Back to Journals » Drug Design, Development and Therapy » Volume 14
Salvianolic Acid Alleviated Blood–Brain Barrier Permeability in Spontaneously Hypertensive Rats by Inhibiting Apoptosis in Pericytes via P53 and the Ras/Raf/MEK/ERK Pathway
Authors Wu Q, Yuan X, Li B , Han R, Zhang H, Xiu R
Received 14 January 2020
Accepted for publication 15 March 2020
Published 16 April 2020 Volume 2020:14 Pages 1523—1534
DOI https://doi.org/10.2147/DDDT.S245959
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Qingbin Wu,1,* Xiaochen Yuan,1,* Bingwei Li,1,* Ruiqin Han,2 Honggang Zhang,1 Ruijuan Xiu1
1Institute of Microcirculation, Chinese Academy Medical Sciences & Pecking Union Medical College; 2Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaochen Yuan; Honggang Zhang
Institute of Microcirculation, Chinese Academy Medical Sciences & Pecking Union Medical College, 5, Dong Dan San Tiao, Beijing 100005, People’s Republic of China
Tel +0086-10-65126407
Fax +0086-10-65251957
Email [email protected]; [email protected]
Objective: To investigate the effect of salvianolic acid A (SA) on the permeability of blood–brain barrier (BBB) and brain microvascular pericyte apoptosis in spontaneously hypertensive rats (SHR).
Methods: Evans Blue was used to determine the BBB permeability in control rats and SHR. Western blotting was used to evaluate the expression levels of relevant proteins in the pericytes isolated from the differentially treated animals. An in vitro model of hypertension was established by stimulating pericytes with angiopoietin-2 (Ang2). MTT assay was used to assess cell viability, and apoptosis and cell cycle distribution were analyzed by flow cytometry.
Results: SA attenuated BBB permeability in SHR in a dose-dependent manner. It downregulated pro-apoptotic proteins including p53, p21, Fas, FasL, cleaved-caspase 3/caspase 3 and Bax in the pericytes of SHR and upregulated CDK6, cyclin D1, CDK2, cyclin E and Bcl2. In addition, SA activated the Ras/Raf/MEK/ERK pathway in a dose-dependent manner by increasing the levels of Ras, Raf, p-MEK1, p-MEK2, p-ERK1 and p-ERK2. Finally, SA reduced Ang2-induced apoptosis of cerebral microvessels pericytes and decreased the proportion of cells in the G0/G1 phase of the cell cycle by inhibiting the p53 pathway and activating the Ras/Raf/MEK/ERK pathway.
Conclusion: SA reduced BBB permeability in spontaneously hypertensive rats, possibly by inhibiting Ang2-induced apoptosis of pericytes by activating the Ras/Raf/MEK/ERK pathway.
Keywords: salvianolic acid A, blood–brain barrier, hypertension, pericytes, apoptosis
Introduction
Hypertension is a clinical syndrome characterized by increased blood pressure in the systemic arteries, and can progress to terminal damage in the heart, brain and kidneys. More than 200 million patients are currently diagnosed with hypertension in China, and its incidence rate has been increasing among the younger demography as well.1,2 Hypertension is also a risk factor for cardiovascular and cerebrovascular diseases,3 and long-term high blood pressure can damage the blood–brain barrier (BBB), eventually resulting in central nervous system diseases.4 Carnevale5 and Young6 et al showed that hypertension promotes the occurrence and progression of Alzheimer’s disease in mice by increasing the BBB permeability, which can lead to the deposition of amyloid-beta (Aβ) plaques in the brain parenchyma and cerebrovascular wall.7
The BBB prevents the intrusion of substances into the cerebrospinal fluid from the surrounding blood vessels, thereby protecting and maintaining the stability of the brain microenvironment, along with the homeostasis of ions, hormones and neurotransmitters in the brain tissues.8 The BBB consists of the three cellular components of the brain microvascular system, namely endothelial cells, astrocytes and pericytes.8 Studies have identified a high density of covered pericytes in the cerebral vascular wall, indicating an important function in the brain.9,10 Indeed, previous studies10,11 have shown that pericytes can promote BBB function by secreting various soluble factors such as Ang-1.12 In addition, pericyte apoptosis increases BBB permeability, which in turn diminishes the stability of the brain microenvironment, possibly resulting in the development and progression of brain diseases.13,14 The miR-181a alleviates cognitive deficit by slowing pericyte loss and BBB breakdown via inhibition of pericyte apoptosis.15 In addition, the brain microvascular cells of spontaneous hypertensive rats (SHR)16 show alterations in the apoptosis-related p53, MAKP and Jak-STAT pathways, indicating that hypertension can trigger apoptosis in these cells.
