Back to Journals » Cancer Management and Research » Volume 11
Sanguinarine exhibits potent efficacy against cervical cancer cells through inhibiting the STAT3 pathway in vitro and in vivo
Authors Zhang H, Zhang J, Venkat PS, Gu C, Meng Y
Received 18 April 2019
Accepted for publication 23 July 2019
Published 14 August 2019 Volume 2019:11 Pages 7557—7566
DOI https://doi.org/10.2147/CMAR.S212744
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Huijuan Zhang,*,1 Jing Zhang,*,2 Puja S Venkat,3 Chenglei Gu,1 Yuanguang Meng1
1Department of Gynecology and Obstetrics, The First Medical Center, The General Hospital of the People’s Liberation Army, Beijing, People’s Republic of China; 2Department of Gynecology, Guangdong Hydropower Hospital, Guangdong, People’s Republic of China; 3Radiation Oncology, David Geffen School of Medicine, University of California, Los Angeles, CA, USA
*These authors contributed equally to this work
Background: Cervical cancer is the third most common malignancy among female cancer patients worldwide. Signal transducer and activator of transcription 3 (STAT3) is a transcription factor which regulates a variety of cancer cellular physiological activities including cervical cancer. Sanguinarine (SNG) is a natural plant-derived benzophenanthridine alkaloid that possesses antitumor activities in several cancer cells. However, its anticancer effect on human cervical cancer cells and the underlying mechanisms have not been fully defined.
Methods: In this study, the inhibitory effect of SNG on the proliferation and growth of HeLa cell was detected by MTT assay. Next, cell cycle and apoptosis of HeLa cells was analyzed using Annexin-V/PI double staining and flow cytometry. Then, we measured intracellular ROS generation induced by SNG in HeLa cells by DCFH-DA (10 μM) staining, and the expression level of p-STAT3 and STAT3 was detected by Western blot. Finally, in order to study the effect of SNG on tumor growth in vivo, athymic nude mice were used in the vivo experiments.
Result: This study showed that SNG dose-dependently decreased the tumor cell proliferation and induced a marked increase in cell apoptosis in HeLa cells. Western blot analysis results revealed that SNG-induced antitumor effect might be mediated by STAT3 inhibition. SNG increased the expression of the proapoptotic protein Bax and reduced the expression of the antiapoptotic protein Bcl-2. We further found that SNG dose-dependently increased ROS level in Hela cells. Moreover, pretreatment with N-acetyl-l-cysteine, a scavenger of ROS, almost reversed the SNG-induced anticancer effect. In addition, SNG inhibited human cervical cancer xenograft growth without exhibiting toxicity in vivo.
Conclusion: Our findings highlight STAT3 as a promising therapeutic target. We also demonstrate that SNG is a novel anticancer drug for the treatment of cervical cancer.
Keywords: sanguinarine, STAT3, ROS, apoptosis, cervical cancer
Introduction
Cervical cancer has become the second common malignant tumor in young and middle-aged women worldwide.1 There are 131,500 new cases of cervical cancer every year in China, accounting for more than 28% of the global cases.2 Currently, the traditional three treatment methods of cervical cancer, namely surgery, chemotherapy, and radiotherapy, are unable to achieve satisfactory therapeutic effects.3 Although radiotherapy is effective as a standard treatment to reduce surgical complications, it also triggers side effects which can be divided into 2 types.4 Early side effects include erythema of the skin, wet skin peeling, and mucositis. Meanwhile, late side effects include radiation-induced fibrosis, atrophy, neural damage, and a range of endocrine and growth-related effects.5–7 In particular, drug resistance in the treatment of cervical cancer is a major difficulty in clinical treatment. Therefore, it is an urgent problem to be solved to find a reasonable and effective treatment method for cervical cancer.
Natural products are important sources of existing drugs and play a significant role in the discovery of antitumor drugs.8,9 In terms of antineoplastic drugs alone, about two-thirds come from natural products.10 Sanguinarine (SNG), an extract from the poppy family, has a variety of pharmacological activities, including antimicrobial, anti-inflammatory, antioxidative, and antifungal, and can improve liver function and immunity.11–14 In recent years, it has been found that SNG has antitumor effects on colorectal cancer, pancreatic cancer, and breast cancer.15–17 The mechanism of action of SNG on cervical cancer has rarely been reported.
