Back to Journals » Infection and Drug Resistance » Volume 15
Salmonella enteritis Spondylitis with Brucella melitensis Infection: A Rare Case of Mixed Infections of Spine
Authors Zhang W, Wang J , Zhang Y, Ma R, Zhang Q
Received 10 August 2022
Accepted for publication 2 November 2022
Published 7 November 2022 Volume 2022:15 Pages 6525—6531
DOI https://doi.org/10.2147/IDR.S385759
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
WenSheng Zhang,* Jie Wang,* Yao Zhang, Rui Ma, Qiang Zhang
Department of Orthopedics, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiang Zhang, Email [email protected]
Background: As a widespread back condition in orthopedics, spondylitis is rarely caused by Salmonella. Here, we report a rare case of spondylitis caused by Salmonella enteritis associated with Brucella melitensis.
Case Presentation: Salmonella septicemia was initially diagnosed in a 27-year-old woman with high fever and low back pain, but her symptoms did not improve after 3 days of antibiotic treatment. The patient was then referred to our hospital’s Department of Infectious Diseases. This patient had mild anemia. There were no positive results for tuberculosis antibody and Rose Bengal plate agglutination (RBPT). When the patient’s symptoms did not improve after diagnostic anti-tuberculosis treatment, he was transferred to our Orthopaedics department for lumbar posterior lesion removal, decompression, internal fixation, cage implantation, and bone grafting fusion under general anesthesia. Following the operation, a postoperative specimen culture and a real-time polymerase chain reaction (real-time-PCR) indicated Salmonella enteritis with Brucella melitensis (B. melitensis) infection. The symptoms improved and inflammatory markers returned to normal after 2 weeks of treatment with levofloxacin, rifampicin, and doxycycline.
Conclusion: Anaemic patients with immunocompromised conditions should be given special attention in the diagnosis of Salmonella spondylitis. Surgery should be considered if antibiotic therapy fails to identify the pathogen that is infecting the patient with infectious spondylitis.
Keywords: Salmonella, brucellosis, spondylitis, mixed infection
Background
Salmonella is a gram-negative intracellular bacterium. Enteritis is usually caused by Salmonella enteritis, which lives in the intestines of humans and animals. For patients with underlying diseases or factors suppressing their resistance to infection, it may occasionally affect extraintestinal organs, like the brain, thyroid, and musculoskeletal system.1 Salmonella-related spondylitis accounts for 0.45% of osteomyelitis cases.2 Immunocompromised patients, especially those with sickle cell disease, are prone to Salmonella enteritis osteomyelitis.3 Multiple organs can be affected by Brucellosis; osteoarticular involvement is the most common brucellosis complication, manifesting as spondylitis, arthritis, sacroiliitis, discitis, osteomyelitis, tenosynovitis, and bursitis, involvement of the lumbar and thoracic vertebrae can lead to symptoms such as lumbar and thoracic spondylitis and discitis.4–6 In this report, we describe the rarity of Salmonella enteritis spondylitis complicated by B. melitensis infection. No previous literature has described this co-infection.
Case Presentation
A 27-year-old female with a maximum body temperature of 40.4°C developed low back pain and percussive pain in the renal area about 3 weeks ago. Cotrimoxazole was sensitive to bovine Salmonella enteritis in blood cultures obtained at Beijing Wangjing Hospital (Beijing, China). A combination of antibiotics etimicin sulfate (300mg po qd), cefoxitin (2g po q12h) and cotrimoxazole (4g po qd) was administered. In spite of 3 days of treatment, her symptoms did not improve, so she sought further treatment at our hospital. Previously, the patient had been healthy and did not have any significant medical or surgical history. In addition to eating burgers from unknown vendors before fever, she had no history of epidemic areas, livestock, contact with cattle, sheep, or habit of consuming unpasteurized milk, tuberculosis, smoking, intravenous drug use, or any other HIV infection factors.