Salvianolic acid A (SA) is a water-soluble phenolic acid compound extracted from the dried roots and rhizomes of Salvia miltiorrhiza bunge. Studies show significant antioxidant,17 anti-thrombosis,18 neuroprotective,19 cardioprotective,20 and anti-apoptotic effects of SA. In addition, SA can also alleviate cerebral ischemia-induced damage of neuronal and vascular cells through its anti-apoptotic effects.21,22 In this study, we investigated the effects of SA on BBB permeability and brain microvascular pericyte apoptosis in SHR rats. SA restored the permeability of BBB in the SHR rats by inhibiting apoptosis of pericytes via the p53 and the Ras/Raf/MEK/ERK pathways. Taken together, SA is a potential therapeutic agent that can prevent brain diseases in patients with hypertension.
Materials and Methods
Treatment of SHR Rats
All animal experiments were approved and performed in accordance with relevant guidelines and regulations by the Laboratory Animal Welfare and Ethics Committee of the Institute of Microcirculation, Peking Union Medical College & Chinese Academy of Medical Sciences. Thirteen-week-old male SHR and Wistar Kyoto (WKY) rats were purchased from Vital River Laboratory Animal Technology Co. Ltd (License No. SCXK2014-0004), and divided into the control (WKY), SHR, SHR+SA-L (low dose), SHR+SA-M (medium dose) and SHR+SA-H (high dose) groups. Accordingly, the animals were injected daily with 2.5 mg/kg, 5 mg/kg and 10 mg/kg SA (E-0539, Tauto Biotech, Shanghai, China) via the intraperitoneal route for 4 weeks.21,23 The control rats were injected with the same volume of saline.
Assessment of BBB Permeability
BBB permeability was assessed by Evans Blue (EB) extravasation as described previously.24 Briefly, 2% (w/v) EB in saline (Sigma-Aldrich, St Louis, MO) was administrated to the animals by intraperitoneal injection. After 3h, mice were anesthetized by pentobarbital sodium and transcardially perfused with 4% paraformaldehyde in saline. The brains were removed, dried, weighed and subsequently homogenized in 50% trichloroacetic acid for 72h at room temperature, and centrifuged at 10,000×g for 10 min. The fluorescence of the supernatants was detected at excitation and emission wavelengths of 620 and 680 nm, respectively, and the dye concentrations were calculated based on the standard curve of EB (0, 50, 100, 200, 400, 800, 1600, 3200 and 6400μg in trichloroacetic acid) relative to the amount of tissue (μg EB/mg of tissue).