The signal transducer and activator of transcription (STAT) family has dual roles of signal transduction and transcriptional activation and is a vital link of the JAK-STAT signaling pathway.18 As a member of the STAT family, the continuous activation of STAT3 is closely related to malignant transformation of tumors. STAT3 can inhibit proinflammatory cytokines produced by tumor cells, contribute to immune escape, and promote tumor invasion and metastasis.19 Therefore, the inhibition of STAT3 activity has a potential antitumor effect.
In this study, cervical cancer cells HeLa were taken as the research object to explore the mechanism of the effect of SNG on the ability of proliferation, apoptosis, and invasion of cervical cancer cells, so as to provide a new method for the treatment of cervical cancer.
Materials and methods
Reagents and antibodies
SNG was obtained from MedChem Express. The compounds used in vitro were dissolved in dimethylsulfoxide (DMSO). Antibodies including anti-Bax, anti-Bcl-2, anti-GAPDH, donkey anti-rabbit IgG-HRP and goat anti-mouse IgG-HRP horseradish peroxidase were purchased from Santa Cruz Biotechnology. Antibodies including p-STAT3 and STAT3 were obtained from Cell Signaling Technology. N-acetyl-L-cysteine (NAC), DMSO, and MTT were purchased from Sigma-Aldrich. ROS probe 2′,7′-dichlorodihydro fluorescein diacetate (DCFH-DA) was purchased from Thermo Fisher. FITC Annexin V Apoptosis Detection Kit I and propidium iodide (PI) were purchased from BD Pharmingen. A protease phosphatase inhibitor mixture was obtained from Applygen Technologies.
Cell culture of human cervical cancer cells
Human cervical cancer cell line HeLa was purchased from the American Type Culture Collection and cultured in minimum essential media (MEM) (Thermo Fisher Scientific, Waltham, MA, USA) with 10% FBS (Thermo Fisher Scientific). Cells were cultured in a humidified cell incubator with an atmosphere of 5% CO2 at 37°C.
Cell viability assay
We used the MTT assay to determine the effect of SNG on cell growth. Cervical cells were plated in 96-well plates at a density of 8000 cells per well and cultured overnight at 37°C. The next day, cells were treated with required concentrations of SNG (0.5, 1, 2.5, 5, 10, and 20 μM) for 24 hrs and 48 hrs. After the treatment, MTT reagent was added to each well and incubated for 3 hrs at 37°C. Later, DMSO was added per well to stop the reaction, and the absorbance was read at 490 nm.
Colony formation assays
To assess cervical cancer colony formation, HeLa cells were treated with SNG at various concentrations (0.5, 1, and 2 μM) for 24 hrs. Subsequently, the SNG-containing medium was removed, and the cells were incubated for an additional 7 days in a normal medium. Emerging colonies were stained with crystal violet solution. A colony was defined as a cluster of at least 50 cells that can be determined microscopically.
Gel electrophoresis and Western blot
Cell or tumor tissue collection and homogenization were performed as previously described.20 Then, the homogenates were centrifuged at 12,000×g for 10 mins at 4°C. Concentrations of protein in whole-cell extracts were determined using the Bradford protein assay. After SDS electrophoresis and transfer, the blots were blocked for 2 hrs at room temperature with fresh 5% nonfat milk in 0.1% Tween 20 (TBST) and then incubated with a specific primary antibody in TBST overnight at 4°C. After being incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hr, the blots were washed with TBST. Finally, the immunoreactive bands were visualized using ECL kit, and the density of the bands was measured by Image J computer software.
Measurement of intracellular ROS levels
DCFH-DA was used for measuring hydrogen peroxide.21 After SNG (2, 4, and 6 μM) treatment, cervical cancer cells were detached and incubated in MEM medium with DCFH-DA (10 μM, 30 mins) at 37°C in the dark. Cells were collected, and the relative fluorescence of DCF was analyzed using Accuri C6 plus flow cytometry.
Annexin V-PI staining
According to the manufacturer’s instruction, cells were collected and washed twice with PBS and then suspended in 500 μL of 1× binding buffer. The cells were stained with 5 μL of Annexin V and 10 μL of PI and then incubated for 20 mins at room temperature in the dark. The stained cells were analyzed by Accuri C6 plus flow cytometry.
