Back to Journals » Drug Design, Development and Therapy » Volume 9
Sample sizes in dosage investigational clinical trials: a systematic evaluation
Authors Huang J , Su Q, Yang J , Lv Y, He Y, Chen J, Xu L, Wang K, Zheng Q
Received 21 October 2014
Accepted for publication 2 December 2014
Published 7 January 2015 Volume 2015:9 Pages 305—312
DOI https://doi.org/10.2147/DDDT.S76135
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Ji-Han Huang,1,* Qian-Min Su,2,* Juan Yang,1 Ying-Hua Lv,1 Ying-Chun He,1 Jun-Chao Chen,1 Ling Xu,1 Kun Wang,1 Qing-Shan Zheng1
1Center for Drug Clinical Research, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Department of Computer, College of Electronic and Electrical Engineering, Shanghai University of Engineering Science, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Abstract: The main purpose of investigational phase II clinical trials is to explore indications and effective doses. However, as yet, there is no clear rule and no related published literature about the precise suitable sample sizes to be used in phase II clinical trials. To explore this, we searched for clinical trials in the ClinicalTrials.gov registry using the keywords “dose-finding” or “dose–response” and “Phase II”. The time span of the search was September 20, 1999, to December 31, 2013.
A total of 2103 clinical trials were finally included in our review. Regarding sample sizes, 1,156 clinical trials had <40 participants in each group, accounting for 55.0% of the studies reviewed, and only 17.2% of the studies reviewed had >100 patient cases in a single group. Sample sizes used in parallel study designs tended to be larger than those of crossover designs (median sample size 151 and 37, respectively). In conclusion, in the earlier phases of drug research and development, there are a variety of designs for dosage investigational studies. The sample size of each trial should be comprehensively considered and selected according to the study design and purpose.
Keywords: sample number, dose-finding, dose–response, systematic review
Introduction
The stages of clinical trials for drugs in development can be divided into four phases. The main purpose of the first clinical stage, phase I, is to observe the tolerance and pharmacokinetic characteristics of the drug in the human body and to provide evidence to establish the phase II administration protocol. The purpose of phase II clinical trials is to evaluate the efficacy and safety of the drug in patients with the target indication. In phase III, the efficacy and safety of the drug in patients with the target indication is further validated, providing the basis of the evidence used for review during the drug registration and application process. The phase IV clinical trial, which takes place during the postmarketing period, provides further evidence regarding the drug’s efficacy and any emerging adverse reactions under conditions of real-life use in large numbers of patients. This enables the benefit–risk profile of the drug in normal or special patient populations and any required changes, such as to dose administration, to be determined, along with any changes that may be required, for example, dose administration.
Generally, the sample size of confirmatory trials is determined according to the results of primary investigational trials, which should comply with statistical requirements. The sample size is generally based on the primary outcome measures of the trial, and the trial design should also be taken into consideration. The sample size calculation is based on factors including the study design, the features of the primary outcome measures (metric index or classificatory index), difference value with clinical significance, the statistical tests to be used, and the probabilities of type I and type II errors.1–3
Phase II clinical trials of new drugs are a series of studies on aspects of drug use such as the target indication, dosage regimens, and treatment course. However most existing dose-finding designs have been proposed for phase I oncology trials, where the main outcome is toxicity, and dose escalation is guided by ethical considerations. The main purpose of investigational phase II clinical trials is to study the indications and the effective dose, with the aim of informing the design of phase III confirmative trials. Generally, the phase II clinical trial is a multidose and parallel-design study. Theoretically, larger sample sizes are preferable, but there is no uniform rule on sample sizes, and they can range from dozens to hundreds of cases.4–6 Larger sample sizes increase development costs and the length of time taken, whereas sample sizes that are too small may not achieve the purpose of the trial.
Because of this lack of clear rules and published literature on suitable sample sizes, the aim of this study is to conduct a quantitative and systematic evaluation of sample sizes used in phase II investigational multidose clinical trials registered in ClinicalTrials.gov, to provide guidance on the design of new drug clinical trials.