Isolation, Culture and Identification of Pericytes
After the treatment regimen, SHR rats were decapitated and their brains were resected under sterile conditions. The tissues were immersed in pre-chilled PBS and the pericytes were isolated and purified as previously described.25,26 Briefly, meninges and large pial vessels were removed, and the gray matter was isolated under a dissecting microscope. The tissues were minced in ice cold Dulbecco’s modified Eagle’s medium (DMEM) supplemented with collagenase type II (1 mg/mL), DNase I (15 µg/mL) and gentamicin C (50 µg/mL), and digested for 1.5 h at 37°C. The digested microvessels were precipitated by centrifugation in 20% bovine serum albumin/DMEM at 1000 g for 20 min. After digesting further for 1h at 37°C using DNase I (6.7 µg/mL) and collagenase/dispase (1 mg/mL; Roche, Switzerland), the microvessel clusters were separated on a 33% continuous Percoll (GE Healthcare, UK) gradient (1000 g, 10 min), and washed twice with DMEM. The isolated microvessels were cultured in Pericyte Medium (Catalog Number: 1201, ScienCell) consisting of 500 mL basal medium, 10 mL fetal bovine serum, 5 mL pericyte growth supplement, and 5 mL penicillin/streptomycin solution. After 14 days of culture, the pericytes were identified by immunostaining with PDGFRβ and NG2 as previously described.27
Western Blotting
The fibroblast-like synoviocytes and synovial tissues were homogenized in RIPA Lysis Buffer (P0013K, Beyotime, ShangHai, China), and the protein concentration in the lysates was analyzed using the BCA Protein Assay Kit (P0010S, Beyotime, ShangHai, China). Equal amounts (50 µg) of protein per sample were separated by 10% SDS-PAGE and transferred to PVDF membrane. After blocking with 5% skim milk powder for 1h at room temperature, the blots were probed overnight with anti-p53 (1:500, ab131442), anti-p21 (1:1000, ab109199), anti-Cdk2 (1:2000, ab32147), anti-Cdk6 (1:2000, ab241554), anti-cyclin D1 (1:3000, ab40754), anti-cyclin E1 (1:2000, ab71535), anti-Fas (1:1000, ab82419), anti-Fas ligand (1:500, ab231011), anti-caspase-3 (1:500, ab13847), anti-cleaved caspase-3 (1:1000, ab2302), anti-Bax (1:1500, ab32503), anti-Bcl-2 (1:500, ab59348), anti-Ras (1:500, ab52939), anti-Raf1 (1:1000, ab50858), anti-MEK1 (1:1000, ab96379), anti-MEK2 (1:500, ab30622), anti-Erk1 (pT202/pY204) + Erk2 (pT185/pY187) (1:5000, ab50011) and anti-β-actin (1:3000, ab8227) primary antibodies at 4°C. Following incubation with HRP-conjugated goat anti-rabbit IgG H&L (ab6721, 1:1000, abcam, Cambs, UK) or HRP-rabbit anti-mouse IgG H&L (ab6728, 1:1000, abcam, Cambs, UK) for 1 hour at room temperature, the positive bands were visualized using the BeyoECL Plus chromogenic kit (P0018S, Beyotime, ShangHai, China), and the densities were analyzed by the Beckman Coulter Immunoassay System (UniCel DxI 800, Beckman, CA, USA).
MTT Assay
Pericytes were seeded in a 96-well culture plate at the density of 2×103 cells/well, and treated with varying concentrations of Ang2 (0.01 µM, 0.1 µM, 1 µM and 10 µM) or SA (1 µM, 5 µM, 10 µM and 20 µM) for 3, 6, 12 and 24h. After suitable treatment, 10μL MTT (10mg/mL) was added to each well, and the cells were incubated for 4 hours. The supernatants were then aspirated, and the formazan crystals were dissolved with 100μL DMSO per well. After 30 min, optical density (OD) was measured at 570 nm using a plate reader spectrophotometer.
Apoptosis and Cell Cycle Analysis
Pericytes were treated with 1μM Ang2 for 12h with/out a 6h SA (20µM) pre-treatment. The control, Ang 2 and Ang2+SA cells were harvested and incubated with 100µL propidium iodide (PI) and 300µL Annexin V‑FITC staining solution (Sigma‑Aldrich; Merck‑Millipore) for 15 min in the dark. The apoptotic cells were analyzed by flow cytometry (Accuri C6 Flow Cytometer, BD Biosciences, Franklin Lakes, NJ, USA). To evaluate the cell cycle, the harvested cells were fixed overnight with 70% chilled at 4°C. After washing with PBS, the cells were stained with PI and analyzed by flow cytometry.
Statistical Analysis
SPSS20.0 was used for statistical analysis. Student’s t test and ANOVA with Duncan’s post hoc test were, respectively, used to compare two or multiple groups. P values <0.05 were considered statistically significant.