In vivo antitumor study
All mouse experiments were approved by the Ethics Committee of Chinese People’s Liberation Army General Hospital. Tumors were established by orthotopic injection of cells (5×106 cells were mixed with PBS in 100 μL) into the right flanks of mice (athymic nude mice, male, 4–5 weeks old). Once the tumor volume reached about 50 mm3, these mice were randomly divided into two groups, with 6 per group. The control group received an intraperitoneal injection of saline solution, and the experimental group received an intraperitoneal injection (once daily) of SNG at a dose of 5 mg/kg. Tumor growth was measured twice weekly, and the volume of the tumors was calculated as Volume = length *× width2/2. At the end of the experiment, the mice were sacrificed, and the tumors, liver, and kidney were resected for use in Western blot and histology analysis. All animals, chosen from Experimental Animal Center of the People’s Liberation Army General Hospital, were handled according to the guidelines of the Institutional Animal Care and Use Committee.
MDA assay
The tumor tissue lysates were centrifuged at 12,000×g for 10 mins at 4°C. The total protein concentrations were determined using the Bradford protein assay. Tumor tissue proteins were normalized and subjected to MDA assay as described in the Lipid Peroxidation MDA assay kit (Beyotime, Shanghai, China). MDA levels were detected using multimode microplate readers at 535 nm.
H&E staining
For histologic analysis, the harvested liver and kidney tissues of mice were fixed overnight and paraffin embedded. The paraffin tumor tissue sections (4 μm) were stained with H&E. Each image of the sections was captured using a light microscope.
Statistical analysis
All data represent at least three independent experiments and are expressed as the mean ± SD. Statistical comparisons were made using one-way ANOVA. P-values <0.05 were considered statistically significant.
Results
SNG suppresses cell viability and induces apoptosis in HeLa cells
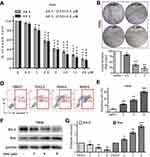
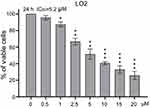
In order to find out the effect of SNG on cell viability, HeLa cells were treated with SNG at different concentrations (0, 0.5, 1, 2.5, 5, 10, and 20 μM) for 24 hrs or 48 hrs. As the dose of SNG increased from 1 to 20 μM, the viability of cells was decreased in a dose-dependent manner in all HeLa cell lines (Figure 1A). Meanwhile, SNG treatment also reduced the survival rate of nontumor cell line (LO2). However, the half-maximal inhibitory concentration (IC50) value in LO2 cells was higher than that in Hela cells (IC50=5.2 μM vs IC50=3.5 μM), indicating that SNG was less toxic to normal cells (Figure S1). Then, we analyzed the effect of SNG on HeLa cell colony formation. Under the treatment of SNG (0.5, 1, and 2 μM), the number of malignant cell colony formation was significantly reduced (Figure 1B and C).
In order to find out whether the inhibitory effect of SNG on HeLa cells was due to the apoptosis or cell cycle, Annexin-V/PI staining and flow cytometry were performed. As shown in Figure 1D and E, the apoptosis rate of HeLa cells was increased from 3.5%±0.8% in control to 15.5±3.9%, 21.8±5.9%, and 40.8±7.6%, with 2, 4, 6 μM SNG, respectively. In addition, SNG treatment significantly elevated the expression of Bax and suppressed the expression of Bcl-2 (Figure 1F and G). These results suggest that the treatment of SNG could inhibit proliferation and induce apoptosis of cervical cancer cells.
SNG induces ROS accumulation and ROS-dependent apoptosis in HeLa cells
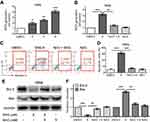
ROS plays an important role in promoting apoptosis of cancer cells.22–24 To investigate whether SNG-induced apoptosis was related to the changes of ROS level, we measured intracellular ROS generation after SNG treatment (2, 4, and 6 μM) for 3 hrs in HeLa cells by staining with DCFH-DA (10 μM). Compared with DMSO group, the production of ROS with SNG (6 μM) treatment was significantly increased (P<0.001) (Figure 2A). However, after the pretreatment of NAC (5 mM), a ROS scavenger, the levels of ROS generation in SNG-treated HeLa cells were significantly reversed (P<0.01) (Figure 2B). Moreover, SNG-induced apoptosis effect was almost abolished when ROS levels were blocked by NAC (Figure 2C and D). In addition, we also found that the combined treatment of NAC and SNG decreased the Bax expression and increased the Bcl-2 expression compared with SNG alone (P<0.01) (Figure 2E and F). All these findings indicate that the production of ROS is a key regulator of SNG-induced apoptosis in cervical cancer cells.
