela JL, Berenguer J, Guarro J, et al. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med Mycol. 2009;47(4):359–370. doi:10.1080/13693780802524506
11. Seidel D, Meissner A, Lackner M, et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope((R)). Crit Rev Microbiol. 2019;45(1):1–21. doi:10.1080/1040841X.2018.1514366
12. Watanabe S, Anzawa K, Mochizuki T. Case of mycotic cyst caused by Scedosporium apiospermum developed liver dysfunction following administration of voriconazole. J Dermatol. 2017;44(11):e296–e297. doi:10.1111/1346-8138.13980
13. He XH, Wu JY, Wu CJ, Halm-Lutterodt NV, Zhang J, Li CS. Scedosporium Apiospermum Infection after Near-drowning. Chin Med J. 2015;128(15):2119–2123. doi:10.4103/0366-6999.161401
14. David NGHFC, Michael SS. The Sanford Guide to Antimicrobial Therapy 2020. 范洪伟.
15. Shand JM, Albrecht RM, Burnett HF 3rd, Miyake A. Invasive fungal infection of the midfacial and orbital complex due to Scedosporium apiospermum and mucormycosis. J Oral Maxillofac Surg. 2004;62(2):231–234. doi:10.1016/j.joms.2003.04.013
16. Vacher G, Niculita-Hirzel H, Roger T. Immune responses to airborne fungi and non-invasive airway diseases. Semin Immunopathol. 2015;37(2):83–96. doi:10.1007/s00281-014-0471-3
17. Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(1):26–34. doi:10.1016/j.cmi.2018.07.011
18. Sharma A, Goel A. Mucormycosis: risk factors, diagnosis, treatments, and challenges during COVID-19 pandemic. Folia Microbiol. 2022. doi: 10.1007/s12223-021-00934-5
19. DeSimone MS, Crothers JW, Solomon IH, Laga AC. Scedosporium and lomentospora infections are infrequent, difficult to diagnose by histology, and highly virulent. Am J Clin Pathol. 2021;156(6):1044–1057. doi:10.1093/ajcp/aqab070
20. Kaur H, Ghosh A, Rudramurthy SM, Chakrabarti A. Gastrointestinal mucormycosis in apparently immunocompetent hosts-A review. Mycoses. 2018;61(12):898–908. doi:10.1111/myc.12798
21. Lamoth F, Kontoyiannis DP. Therapeutic challenges of non-aspergillus invasive mold infections in immunosuppressed patients. Antimicrob Agents Chemother. 2019;63(11):23.
22. Husain S, Capitano B, Corcoran T, et al. Intrapulmonary disposition of amphotericin B after aerosolized delivery of amphotericin B lipid complex (Abelcet; ABLC) in lung transplant recipients. Transplantation. 2010;90(11):1215–1219. doi:10.1097/TP.0b013e3181f995ea
23. Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60. doi:10.1093/cid/ciw326
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.