Results
SA Attenuated the Permeability of BBB in SHR Rats
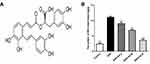
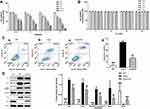
The permeability of BBB was measured in terms of EB leakage. As shown in Figure 1, the EB content was significantly higher in the SHR compared to the healthy control rats. However, SA markedly lowered EB extravasation in the SHR rats in a dose-dependent manner.
SA Restored the Expression of Cell Cycle- and Apoptosis-Related Proteins in the Cerebral Pericytes of SHR
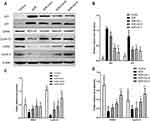
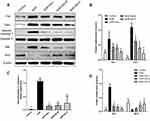
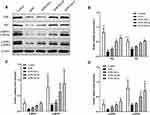
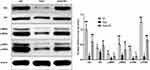
Morphology of pericytes from WKY and SHR and expression of generic markers had been revealed. After 3 days of cultivation, part of pericytes had crawled out of the brain microvessels, and 14 days later, reached almost full confluence. Co-expression of PDGFRβ and NG2, as determined by immunofluorescence, identified the isolated cells as pericytes (Figure 2). The in situ p53 and p21 expression was significantly higher in the cerebral pericytes of SHR compared to normal control rats, and were downregulated by SA in a dose-dependent effect (Figure 3). In contrast, the cell cycle progression-related CDK6, cyclin D1, CDK2 and cyclin E proteins were significantly lower in the SHR compared to the controls, and upregulated by SA in a dose-dependent manner (Figure 3). The pericytes of SHR also expressed high levels of pro-apoptotic proteins, such as Fas, FasL, cleaved-caspase 3/caspase 3 and Bax, while the anti-apoptotic Bcl2 was downregulated. SA treatment restored the levels of all apoptosis-related proteins (Figure 4). Consistent with this, the pro-survival Ras/Raf/MEK/ERK signaling pathway was inhibited in the SHR, with significantly lower levels of Ras, Raf, p-MEK1, p-MEK2, p-ERK1 and p-ERK2 compared to the control rats, which increased after SA treatment (Figure 5). Taken together, SA alleviated apoptosis in the cerebral pericytes of rats with hypertension, and promoted cell cycle progression.
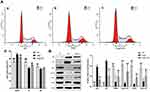
SA Reduced Ang2-Induced Apoptosis of Cerebral Microvessels Pericytes
Ang2 induced apoptosis in the cerebral pericytes in a time- and dose-dependent manner (Figure 6A), which was significantly alleviated by SA (Figure 6C) without any inherent toxicity (Figure 6B). Consistent with this, the Ang2-induced changes in the expression levels of apoptosis-related proteins (Fas, FasL, cleaved-caspase 3/caspase 3, Bax and Bcl2) were restored by SA (Figure 6D). Ang2 also increased the proportion of cells in the G0/G1 phase and decreased that in the S and G2 phase, which corresponded to the upregulation of the checkpoint inhibitors p53 and p21, and downregulation of CDK6, cyclin D1, CDK2 and cyclin E compared to the control pericytes. Pre-treatment with SA reversed the Ang 2-induced changes in cell cycle (Figure 7A), as well as the expression levels of the key proteins, reduced the expression level of these proteins (Figure 7B). Mechanistically, SA activated the Ras/Raf/MEK/ERK pathway by upregulating Ras, Raf, p-MEK1, p-MEK2, p-ERK1 and p-ERK2 in the Ang2-treated pericytes (Figure 8).