SNG inhibits STAT3 phosphorylation in HeLa cells
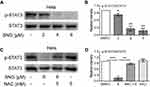
The transcription factor STAT is a newly discovered signal transduction pathway in recent years.25 As a member of the STAT family, STAT3 is involved in the process of cell proliferation and apoptosis.26 Western blot assay was used to examine STAT3 and STAT3 phosphorylation. Compared with the DMSO group, SNG treatment significantly inhibited STAT3 phosphorylation at tyrosine 705 in a dose-dependent manner (Figure 3A and B). Besides, the STAT3 inhibition effects of SNG were reversed by pretreatment with NAC (Figure 3C and D). These results demonstrate that SNG can inhibit STAT3 signaling pathway through ROS generation.
SNG inhibits HeLa xenograft tumor growth in vivo
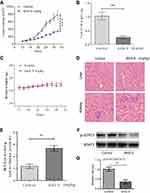
To study the effects of SNG on tumor growth in vivo, athymic nude mice were used in the vivo experiments. As shown in Figure 4A and B, the tumors’ volume and weight in SNG-treated mice were significantly lower than the control group (P<0.001). Meanwhile, no significant weight loss in mice was observed after the treatment of SNG (5 mg/kg) for 32 days (Figure 4C). H&E staining results showed that the cytoplasm was normal without obvious vacuoles and defects, which revealed that the SNG had little toxicity and had little effect on liver and kidney of nude mice (Figure 4D). Moreover, SNG treatment increased significantly the MDA level in tumor tissues (Figure 4E). Additionally, SNG treatment reduced the protein expression levels of p-STAT 3 and STAT3 in tumor tissues (Figure 4F and G). Taken together, these findings revealed that SNG inhibited tumor growth by inducing ROS production and STAT3 signaling pathway inhibition in vivo.
Discussion
At present, the treatment of cervical cancer is mainly based on surgical treatment, supplemented by radiotherapy.27,28 The purpose of this study was to elucidate the molecular mechanism of SNG-induced anticancer effects in cervical cancer cells and to explore its effects on the growth of cervical cancer cells in vivo using the HeLa xenograft model. To our knowledge, SNG has good antitumor activity, and its antitumor mechanism is complex, involving many signal transduction pathways. Yi M et al29 have indicated that SNG has an inhibitory effect on pancreatic cancer through Shh-Gli-Nanog pathway. Meanwhile, a flow cytometry analysis conducted by R. Zhang et al30 have demonstrated that overexpression of SNG inhibits gastric cancer cell viability and induces cell apoptosis via DUSP4/ERK pathway. In the present study, we found that SNG significantly reduced the proliferation of HeLa cells at low concentration (2.5 μM). Moreover, our results demonstrated that SNG blocked the STAT3 signaling pathway in a dose-dependent manner so as to promote the apoptosis rate of cervical cancer cells.
STAT3 is a very important cell signaling pathway, leading to the occurrence and development of various tumors.11 Stimulated by the cytokine growth factor, this pathway can act on specific DNA fragments in the nucleus to regulate the transcription of target genes, thus activating the pathway.26 Under normal circumstances, activation of STATs is rapid and transient, but when specific STAT proteins continue to be expressed, especially STAT3 and STAT5, multiple tumors can develop.31,32 STAT3 provides survival signal for tumor cells and inhibits their apoptosis, which is mediated by the expression of Bcl-2 and Bcl-xl. In this study, Annexin-V/PI staining and flow cytometry revealed an increased apoptosis rate of HeLa cells after SNG treatment. Meanwhile, the expression of the proapoptotic protein Bax was increased, while the expression of the antiapoptotic protein Bcl-2 was reduced. The ratio of Bax/Bcl-2 controls the switch of mitochondrial permeability transition pore (MPTP); when the ratio of Bax/Bcl-2 increases, the opening of MPTP causing the permeability of mitochondrial membrane decreases.33 Pharmacological and genetic in vivo and in vitro studies have showed that the destruction of the STAT3 signaling pathway can inhibit the expression of Bcl-2, activate the apoptotic gene Bax, and lead to the apoptosis of tumor cells,34 which is consistent with our study.