At admission, the body temperature was 38.4°C. During the physical examination, there were palpable pains in the spinous processes of the lumbar 4 and 5 vertebrae, lumbar forced positions, lumbar back pain, and limited lumbar flexion. Neither inguinal nor axillary lymph nodes were found, nor was there any evidence of hepatosplenomegaly. Laboratory tests were as follows: hemoglobin (Hb) 96 g/L, red blood cell count (RBC) 2.84×1012/L, serum ferritin 12μg/L, mean corpuscular volume (MCV) was 74.6 fL, mean corpuscular hemoglobin content (MCH) was 23.8 pg, and mean corpuscular hemoglobin concentration (MCHC) was 302g/L, white blood cell (WBC) 8.05×109/L accompanied by neutrophil elevation, platelet 344×109/L, erythrocyte sedimentation rate (ESR) 117mm/h, C-reactive protein (CRP) 75.7mg/L, albumin 34.3g/L, fasting blood glucose 5.6 mmol/L, HIV antibody negative, complement C3 1290mg/L, complement C4 330 mg/L, IgM 1.04 mg/L, IgG 9.66 mg/L, IgA 1.09 mg/L. In the absence of tuberculosis antibodies, the RBPT and acid-fast staining of the sputum smear were also negative, and the chest X-ray was normal. The blood culture was also free of bacteria and fungi after 5 days.
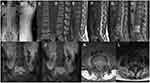
The L4/5 vertebral space was narrowed on the preoperative X-ray (Figure 1A and B). Preoperative CT showed the intervertebral disc of L4/5 and adjacent vertebral body were heavily damaged with dense pores (Figure 1C and D). Preoperative sagittal MRI showed vertebral body and intervertebral disc lesions with low signal on T1-weighted imaging (T1WI), high signal on T2-weighted imaging (T2WI), and high signal on fat-suppressed T2-weighted imaging (FS-T2WI), which was significantly enhanced after enhancement (Figure 1E–H). Coronal and transverse MRI showed vertebral body damage of L4 and L5 complicated by intervertebral disc damage of L4/5 (Figure 1I–L).
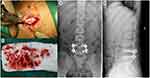
The patient’s symptoms did not improve after diagnostic anti-tuberculosis treatment at the Department of Infectious Diseases of our hospital. As a result, he was transferred to Department of Orthopedics for surgical treatment. Preliminary diagnosis is suppurative spondylitis, although brucellosis spondylitis and spinal tuberculosis cannot be ruled out. Following general anesthesia, lumbar posterior lesion removal, decompression, internal fixation, cage implantation, and bone graft fusion were performed (Figure 2A–D). Real-time PCR of the nucleus pulposus samples showed that there was B. melitensis (Figure 3).
Salmonella enteritis was detected in intraoperative pus secretions 7 days after surgery, and the pathological Giemsa staining of intraoperative tissues (nucleus pulposus, endplate cartilage) showed Brucella contamination (Figure 4). Due to the long-term use of antibiotics, a stool smear revealed possible yeast growth. The patient was finally diagnosed with mixed spinal infection of Salmonella enteritis and B. melitensis. Afterwards, she was prescribed levofloxacin, rifampicin, and doxycycline. Antifungal fluconazole was administered orally. Oral ferrous sulfate tablets for the treatment of iron deficiency anemia. Two weeks after treatment, the patient was no longer experiencing fever or sweating, and was able to perform daily activities using a lumbar brace, CRP returned to normal and ESR (39mm/h) was significantly lower than before, Hb 112g/L and RBC 3.84×1012/L, MCV 91.4 fL, MCH 30.2 pg, MCHC 326. A 6-month treatment course of levofloxacin, rifampicin, and doxycycline was continued after the patient was discharged. A follow-up 2 years later revealed normal CRP and ESR, Hb 119 g/L, and RBC 3.95×1012/L; X-ray showed a good position of internal fixation (Figure 5A and B); CT showed well fusion of L4 and L5 vertebral body, suggesting the formation of new bone in L4/5 disc space (Figure 5C–F); MRI demonstrated a good repair of the lesion (Figure 5G–J).