Discussion
Acute and chronic hypertension are known to disrupt the BBB,28,29 and alterations in brain microvascular permeability and BBB integrity often precede a chronic hypertensive state.30,31 In the present study, the brain tissues of the SHR were significantly more permeable compare to that of control mice. Although the exact molecular mechanism underlying hypertension-induced BBB damage is unknown, studies show that chronic hypertension causes functional changes in the cerebral endothelial cells, astrocyte termini and pericytes.8 Pericytes are located on the microvascular wall of the brain. They are surrounded by the basement membrane, and are closely linked to endothelial cells and astrocyte terminal foot processes.32,33 Pericytes regulate cerebral blood flow and maintain BBB integrity, along with regulating neuroimmunity and pluripotent stem cell differentiation.32,33 Pericyte dysfunction is associated with several central nervous system diseases, such as Alzheimer’s disease,34 ischemic stroke,35 subarachnoid hemorrhage36 and epilepsy.37 In a previous study, we found that the expression of key proteins involved in p53/MAPK/JAK-STAT pathways, as well as cell cycle-related proteins, was significantly altered in the brain microvascular pericytes of SHR.16
Salvianolic acid A and salvianolic acid B (SB) are water-soluble extracts of Salvia miltiorrhiza, and have similar chemical structures and pharmacological effects. SA attenuated the increased BBB permeability in SHR, and inhibited apoptosis in the microvascular pericytes of the hypertensive animals. Mechanistically, SA restored cell cycle progression and the MAPK pathway that were downregulated in the SHR pericytes.
TP53 is the master regulator of apoptosis and cell cycle,38 and is activated following a stressful stimuli. The wild-type p53 protein interacts with the cell cycle inhibitor p2139,40 that blocks multiple cyclin-CDK complexes such as cyclin D1-CDK6, cyclin E-CDK2 and cyclin A-CDK2, and interrupts the cell cycle at the G1 phase. This stalls DNA replication and allows the damaged cells sufficient time to repair.39,40 In case, the DNA damage is extensive and cannot be repaired, p53 activates the apoptosis program through different pathways. It transcriptionally inhibits the pro-survival Bcl2,41,42 and activates the pro-apoptotic Bax.43 In addition, p53 can also induce apoptosis via the death signaling receptor protein pathway. A previous study showed that p53 transiently sensitized cells to Fas-induced apoptosis by inducing Fas-FADD binding.44 Ang2 is a growth factor belonging to the angiopoietins and is involved in endothelial physiology and cardiovascular remodeling. Ang2 expression is triggered by inflammatory conditions, such as hypertension.45 Hypertensive patients had higher levels of plasma Ang-2.46 Ang2 can induce pericyte and astrocyte apoptosis.47,48 There are reported that Ang2 induces pericyte loss in normal mice retina and in mice overexpressing Ang2.49,50 The major receptor in the angiopoietin/Tie signaling pathway is Tie2. However, Tie2 is not always responsible for Ang2-induced functions, and an interaction with other transmembrane molecules has been identified. There are reported that Ang2 induced pericyte apoptosis via Ang2/α3β1-integrin/p53 signaling pathway and Ang2 induced astrocyte apoptosis under high glucose via αvβ5-integrin/GSK-3β/β-catenin pathway.47,48 Furthermore, SB can inhibit acute ethanol-induced hepatocyte apoptosis via the p53 pathway.51 These findings indicate that SA also alleviates pericyte apoptosis in SHR rats by targeting the p53 pathway.
In the present study, SA upregulated the Ras/Raf/MEK/ERK signaling pathway in brain microvascular pericytes in SHR, and also reversed Ang2-induced inhibition of this pathway. Activation of the Ras/Raf/MEK/ERK pathway induces cyclin D1, which is essential for G1/S phase transition, as well as formation of the cyclin B/CDK1 complex that further promotes cell entry into the M phase. Therefore, constitutive activation of ERK can prevent apoptosis by promoting cell cycle progression.52–54 Previous studies have shown that SA promotes metastasis of squamous cell carcinoma through the c-Raf/MEK/ERK pathway.55 Furthermore, SB can also promote osteogenesis of human mesenchymal stem cells and prevent oxidative stress-induced apoptosis in rat bone marrow stem cells by activating the MEK/ERK pathway.56,57 Taken together, SA maintains pericyte survival and is a promising therapeutic agent against hypertension.
Conclusion
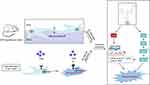
Hypertension increased BBB permeability in a rat model, which was alleviated by SA via inhibition of pericyte apoptosis. In addition, SA also reversed Ang2-induced apoptosis of cerebral microvessel pericytes by inhibiting p53 and activating the Ras/Raf/MEK/ERK pathway (Figure 9).