We investigated the effect of SNG on the constitutive active STAT3 in HeLa cells, finding that the inhibition of STAT3 phosphorylation by SNG was dependent on ROS generation. ROS are derived from normal metabolites in the cell. Due to their different activities, these ROS have different physiological effects on cells, and the signal transduction pathway is the main physiological role.35 Studies have shown that high levels of ROS accumulation in cells induce oxidative stress and ultimately kill cells by activating apoptotic signaling pathways.17,36 In this study, the production of ROS played a vital part in the proapoptotic effect of SNG on cervical cancer cells. Treatment with SNG resulted in a dose-dependent increase in ROS levels in HeLa cells. However, after the treatment of ROS scavenger NAC, the levels of ROS generation in SNG-treated HeLa cells were significantly reversed. SNG also decreased the protein expression levels of p-STAT3 and STAT3, while NAC could reverse all these effects.
The key role of continuous activation of STAT3 in the process of tumorigenesis and development confirmed that STAT3 is a proto-oncogene and can be used as a target for anticancer drugs.37 Unlike traditional cytotoxic drugs which are characterized by poor selectivity, strong toxic and side effects, and easy drug resistance, target-specific antitumor drugs target the differences between normal cells and tumor cells and have killing effects on tumor cells with little effect on normal cells.38 In order to verify the idea, we studied the toxic effect of SNG on nontumor cells (LO2). The results showed that the IC50 value in LO2 cells was higher than that in Hela cells (IC50=5.2 μM vs IC50=3.5 μM), indicating that SNG had little toxicity on normal cells. In vivo, SNG treatment reduced tumor volume and weight with no significant side effects in mice. Therefore, blocking transcription factors may be one of the most effective methods to treat tumors.
In addition to various chemotherapy drugs, some techniques such as nanoparticles also play a vital role in the target treatment of cervical cancer. As a means of drug delivery, nanoparticles could enhance the water solubility, stability, and bioavailability of drugs, so as to prolong their circulation in blood compartments.39 A study conducted by G B et al40 has indicated that the prepared nanoparticle containing a chemotherapeutic drug paclitaxel could locally release the paclitaxel at the target site to reduce the dose into the systemic circulation. Therefore, whether SNG-loaded nanocapsules can target to treat cervical cancer and improve its bioavailability needs to be further studied.
Conclusion
In conclusion, we studied the anticancer effects and potential mechanisms of SNG in cervical cancer cells. This experiment clarified that SNG regulates mitochondria-mediated apoptosis pathway through ROS generation, inhibits STAT3 signaling pathway to promote HeLa cell apoptosis, and provides new ideas and methods for the treatment of cervical cancer. In addition, our results suggest that screening and optimizing STAT3 inhibitors from natural products could be a target for developing new treatments for tumors with high STAT3 expression.
Acknowledgment
The study was supported by funding from National Natural Science Foundation (81571411).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Medina-Alarcón KP, Voltan AR, Fonseca-Santos B, et al. Highlights in nanocarriers for the treatment against cervical cancer. Mater Sci Eng C. 2017;80(undefined):748–759. doi:10.1016/j.msec.2017.07.021
2. Global Burden of Disease Cancer C, Fitzmaurice C, Dicker D, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–527. doi:10.1001/jamaoncol.2015.0735
3. Priyadarsini RV, Murugan RS, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol. 2010;649(1):84–91. doi:10.1016/j.ejphar.2010.09.020
4. Ota T, Takeshima N, Tabata T, Hasumi K, Takizawa K. Treatment of squamous cell carcinoma of the uterine cervix with radiation therapy alone: long-term survival, late complications, and incidence of second cancers. British Journal of Cancer. 2007;97(8):1058–1062. doi:10.1094/PDIS-91-4-0467B
5. Patel V, Mcgurk M. Use of pentoxifylline and tocopherol in radiation-induced fibrosis and fibroatrophy. Br J Oral Maxillofac Surg. 2017;55(3):235–241. doi:10.1016/j.bjoms.2016.11.323
6. Mutrikah N, Winarno H, Amalia T, Djakaria M. Conventional and conformal technique of external beam radiotherapy in locally advanced cervical cancer: dose distribution, tumor response, and side effects. J Phys. 2017;884:012122. doi:10.1088/1742-6596/884/1/012122
7. Chemaitilly W, Cohen LE. Diagnosis of endocrine disease: endocrine late-effects of childhood cancer and its treatments. Eur J Endocrinol. 2017;176(4):R183–R203. doi:10.1530/EJE-17-0054
8. AlQathama A, Prieto JM. Natural products with therapeutic potential in melanoma metastasis. Nat Prod Rep. 2015;32(8):1170–1182. doi:10.1039/c4np00130c
9. Wong-Arce A, Gonzalez-Ortega O, Rosales-Mendoza S. Plant-made vaccines in the fight against cancer. Trends Biotechnol. 2017;35(3):241–256. doi:10.1016/j.tibtech.2016.12.002
10. Shanchao WU, Sheng C, Zhang W, Pharmacy SO. Advance in anti-cancer lead-compounds derived from natural products. J Pharm Pract. 2014;32(5):337–341.