Search methods and data extraction
Search strategy
Registered clinical trials in the US trial database (http:www.clinicaltrials.gov) were searched using the keywords “dose-finding” OR “dose-response” AND “Phase II” from September 20, 1999, to December 31, 2013.
Quantitative evaluation
A standard database (Microsoft® Excel®) was established, and two evaluators individually read the title and full-text view, after excluding those clinical trials that obviously did not meet the inclusion criteria, to confirm whether the clinical trial was suitable for inclusion. Where it was ambiguous or difficult to determine whether a study should be included, it was discussed by the two researchers and decided by a third researcher if consensus could not be reached.
A standard database was established and the following information was extracted: status of the trial, study title, ClinicalTrials.gov identifier, study type, study design (including allocation, endpoint classification, intervention model, masking, primary purpose), primary outcome measures, condition, number of arms, and sample size.
Findings
Inclusion process
A total of 8,401 trials were retrieved in the initial search. Of these, 6,298 were excluded for the following reasons: no grouping information (739), studies with a single group (3,852), number of recruited cases not stated (69), and noninvestigational clinical trials (1,638). After careful reading of the title and full-text review, 2,103 clinical trials were finally included in our review (Figure 1).
  | Figure 1 Screening process and search results. |
Overall trial characteristics of clinical trials
Characteristics of the included studies are shown in Table 1. Of the 2,103 clinical trials, completed trials accounted for 58.6%, active, not recruiting trials for 11.8%, recruiting trials for 18.7%, terminated trials for 7.9%, withdrawn trials for 0.4%, suspended trials for 0.6%, and not yet recruiting trials for 1.8%. All included studies were interventional. A total of 116 had published results, accounting for 5.5%. Results had not been published for 94.5% of studies.
  | Table 1 General characteristics of the included clinical trials |
When the clinical trials were classified according to the allocation type, randomized clinical trials accounted for 84.2%, nonrandomized for 15.3%, and trials with unclear distribution type for 0.5%.
When classified according to the intervention model, parallel assignment accounted for 83.1%, crossover assignment for 5.0%, factorial assignment for 2.2%, and single-group assignment for 9.2%. Trials for which this information was not provided comprised 0.5%.
Regarding masking, 56.8% of trials were double blind, 4.7% were single blind, and 38.3% were open label. This information was not provided for 0.1% of studies.
When classifying the studies according to primary purpose, treatment accounted for 83.3%, supportive care for 1.0%, prevention for 12.7%, diagnostic for 0.8%, basic science for 0.9%, health services research for 0.3%, and screening for 0.05%. This information was not provided for 1.0% of studies.
When the trials were classified by endpoint, safety/efficacy accounted for 68.8%, efficacy for 16.5%, safety for 4.2%, pharmacokinetics/pharmacodynamics for 1.7%, pharmacokinetics for 0.8%, pharmacodynamics for 0.7%, bioavailability for 0.4%, and bioequivalence for 0.4%. This information was not provided for 6.9% of studies.
When classifying studies according to study phase, phase I phase II trials accounted for 18.6% of studies and phase II for 81.4%.
The distribution of the disease conditions studied in the trials is shown in Table 2. The therapeutic areas covered by the studies included in our review could be divided into 22 categories. When the trials were classified by disease conditions, bacterial and fungal diseases accounted for 5.3%; behaviors and mental disorders for 4.8%; cancers and other neoplasms for 28.5%; muscle, bone, and cartilage diseases for 8.3%; nutritional and metabolic diseases for 5.1%; urinary tract, sexual organs, and pregnancy conditions for 13.7%; wounds and injuries for 5.3%; and other disease conditions comprised <5% of studies. Clinical trials of cancers and other neoplasms accounted for the largest proportion (Table 2).
  | Table 2 Disease conditions covered in the included trials |
Sample size
The number of cases varied significantly among the trials. Of the 2,103 included, 891 (42.4%) had <100 participants, and 409 (19.4%) had >300 participants (Figure 2).
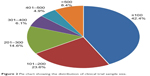  | Figure 2 Pie chart showing the distribution of clinical trial sample size. |
The number of participants in a trial showed a relationship with the number of trials in that group (Figure 3). In summary, 54.9% of trials included <40 participants, whereas only 17.2% included >100 participants.