Acknowledgments
This study was supported by the innovation fund of the Chinese Academy of Medical Sciences and Peking Union Medical College (Nos. 3332014006 and 3332015123), the CAMS Initiative for Innovative Medicine (CAMS-I2M) (No. 2016-I2M-3-006) and National Natural Science Foundation of China (81801433).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Weiwei C, Runlin G, Lisheng L, et al. Outline of the report on cardiovascular diseases in China, 2014. Eur Heart J Suppl. 2016;18(SupplF):F2–F11. doi:10.1093/eurheartj/suw030
2. Chen WW, Gao RL, Liu LS, et al. China cardiovascular diseases report 2015: a summary. J Geriatr Cardiol. 2017;14(1):1–10. doi:10.11909/j.issn.1671-5411.2017.01.012
3. James JE. Hypertension control and cardiovascular disease. Lancet. 2017;389(10065):154. doi:10.1016/S0140-6736(17)30018-1
4. Ueno M, Sakamoto H, Tomimoto H, et al. Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol. 2004;107(6):532–538. doi:10.1007/s00401-004-0845-z
5. Carnevale D, Mascio G, D’Andrea I, et al. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60(1):188–197. doi:10.1161/HYPERTENSIONAHA.112.195511
6. Young Park J, Wook Yun J, Whan Choi Y, et al. Antihypertensive effect of gomisin A from Schisandra chinensis on angiotensin II-induced hypertension via preservation of nitric oxide bioavailability. Hypertens Res. 2012;35(9):928–934. doi:10.1038/hr.2012.50
7. Zenaro E, Piacentino G, Constantin G. The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis. 2017;107:41–56. doi:10.1016/j.nbd.2016.07.007
8. Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi:10.1016/j.neuron.2008.01.003
9. Greenberg JI, Shields DJ, Barillas SG, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456(7223):809–813. doi:10.1038/nature07424
10. Stapor PC, Sweat RS, Dashti DC, Betancourt AM, Murfee WL. Pericyte dynamics during angiogenesis: new insights from new identities. J Vasc Res. 2014;51(3):163–174. doi:10.1159/000362276
11. Sims DE. Recent advances in pericyte biology–implications for health and disease. Can J Cardiol. 1991;7(10):431–443.
12. Yan M, Hu Y, Yao M, Bao S, Fang Y. GM-CSF ameliorates microvascular barrier integrity via pericyte-derived Ang-1 in wound healing. Wound Repair Regen. 2017;25(6):933–943. doi:10.1111/wrr.12608
13. Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. doi:10.1038/nature09522
14. Liu S, Agalliu D, Yu C, Fisher M. The role of pericytes in blood-brain barrier function and stroke. Curr Pharm Des. 2012;18(25):3653–3662. doi:10.2174/138161212802002706
15. Wu Q, Yuan X, Bai J, et al. MicroRNA-181a protects against pericyte apoptosis via directly targeting FOXO1: implication for ameliorated cognitive deficits in APP/PS1 mice. Aging (Albany NY). 2019;11(16):6120–6133. doi:10.18632/aging.102171
16. Yuan X, Wu Q, Liu X, Zhang H, Xiu R. Transcriptomic profile analysis of brain microvascular pericytes in spontaneously hypertensive rats by RNA-Seq. Am J Transl Res. 2018;10(8):2372–2386.