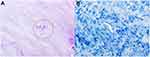 |
Figure 4 In postoperative pathology, Brucella (red circle) was positive for Giemsa staining (A). Mycobacterium tuberculosis was negative for acid-fast staining of the intervertebral disc of L4/5 (B). |
 |
Figure 5 Radiological studies 2 years after discharge. Plain radiographs showed no abnormal changes in the fixed position of L4–L5 vertebral segments (A and B). No abnormalities on CT and MRI (C–J). |
Discussion and Conclusions
Spinal infection is classified as specific infection (B. melitensis, Mycobacterium tuberculosis, syphilis, fungi) and non-specific infection (Staphylococcus aureus, Escherichia coli, Staphylococcus saprophyticus, Streptococcus pneumoniae, etc).7 There has been an increase in Salmonella-induced spondylitis in recent years.6,8–16 Spondylitis caused by Salmonella enteritis and B. melitensis has not been reported to date. An unusual mixed infection characterizes this case.
Infections in the gastrointestinal tract are usually caused by Salmonella enteritis, a gram-negative bacterium. Microorganisms in contaminated water and food can cause Salmonella enteritis infection. There are usually conservative treatments available for symptoms of acute infections, such as diarrhea, vomiting, and abdominal pain. Salmonella enteritis, however, can persist in host cells and cause chronic infections. Those suffering from anemia (especially sickle cell anemia), systemic lupus erythematosus, diabetes, and those receiving immunosuppressive medications are more likely to contract Salmonella enteritis spondylitis.17,18
In patients with low immunity, Salmonella enteritis can cause extraintestinal infections; non-typhoid salmonellosis does not always present with gastrointestinal symptoms, especially in patients with low immunity, because diarrhea is a defense mechanism that depends on a normal immune response.19 Spinal tuberculosis and Salmonella spondylitis are easily confused—It has been shown that fever, a higher white blood cell count, CRP, alkaline phosphatase, and a higher proportion of neutrophils are all indications of suppurative spondylitis; the symptoms and signs of spinal tuberculosis are less obvious than those of Salmonella spondylitis.20,21 It is possible to distinguish tuberculous spondylitis from suppurative spondylitis with MRI—There are often paraspinal abnormalities, paraspinal abscesses with thin and smooth walls involving thoracic vertebrae in tuberculous spondylitis.22 A blood culture is essential for diagnosing spinal infections, but only 20% to 60% of the patients have a positive culture, the gold standard for diagnosis is percutaneous biopsy, culture of the lesion site, and histopathological examination.23
Salmonella osteomyelitis is commonly treated with chloramphenicol, third-generation cephalosporins, and fluoroquinolones, although there are no standard antimicrobial treatment guidelines, antibiotic treatment should be continued for at least 6 weeks to avoid recurrence and failure.24,25 Surgical indications of Salmonella spondylitis include neurological impairment, large intraspinal and paraspinal abscesses, extensive bone destruction and spinal instability.26,27
Around 500,000 cases of brucellosis spondylitis occur each year worldwide, making it the most common zoonotic disease; infection occurs mainly through contact with animals or through consumption of unpasteurized milk and fresh cheese.28 Brucellosis spondylitis is a nonspecific disease that causes fevers, night sweats, fatigue, and is mainly found in northern provinces of China.29 It is difficult to differentiate from spinal tuberculosis based on these non-specific symptoms. Thus, brucellosis spondylitis is often misdiagnosed as spinal tuberculosis in areas with low brucellosis incidence. There are many subtle differences between the