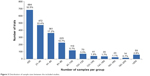  | Figure 3 Distribution of sample sizes between the included studies. |
Regarding dosage groups, the number of trials with two to four dosage groups was 1,511 (71.9%). Only 30 trials (1.4%) had >10 dosage groups (Figure 4).
  | Figure 4 Distribution of group size between the included studies. |
The number of participants in a trial was related to the study design (Figure 5). The sample size of parallel-design studies was larger than that of crossover-design studies (median sample size 151 [interquartile range (IQR) 71–288] and 37 [21–94], respectively). The sample sizes of factorial-design and single-group studies were more similar (median 85 [50–180] and 52 [28–98], respectively).
  | Figure 5 Median (interquartile range) of sample size in trials with different study designs. |
In terms of group sizes in the included studies, the number of cases per group (median [IQR]) was 30 [15–51], 35 [16–68], 38 [17–69], 39 [16–65], 33 [12–65], 31 [16–73], and 24 [10–64], with group numbers of 2, 3, 4, 5, 6, 7, and >7, respectively. The more groups a clinical trial had, the fewer cases were included in each group (Figure 6).
The range of disease types covered by the included studies was broad and could be classified into 22 groups based on the ClinicalTrials.gov classification method. The number of cases also varied according to the disease type. The median (IQR) number of cases per group was 58.7 (25–111) in bacterial and fungal diseases trials, 50 (26.7–70) in trials of muscle, bone, and cartilage diseases, 40 (18.6–79.1) in trials of viral diseases, and the median (IQR) of sample size in cancers and other neoplasms was 27.7 (12.5–50) per group. The number of cases was small for parasitic diseases and gland hormone–related diseases, but there were fewer clinical trials related to these conditions (Figure 7).
  | Figure 7 Median (interquartile range) of sample size according to disease type per group. |
Discussion
The phase II clinical trial study is an important component of drug research and development, for which a series of investigational trials are needed. The specific sample size required relates to the purpose of the clinical trial.7 Generally, the sample size of investigational trials tends not to be large, and 10–40 cases per group and three to four groups should be suitable for requirements.8–11 In this systematic evaluation, of the 2,103 clinical trials examined in detail, 1,156 clinical trials had <40 cases in each group, accounting for 54.9% of included trials. Trials of different designs may require different sample size. For example, the sample sizes for crossover trials were relatively small (median 37 cases), whereas those for parallel trials tended to be larger (median 151 cases). Sample size also varied according to the disease type. The median sample size for the bacterial and fungal diseases trials was 58.7 cases per group, whereas that for cancers and other neoplasms diseases trials was comparatively small (median 27.7 cases per group). Bacterial and fungal diseases are easy to cure, and so clinical trials for these can include larger sample sizes. Cancers and other neoplasms are complex and difficult to cure, and so, for ethical reasons, dose exploration trials for these generally include fewer participants.
In the early stages of clinical development, dose–response relationship studies can reduce the failure rate of phase III trials and speed up the process of drug development. The types of designs for dosage investigational trials include parallel, crossover, factorial, and single-group assignment. The parallel design is the most common, and each design has its advantages. Generally, parallel designs require a larger sample size that can only obtain information on general and average dose–response relationships, but not the features of individual dose–response relationships. The crossover design can control individual differences, thus reducing the required number of subjects. Each subject is administered various dosages; therefore, as well as evaluating the average population dose–response relationships, information can also be gathered on the individual dose–response relationships.A limitation of crossover designs is that a long enough washout period is required after each trial treatment to remove the carryover effect from one stage to the next. Factorial designs allow evaluation of the difference between the level of each factor and the interactive effects between each factor. The advantage of factorial designs is that the effects of several factors can be observed simultaneously so that experimental efficiency can be increased, and interactive effects between each factor can be evaluated.