17. Huang J, Qin Y, Liu B, Li GY, Ouyang L, Wang JH. In silico analysis and experimental validation of molecular mechanisms of salvianolic acid A-inhibited LPS-stimulated inflammation, in RAW264.7 macrophages. Cell Prolif. 2013;46(5):595–605. doi:10.1111/cpr.12056
18. Fan HY, Fu FH, Yang MY, Xu H, Zhang AH, Liu K. Antiplatelet and antithrombotic activities of salvianolic acid A. Thromb Res. 2010;126(1):e17–e22. doi:10.1016/j.thromres.2010.04.006
19. Wang T, Shan SY, Han B, Zhang LM, Fu FH. Salvianolic acid A exerts antiamnesic effect on diazepam-induced anterograde amnesia in mice. Neurochem Res. 2011;36(1):103–108. doi:10.1007/s11064-010-0270-8
20. Wang SB, Tian S, Yang F, Yang HG, Yang XY, Du GH. Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarction in rats. Eur J Pharmacol. 2009;615(1–3):125–132. doi:10.1016/j.ejphar.2009.04.061
21. Teng F, Yin Y, Cui Y, et al. Salvianolic acid A inhibits endothelial dysfunction and vascular remodeling in spontaneously hypertensive rats. Life Sci. 2016;144:86–93. doi:10.1016/j.lfs.2015.06.010
22. Tang H, Pan CS, Mao XW, et al. Role of NADPH oxidase in total salvianolic acid injection attenuating ischemia-reperfusion impaired cerebral microcirculation and neurons: implication of AMPK/Akt/PKC. Microcirculation. 2014;21(7):615–627. doi:10.1111/micc.12140
23. Jiang B, Li D, Deng Y, et al. Salvianolic acid A, a novel matrix metalloproteinase-9 inhibitor, prevents cardiac remodeling in spontaneously hypertensive rats. PLoS One. 2013;8(3):e59621. doi:10.1371/journal.pone.0059621
24. Yuan X, Wu Q, Wang P, et al. Exosomes derived from pericytes improve microcirculation and protect blood-spinal cord barrier after spinal cord injury in mice. Front Neurosci. 2019;13:319. doi:10.3389/fnins.2019.00319
25. Dohgu S, Takata F, Yamauchi A, et al. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038(2):208–215. doi:10.1016/j.brainres.2005.01.027
26. Nakagawa S, Deli MA, Nakao S, et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27(6):687–694. doi:10.1007/s10571-007-9195-4
27. Wu Q, Jing Y, Yuan X, et al. The distinct abilities of tube-formation and migration between brain and spinal cord microvascular pericytes in rats. Clin Hemorheol Microcirc. 2015;60(2):231–240. doi:10.3233/CH-141856
28. Lippoldt A, Kniesel U, Liebner S, et al. Structural alterations of tight junctions are associated with loss of polarity in stroke-prone spontaneously hypertensive rat blood-brain barrier endothelial cells. Brain Res. 2000;885(2):251–261. doi:10.1016/S0006-8993(00)02954-1
29. Oztas B, Turkel N. Influence of an abrupt increase in blood pressure on the blood-brain barrier permeability during acute hypertension and epileptic seizures. Pharmacol Res. 2001;44(3):209–212. doi:10.1006/phrs.2001.0841
30. Qi X, Inagaki K, Sobel RA, Mochly-Rosen D. Sustained pharmacological inhibition of deltaPKC protects against hypertensive encephalopathy through prevention of blood-brain barrier breakdown in rats. J Clin Invest. 2008;118(1):173–182. doi:10.1172/JCI32636
31. Yamagata K, Tagami M, Yamori Y. Neuronal vulnerability of stroke-prone spontaneously hypertensive rats to ischemia and its prevention with antioxidants such as vitamin E. Neuroscience. 2010;170(1):1–7. doi:10.1016/j.neuroscience.2010.07.013
32. Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32(4):687–698. doi:10.1016/S0008-6363(96)00063-6
33. Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi:10.1016/j.devcel.2011.07.001
34. Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol. 2014;24(4):371–386. doi:10.1111/bpa.12152
35. Kamouchi M, Ago T, Kuroda J, Kitazono T. The possible roles of brain pericytes in brain ischemia and stroke. Cell Mol Neurobiol. 2012;32(2):159–165. doi:10.1007/s10571-011-9747-5
36. Chen Y, Li Q, Tang J, Feng H, Zhang JH. The evolving roles of pericyte in early brain injury after subarachnoid hemorrhage. Brain Res. 2015;1623:110–122. doi:10.1016/j.brainres.2015.05.004
37. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–738. doi:10.1038/nrn3114
38. Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16(7):393–405. doi:10.1038/nrm4007
39. Tang Y, Luo J, Zhang W, Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol Cell. 2006;24(6):827–839. doi:10.1016/j.molcel.2006.11.021
40. Horvath MM, Wang X, Resnick MA, Bell DA. Divergent evolution of human p53 binding sites: cell cycle versus apoptosis. PLoS Genet. 2007;3(7):e127. doi:10.1371/journal.pgen.0030127