two diseases: intermittent high fever of brucellosis spondylitis is more common than low fever of spinal tuberculosis; patients with spinal tuberculosis were more likely to experience vertebral body destruction, kyphosis, paraspinal abscess, and spinal cord compression.30,31 The RBPT is mainly used for Brucella infection screening.32 Presently, blood culture is the gold standard for diagnosing brucellosis, but positive detection rate in chronic bacteremia is low.33 As compared with other detection techniques, PCR is highly sensitive and specific for identifying Brucella species from peripheral blood and other tissues.34
In the acute phase of Brucellosis, rifampicin and doxycycline are the most commonly used drugs. The clinical treatment of brucellosis in China is mainly a triple-drug regimen, and enduring drug treatment for more than 3 months can reduce the risk of recurrence.35–37 When drug treatment does not seem to be working, surgical intervention may be necessary for patients with brucellosis spondylitis. The surgical treatment has been demonstrated in clinical studies to be effective in removing lesions, relieving pain, improving local blood flow to lesions, maintaining and rebuilding spinal stability, reducing complications, promoting early rehabilitation and cure of lesions, and improving clinical outcomes.38,39
Anemia, a risk factor for Salmonella, was moderate in our case. As we speculated, the patient probably had Salmonella enteritis and B. melitensis infection through the digestive tract after eating a burger from an unidentified vendor prior to the fever. After antibiotic treatment, the patient’s symptoms were not relieved, and the preoperative laboratory examination was unable to identify the infection bacteria. The infectious bacteria were finally identified through surgery and laboratory tests. Our case is characterized by the following points: 1) Salmonella enteritis spondylitis complicated by B. melitensis infection is extremely rare; 2) This patient had no obvious gastrointestinal symptoms; 3) All preoperative tests were negative, and pathogens were not detected; 4) Due to atypical manifestations, the patient was originally misdiagnosed as having spinal tuberculosis.
As a result, patients with spondylitis who are clinically immunocompromised should be cautious of Salmonella infections. In spite of the fact that Brucellosis spondylitis often complicates with Mycobacterium tuberculosis, when the diagnosis is unclear and conservative treatment is ineffective, surgical intervention should be considered as soon as possible.
Patient Consent and Ethics Statement
The patient provided informed consent for publication of this study and accompanying images. The Ethics Committee of the Beijing Ditan Hospital of Capital Medical University approved the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work received no funding.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Shrestha P, Mohan S, Roy S. Bug on the back: vertebral osteomyelitis secondary to fluoroquinolone resistant Salmonella typhi in an immunocompetent patient. BMJ Case Rep. 2015;2015:bcr2015212503. doi:10.1136/bcr-2015-212503
2. Huang ZD, Wang CX, Shi TB, et al. Salmonella osteomyelitis in adults: a systematic review. Orthop Surg. 2021;13(4):1135–1140. doi:10.1111/os.12912
3. Chen M, Wang R, Shan J, et al. Salmonella enteritis spondylitis of thoracic spine: a case report and review of the literature. BMC Surg. 2020;20(1):180. doi:10.1186/s12893-020-00841-5
4. Esmaeilnejad-Ganji SM, Esmaeilnejad-Ganji SMR. Osteoarticular manifestations of human brucellosis: a review. World J Orthop. 2019;10(2):54–62. doi:10.5312/wjo.v10.i2.54
5. Ebrahimpour S, Bayani M, Moulana Z, et al. Skeletal complications of brucellosis: a study of 464 cases in Babol, Iran. Caspian J Intern Med. 2017;8(1):44–48.