11. Zhencai L, Ping J, Wang Q, et al. Study on effect mechanism of sanguinarine on proliferation, apoptosis, invasion and migration of cervical cancer cells. Chongqing Med. 2017;46(22):3039–3042.
12. Wang Q, Dai P, Bao H, et al. Anti-inflammatory and neuroprotective effects of sanguinarine following cerebral ischemia in rats. Exp Ther Med. 2017;13(1):263–268. doi:10.3892/etm.2016.3947
13. Cecen E, Altun Z, Ercetin P, Aktas S, Olgun N. Promoting effects of sanguinarine on apoptotic gene expression in human neuroblastoma cells. Asian Pac J Cancer Prev. 2014;15(21):9445–9451. doi:10.7314/apjcp.2014.15.21.9445
14. Yang X-J, Miao F, Yao Y, et al. In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi. Molecules. 2012;17(11):13026–13035. doi:10.3390/molecules171113026
15. Singh CK, Kaur S, George J, et al. Molecular signatures of sanguinarine in human pancreatic cancer cells: A large scale label-free comparative proteomics approach. Oncotarget. 2015;6(12):10335–10348. doi:10.18632/oncotarget.3231
16. Park SY, Jin ML, Kim YH, Lee SJ, Park G. Sanguinarine inhibits invasiveness and the MMP-9 and COX-2 expression in TPA-induced breast cancer cells by inducing HO-1 expression. Oncol Rep. 2014;31(1):497–504. doi:10.3892/or.2013.2843
17. Gong X, Chen Z, Han Q, et al. Sanguinarine triggers intrinsic apoptosis to suppress colorectal cancer growth through disassociation between STRAP and MELK. BMC Cancer. 2018;18(1):578. doi:10.1186/s12885-018-4242-8
18. Lopez TV, Lappin TRJ, Maxwell P, et al. Autocrine/paracrine erythropoietin signalling promotes JAK/STAT-dependent proliferation of human cervical cancer cells. Int J Cancer. 2011;129(11):2566–2576. doi:10.1002/ijc.25935
19. Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11(12):1314–1321. doi:10.1038/nm1325
20. Ma D, Lu B, Feng C, et al. Deoxypodophyllotoxin triggers parthanatos in glioma cells via induction of excessive ROS. Cancer Lett. 2016;371(2):194–204. doi:10.1016/j.canlet.2015.11.044
21. Zhang C, Yang L, Wang X-B, et al. Calyxin Y induces hydrogen peroxide-dependent autophagy and apoptosis via JNK activation in human non-small cell lung cancer NCI-H460 cells. Cancer Lett. 2013;340(1):51–62. doi:10.1016/j.canlet.2013.06.021
22. Lu Z, Zhang G, Zhang Y, et al. Isoalantolactone induces apoptosis through reactive oxygen species-dependent upregulation of death receptor 5 in human esophageal cancer cells. Toxicol Appl Pharmacol. 2018;352:46–58. doi:10.1016/j.taap.2018.05.026
23. Li S, Zhuang Z, Wu T, et al. Nicotinamide nucleotide transhydrogenase-mediated redox homeostasis promotes tumor growth and metastasis in gastric cancer. Redox Biol. 2018;18:246–255. doi:10.1016/j.redox.2018.07.017
24. Xiang T, Du L, Pham P, Zhu B, Jiang S. Nelfinavir, an HIV protease inhibitor, induces apoptosis and cell cycle arrest in human cervical cancer cells via the ROS-dependent mitochondrial pathway. Cancer Lett. 2015;364(1):79–88. doi:10.1016/j.canlet.2015.04.027
25. Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–248. doi:10.1038/nrclinonc.2018.8
26. Liu S, Sun J, Cai B, et al. NANOG regulates epithelial-mesenchymal transition and chemoresistance through activation of the STAT3 pathway in epithelial ovarian cancer. Tumor Biol. 2016;37(7):1–10.