41. Miyashita T, Harigai M, Hanada M, Reed JC. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994;54(12):3131–3135.
42. Kim EM, Jung CH, Kim J, Hwang SG, Park JK, Um HD. The p53/p21 Complex regulates cancer cell invasion and apoptosis by targeting Bcl-2 family proteins. Cancer Res. 2017;77(11):3092–3100. doi:10.1158/0008-5472.CAN-16-2098
43. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80(2):293–299. doi:10.1016/0092-8674(95)90412-3
44. Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282(5387):290–293. doi:10.1126/science.282.5387.290
45. Patel JV, Lim HS, Varughese GI, Hughes EA, Lip GY. Angiopoietin-2 levels as a biomarker of cardiovascular risk in patients with hypertension. Ann Med. 2008;40(3):215–222. doi:10.1080/07853890701779586
46. Nadar SK, Blann A, Beevers DG, Lip GY. Abnormal angiopoietins 1&2, angiopoietin receptor Tie-2 and vascular endothelial growth factor levels in hypertension: relationship to target organ damage [a sub-study of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT)]. J Intern Med. 2005;258(4):336–343. doi:10.1111/j.1365-2796.2005.01550.x
47. Yun JH, Park SW, Kim JH, Park YJ, Cho CH, Kim JH. Angiopoietin 2 induces astrocyte apoptosis via alphavbeta5-integrin signaling in diabetic retinopathy. Cell Death Dis. 2016;7:e2101. doi:10.1038/cddis.2015.347
48. Park SW, Yun JH, Kim JH, Kim KW, Cho CH, Kim JH. Angiopoietin 2 induces pericyte apoptosis via alpha3beta1 integrin signaling in diabetic retinopathy. Diabetes. 2014;63(9):3057–3068. doi:10.2337/db13-1942
49. Hammes HP, Lin J, Wagner P, et al. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53(4):1104–1110. doi:10.2337/diabetes.53.4.1104
50. Pfister F, Feng Y, Vom Hagen F, et al. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008;57(9):2495–2502. doi:10.2337/db08-0325
51. Li M, Lu Y, Hu Y, et al. Salvianolic acid B protects against acute ethanol-induced liver injury through SIRT1-mediated deacetylation of p53 in rats. Toxicol Lett. 2014;228(2):67–74. doi:10.1016/j.toxlet.2014.04.011
52. Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001;93(1–2):53–62. doi:10.1016/S0248-4900(01)01125-X
53. Chang F, Steelman LS, Shelton JG, et al. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review). Int J Oncol. 2003;22(3):469–480.
54. Thaler S, Hahnel PS, Schad A, Dammann R, Schuler M. RASSF1A mediates p21Cip1/Waf1-dependent cell cycle arrest and senescence through modulation of the Raf-MEK-ERK pathway and inhibition of Akt. Cancer Res. 2009;69(5):1748–1757. doi:10.1158/0008-5472.CAN-08-1377
55. Fang CY, Wu CZ, Chen PN, et al. Antimetastatic potentials of salvianolic acid A on oral squamous cell carcinoma by targeting MMP-2 and the c-Raf/MEK/ERK pathway. Environ Toxicol. 2018;33(5):545–554. doi:10.1002/tox.22542
56. Lu B, Ye Z, Deng Y, Wu H, Feng J. MEK/ERK pathway mediates cytoprotection of salvianolic acid B against oxidative stress-induced apoptosis in rat bone marrow stem cells. Cell Biol Int. 2010;34(11):1063–1068. doi:10.1042/CBI20090126
57. Xu D, Xu L, Zhou C, et al. Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway. Int J Biochem Cell Biol. 2014;51:1–9. doi:10.1016/j.biocel.2014.03.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.