6. Kutlu SS, Kutlu M, Tuzun T, et al. Spondylodiscitis: a common complication of brucellosis. J Infect Dev Countries. 2018;12(07):550–556. doi:10.3855/jidc.10557
7. Skaf GS, Domloj NT, Fehlings MG, et al. Pyogenic spondylodiscitis: an overview. J Infect Public Health. 2010;3(1):5–16. doi:10.1016/j.jiph.2010.01.001
8. Feng ZY, Guo F, Chen Z. Literature review and clinical presentation of cervical spondylitis due to Salmonella enteritidis in immunocompetent. Asian Spine J. 2014;8(2):206–210. doi:10.4184/asj.2014.8.2.206
9. Zaki M, Muhammad Ariffin MH. Single-stage debridement and spinal instrumentation for Salmonella spondylodiscitis of thoracic vertebra. Cureus. 2021;13(9):e18306. doi:10.7759/cureus.18306
10. Farrar H, Abbey A, Patel V, et al. Osteomyelitis, discitis, epidural and psoas abscess secondary to Salmonella enterica in a man with diabetes mellitus and newly diagnosed α-thalassaemia trait. BMJ Case Rep. 2015;2015:bcr2014207008. doi:10.1136/bcr-2014-207008
11. Banerjee B, Madiyal M, Madhava PK, et al. Typhoid spondylodiscitis mimicking tuberculosis in a teenage girl. J Infect Public Health. 2018;11(1):136–137. doi:10.1016/j.jiph.2017.04.004
12. Muhamad Effendi F, Ibrahim MI, Mohd Miswan MF. Salmonella spondylodiscitis of the thoracic vertebrae mimicking spine tuberculosis. BMJ Case Rep. 2016;2016:bcr2016215909. doi:10.1136/bcr-2016-215909
13. Fukuda T, Bouchi R, Minami I, et al. Retrograde pyelonephritis and lumbar spondylitis as a result of Salmonella typhi in a type 2 diabetes patient with neurogenic bladder. J Diabetes Investig. 2016;7(3):436–439. doi:10.1111/jdi.12375
14. Cheng W, Lian K, Luo D, et al. Salmonella potsdam causing lumbar vertebral osteomyelitis: a case report. Medicine. 2018;97(18):e0682. doi:10.1097/MD.0000000000010682
15. Librianto D, Suwarto S, Imran D, et al. An extremely rare case of upper thoracic salmonella infection. Orthop Res Rev. 2021;13:107–112. doi:10.2147/ORR.S319616
16. Myojin S, Kamiyoshi N, Kugo M. Pyogenic spondylitis and paravertebral abscess caused by Salmonella Saintpaul in an immunocompetent 13-year-old child: a case report. BMC Pediatr. 2018;18(1):24. doi:10.1186/s12887-018-1010-5
17. Gupta SK, Pandit A, White DG. Salmonella osteomyelitis of the thoracic spine: an unusual presentation. Postgrad Med J. 2004;80(940):110–111. doi:10.1136/pmj.2002.002592
18. Gondusky JS, Gondusky CJ, Helmers SW. Salmonella osteomyelitis in newonset diabetes mellitus. Orthopedics. 2009;32:690–693.
19. Ortiz D, Siegal EM, Kramer C, et al. Nontyphoidal cardiac salmonellosis: two case reports and a review of the literature. Texas Heart Inst J. 2014;41(4):401–406. doi:10.14503/THIJ-13-3722
20. Kim CJ, Song KH, Jeon JH, et al. A comparative study of pyogenic and tuberculous spondylodiscitis. Spine. 2010;35:E1096–E1100. doi:10.1097/BRS.0b013e3181e04dd3
21. Huang DB, DuPont HL. Problem pathogens: extra-intestinal complications of Salmonella enterica serotype Typhi infection. Lancet Infect Dis. 2005;5(6):341–348. doi:10.1016/S1473-3099(05)70138-9
22. Harada Y, Tokuda O, Matsunaga N. Magnetic resonance imaging characteristics of tuberculous spondylitis vs. pyogenic spondylitis. Clin Imag. 2008;32:303–309. doi:10.1016/j.clinimag.2007.03.015
23. Acosta FL, Chin CT, Quiñones-Hinojosa A, et al. Diagnosis and management of adult pyogenic osteomyelitis of the cervical spine. Neurosurg Focus. 2004;17(6):E2. doi:10.3171/foc.2004.17.6.2