27. Wang W, Liu X, Meng Q, Zhang F, Hu K, Gupta S. Nomograms predicting survival and patterns of failure in patients with cervical cancer treated with concurrent chemoradiotherapy: A special focus on lymph nodes metastases. PLoS One. 2019;14(4):e0214498. doi:10.1371/journal.pone.0214498
28. Ju FJ. Evaluation of the efficacy of chemoradiotherapy in cervical cancer using diffusion-weighted imaging and apparent diffusion coefficient. Onco Targets Ther. 2016;9:7555–7561. doi:10.2147/OTT.S111829
29. Ma Y, Yu W, Shrivastava A, et al. Sanguinarine inhibits pancreatic cancer stem cell characteristics by inducing oxidative stress and suppressing sonic hedgehog-Gli-Nanog pathway. Carcinogenesis. 2017;38(10):1047–1056. doi:10.1093/carcin/bgx070
30. Zhang R, Wang G, Zhang PF, et al. Sanguinarine inhibits growth and invasion of gastric cancer cells via regulation of the DUSP4/ERK pathway. J Cell Mol Med. 2017;21(6):1117–1127. doi:10.1111/jcmm.13043
31. Phesse TJ. Abstract 4014: the gp130/Stat3 pathway enables Wnt induced regeneration and tumorigenesis in the mouse intestine. Cancer Res. 2012;72(8 Supplement):4014.
32. Kim C, Lee S-G, Yang WM, et al. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018;431:123–141. doi:10.1016/j.canlet.2018.05.038
33. Hatok J, Racay P. Bcl-2 family proteins master regulators of cell survival. Biomol Concepts. 2016;7(4):259–270. doi:10.1515/bmc-2016-0015
34. Steve A, Benjamin B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin’s lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9(1):316–326.
35. Ren X, Zhao B, Chang H, Xiao M, Wu Y, Liu Y. Paclitaxel suppresses proliferation and induces apoptosis through regulation of ROS and the AKT/MAPK signaling pathway in canine mammary gland tumor cells. Mol Med Rep. 2018;17(6):8289–8299. doi:10.3892/mmr.2018.8868
36. Ang LI, Xing YQ, Xiao-Xia LI, et al. Redox regulation of FOXO3a transcription factor. Chin Pharmacol Bull. 2016;32(9):1203–1207.
37. Akhtar S, Achkar IW, Siveen KS, et al. Sanguinarine induces apoptosis pathway in multiple myeloma cell lines via inhibition of the JaK2/STAT3 signaling. Front Oncol. 2019;9(undefined):285. doi:10.3389/fonc.2019.00285
38. Lopus M, Panda D. The benzophenanthridine alkaloid sanguinarine perturbs microtubule assembly dynamics through tubulin binding. Febs J. 2010;273(10):2139–2150. doi:10.1111/j.1742-4658.2006.05227.x
39. Letchmanan K, Shen SC, Ng WK, Tan RB. Dissolution and physicochemical stability enhancement of artemisinin and mefloquine co-formulation via nano-confinement with mesoporous SBA-15. Colloids Surf B. 2017;155(undefined):560–568. doi:10.1016/j.colsurfb.2017.05.003
40. Büyükköroğlu G, Şenel B, Başaran E, Yenilmez E, Yazan Y. Preparation and in vitro evaluation of vaginal formulations including siRNA and paclitaxel-loaded SLNs for cervical cancer. Eur J Pharm Biopharm. 2016;109(undefined):174–183. doi:10.1016/j.ejpb.2016.10.017
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.