Sometimes it is necessary for investigational trials to have more flexible methods to design and analyze the data in order to provide relevant information to the later confirmative trial design according to the accumulated results. In principle, it is not necessary to request testing of statistically significant differences in paired comparisons between dosages, but only if the statistical difference trends (ie, ascending slope) of each dosage can be achieved after using all data. However, it is necessary to confirm whether the recommended lowest dosage has a significant clinical effect. The group mean (the mean in the studied population) can be obtained from the parallel dose–response relationship study, but not the distribution or pattern of individual dose-response curves.12
In conventional designs, rates of adverse reactions may increase if doses are too high, leading to higher rates of withdrawal. In contrast, optimum efficacy might not be achieved if the dose is too low, leading to failure of the trial. According to the accumulated information in the clinical trial, adaptive designs can allow revision of some parts of the trial design dynamically without destroying the efficacy and integrity of the trial.13,14 In recent years, with the development of computer technologies, Bayesian model–based adaptive designs have become increasingly important.15–18
In conclusion, in the earlier phases of drug research and development, there are a variety of designs for dosage investigational studies. For general, exploratory clinical trials, 30–40 cases per group should be suitable for requirements. Where the number of groups is more, larger total samples will be needed. However, the number of participants varies according to disease type. For example, studies of cancer tend to have smaller sample sizes than studies of bacterial and fungal diseases. The sample size of each trial should be comprehensively considered and selected according to the study design and purpose. In the field of oncology19 and rare diseases in particular, there is a growing sense that effective doses can be explored using smaller sample sizes.
Acknowledgments
This study was supported by the scientific research fund of young teachers (ZYX-QNQD-001), the Shanghai Education Commission budget item (2013JW19), grants from the Shanghai 085 Project of Higher Education Connotation Construction (085ZY1202), and National Key Project for investigational new drug (2012ZX09303-003).
Disclosure
The authors report no conflicts of interest in this work.
References
Dupont WD, Plummer WD Jr. Power and sample size calculations: a review and computer program. Control Clin Trials. 1990;11(2):116–128. | ||
Sjögren P, Hedström L. Sample size determination and statistical power in randomized controlled trials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(5):652–653. | ||
Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981;2(2):93–113. | ||
MC. Sample size for comparison of changes in the presence of right censoring caused by death, withdrawal, and staggered entry. Control Clin Trials. 1988;9(1):32–46. | ||
Youssef MAFM. Effective sample size calculation: how many patients will I need to include in my study. Middle East Fertil Soc J. 2011;16(4):295–296. | ||
Shih JH. Sample size calculation for complex clinical trials with survival endpoints. Control Clin Trials. 1995;16(6):395–407. | ||
Schmidt R. Dose-finding studies in clinical drug development. Eur J Clin Pharmacol. 1988;34(1):15–19. | ||
Hacke W, Albers G, Al-Rawi Y, et al; DIAS Study Group. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36(1):66–73. | ||
Couch RB, Patel SM, Wade-Bowers CL, Niño D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS One. 2012;7(12):e49704. | ||
Rössig L, Genth-Zotz S, Rau M, et al; ARG-E04 study group. Argatroban for elective percutaneous coronary intervention: the ARG-E04 multi-center study. Int J Cardiol. 2011;148(2):214–219. | ||
Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum. 2005;52(3):916–923. | ||
ICH E8. General Considerations for Clinical Trials. Available from: www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002877.pdf. Accessed December 31, 2013. | ||
Ivanova A, Bolognese JA, Perevozskaya I. Adaptive dose finding based on t-statistic for dose-response trials. Stat Med. 2008;27(10):1581–1592. | ||
Miller F. Adaptive dose-finding: proof of concept with type I error control. Biom J. 2010;52(5):577–589. | ||
Kuen Cheung Y. Sample size formulae for the Bayesian continual reassessment method. Clin Trials. 2013;10(6):852–861. | ||
Fu H, Manner D. Bayesian adaptive dose-finding studies with delayed responses. J Biopharm Stat. 2010;20(5):1055–1070. | ||
Xie F, Ji Y, Tremmel L. A Bayesian adaptive design for multi-dose, randomized, placebo-controlled phase I/II trials. Contemp Clin Trials. 2012;33(4):739–748. | ||
Li Y, Bekele BN, Ji Y, Cook JD. Dose-schedule finding in phase I/II clinical trials using a Bayesian isotonic transformation. Stat Med. 2008;27(24):4895–4913. | ||
Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA. 2012;307(17):1838–1847. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