24. Mcanearney S, Mccall D. Salmonella osteomyelitis. Ulster Med J. 2015;84:171–172.
25. Papaioannou I, Baikousis A, Korovessis P. Multi-foci Salmonella enteritis osteomyelitis of thoracic spine with pleural effusion and fatal outcome. A unique case presentation and review of the literature. J Orthop Case Rep. 2017;7:69–74. doi:10.13107/jocr.2250-0685.694
26. Ikejiri K, Suzuki K, Ito A. Invasive Salmonella Enteritidis infection complicated by bacterial meningitis and vertebral osteomyelitis shortly after influenza a infection in an immunocompetent young adult. J Infect Chemother. 2020;262:269–273. doi:10.1016/j.jiac.2019.08.001
27. Santos EM, Sapico FL. Vertebral osteomyelitis due to salmonellae: report of two cases and review. Clin Infect Dis. 1998;27(2):287–295. doi:10.1086/514668
28. Zou D, Zhou J, Jiang X. Diagnosis and management of spinal tuberculosis combined with brucellosis: a case report and literature review. Exp Ther Med. 2018;15(4):3455–3458. doi:10.3892/etm.2018.5812
29. Zhong Z, Yu S, Wang X, et al. Human brucellosis in the People’s Republic of China during 2005–2010. Int J Infect Dis. 2013;17(5):e289–e292. doi:10.1016/j.ijid.2012.12.030
30. Dasari S, Naha K, Prabhu M. Brucellosis and tuberculosis: clinical overlap and pitfalls. Asian Pac J Trop Med. 2013;6(10):823–825. doi:10.1016/S1995-7645(13)60145-5
31. Kurtaran B, Sarpel T, Tasova Y, et al. Brucellar and Tuberculous spondylitis in 87 adult patients: a descriptive and comparative case series. Infect Dis Clin Prac. 2008;16(3):166–173. doi:10.1097/IPC.0b013e318168ffb3
32. Faddoul L, Azar R, Haddad A, et al. The importance of blood culture and serology for Brucellosis diagnosis and treatment. J Infect Dev Ctries. 2018;12(21):19. doi:10.3855/jidc.10120
33. Bozgeyik Z, Aglamis S, Bozdag PG, et al. Magnetic resonance imaging findings of musculoskeletal brucellosis. Clin Imaging. 2014;38(5):719–723. doi:10.1016/j.clinimag.2014.04.007
34. Wang Y, Wang Z, Zhang Y, et al. Polymerase chain reaction-based assays for the diagnosis of human brucellosis. Ann Clin Microbiol Antimicrob. 2014;13:31. doi:10.1186/s12941-014-0031-7
35. Zheng R, Xie S, Lu X, et al. A systematic review and meta-analysis of epidemiology and clinical manifestations of human brucellosis in China. Biomed Res Int. 2018;2018:5712920. doi:10.1155/2018/5712920
36. Chaofan S, Xinming Y. Lumbar Brucella spondylitis vertebrae fixation, short safety evaluation and efficacy analysis of segmental fixation. Chin Damage J Prosthodont. 2017;12(05):350–356.
37. Ariza J, Bosilkovski M, Cascio A, et al. Perspectives for the treatment of brucellosis in the twenty-first century: the Ioannina recommendations. PLoS Med. 2007;4:e317. doi:10.1371/journal.pmed.0040317
38. Passias PG, Ma Y, Chiu YL, Mazumdar M, Girardi FP, Memtsoudis SG. Comparative safety of simultaneous and staged anterior and posterior spinal surgery. Spine. 2012;37(3):247–255. doi:10.1097/BRS.0b013e31821350d0
39. Colmenero JD, Fernandez-Nebro A, Reguera JM, et al. Psoas abscess secondary to brucellosis. J Infect. 1991;22(1):107–109. doi:10.1016/0163-4453(91)91402-J
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.