Back to Journals » OncoTargets and Therapy » Volume 13
Salinomycin and Sulforaphane Exerted Synergistic Antiproliferative and Proapoptotic Effects on Colorectal Cancer Cells by Inhibiting the PI3K/Akt Signaling Pathway in vitro and in vivo
Authors Liu F, Lv RB , Liu Y, Hao Q , Liu SJ, Zheng YY, Li C, Zhu C, Wang M
Received 21 January 2020
Accepted for publication 22 April 2020
Published 3 June 2020 Volume 2020:13 Pages 4957—4969
DOI https://doi.org/10.2147/OTT.S246706
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Fang Liu,1,2,* Rong-Bin Lv,3,* Yan Liu,4 Qian Hao,1 Shu-Jie Liu,5 Yuan-Yuan Zheng,2 Cui Li,2 Cheng Zhu,1 Min Wang1,6
1Department of Geriatric Gastroenterology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong 250012, People’s Republic of China; 2Department of Gastroenterology, Taian City Central Hospital, Taian, Shandong 271000, People’s Republic of China; 3Department of PET/CT, Taian City Central Hospital, Taian, Shandong 271000, People’s Republic of China; 4Department of Emergency, Dongping Hospital Affiliated to Shandong First Medical University, Taian, Shandong 271500, People’s Republic of China; 5Department of Obstetrics and Gynecology, Zibo Spring Hospital Co., Ltd, Zibo, Shandong 255022, People’s Republic of China; 6Department of General Practice, Qilu Hospital of Shandong University, Jinan, Shandong 250012, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Min Wang
Department of Geriatric Gastroenterology, Qilu Hospital of Shandong University, 107 Wenhuaxi Road, Jinan, Shandong 250012, People’s Republic of China
Tel +86-531-82166601
Email [email protected]
Background: Both salinomycin (SAL) and sulforaphane (SFN) exert their antitumorigenic effects in various types of cancer We investigated whether combining salinomycin (SAL, an antibiotic ionophore) with sulforaphane (SFN, a phytochemical) exerted synergistic antiproliferative and proapoptotic activities in colorectal cancer (CRC) cells in vitro and in vivo by evaluating the proliferative and apoptotic responses of two CRC cell lines.
Materials and Methods: The combination index (CI) was calculated using the Chou-Talalay method, and the effects of the synergistic combination (CI< 1) of lower doses of SAL and SFN were selected for further studies. Anti-tumor effect of the combination of SAL and SFN was tested both in vitro and in vivo.
Results: Cotreatment effectively inhibited proliferation, migration and invasion and enhanced apoptosis. The xenograft model also showed similar results. Furthermore, we evaluated the molecular mechanism behind SAL- and SFN-mediated CRC cell apoptosis. The combination treatment induced apoptosis in Caco-2 and CX-1 cells by inhibiting the PI3K/Akt pathway, which increased the expression of the tumor suppressor protein p53. The treatment also decreased the expression of the survival protein Bcl-2 and increased the expression of the proapoptotic protein Bax, which increased the Bax/Bcl-2 ratio, as well as enhanced poly ADP-ribose polymerase (PARP) cleavage. Upon inhibiting the PI3K/Akt pathway with LY294002 prior to cotreatment, we detected enhanced PARP cleavage compared to that in the cotreatment only group.
Conclusion: We investigated whether the combination of SAL and SFN had antiproliferative and proapoptotic effects in CRC cells both in vitro and in vivo. Cotreatment also significantly decreased migration and invasion compared to that of the control and SAL or SFN monotherapies. This novel combination of SAL and SFN might provide a potential strategy to treat CRC.
Keywords: salinomycin, sulforaphane, colorectal cancer, synergism, apoptosis, PI3K/Akt pathway
Introduction
For both sexes combined, colorectal cancer (CRC) is the third most commonly diagnosed cancer (10.2% of all cases) and the second most common cause of cancer-related death (9.2% of all cancer-related deaths).1 Most patients are in the middle and late stages of the disease when they are first diagnosed. Despite the availability of many chemotherapeutic drugs, the development of resistance to those drugs poses a major challenge for the treatment of CRC. Therefore, there is a constant demand for new treatments or combination therapies.
Sulforaphane (1-isothiocyanato-4-methylsulfinylbutane, SFN), a phytochemical, is a compound found in cruciferous vegetables, such as broccoli and cauliflower. It is well known in cancer therapy for its anticarcinogenic, antiproliferative, proapoptotic, antimetastatic, and antiangiogenic effects. The protective effects of SFN against oxidative damage induced by toxic drugs have been demonstrated and were shown to be mediated by NF-E2-related factor 2 (Nrf2); therefore, it is an indirect antioxidant.2 The same SFN concentration was demonstrated to be protective in normal cells and harmful to tumor cells, suggesting a potential for its use in chemotherapy.3 Many studies have shown the anticancer potential of SFN in several types of cancers.4–6 Additionally, our previous study demonstrated that SFN could induce apoptosis in the human colon cancer cell line Caco-2.7 Numerous reports have stated that the combination of SFN and a classic chemotherapeutic drug (eg, cisplatin or doxorubicin) results in enhanced apoptosis. SFN combined with cisplatin increased apoptosis and inhibited cell proliferation in many kinds of cancers, such as cervical cancer, epidermal squamous cell carcinoma, prostate cancer and pancreatic cancer.8–10 Based on these observations, we suggest SFN as a novel anticancer agent for the treatment of CRC in humans.
Salinomycin (SAL) is a 751-Da monocarboxylic polyether antibiotic that was isolated from the Streptomyces albus strain and is produced by tank fermentation technology.11 It has been reported that salinomycin can selectively kill human breast cancer stem cells (CSCs) and is 100-fold more potent than paclitaxel.12 Various studies have demonstrated that salinomycin exerts anticancer effects in many types of cancer, including colorectal, prostate, ovarian, lung and breast cancer.13–17 SAL also sensitizes drug-resistant cancer cells to chemotherapeutic agents and targets the CSC population in tumors. There are multiple reports stating that SAL enhances the cytotoxic effects of many conventional chemotherapeutic agents, such as cisplatin, gemcitabine, gefitinib and 5-FU.18–20 In addition, SAL also decreases malignant traits (ie, migration and invasion). Based on the above studies, both SAL and SFN exert their antitumorigenic effects in various types of cancer, and SFN has been demonstrated to be protective against normal cells. Thus, we decided to investigate the antiproliferative and proapoptotic effects of SAL and SFN in CRC cells.
In the present study, the combination of SAL and SFN synergistically inhibited proliferation, induced apoptosis and decreased migration and invasion to greater extents than either the control treatment or either drug alone.
Materials and Methods
Cell Culture Conditions and Reagents
The human colorectal adenocarcinoma cancer cell lines Caco-2 and CX-1 were purchased from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. Caco-2 and CX-1 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) and RPMI 1640 (Gibco, Thermo Fisher Scientific), respectively, supplemented with 10% fetal bovine serum (BI, Israel) and 1% penicillin/streptomycin (PEN/STREP 100×, MILLIPORE, USA). Cells were cultured at 37°C in a 5% CO2 and 95% air humidified incubator. Stocks of SAL (S4526, Sigma-Aldrich, USA) and SFN (S4441, Sigma-Aldrich, USA) were prepared via dissolution in dimethyl sulfoxide (DMSO, D2650, Sigma-Aldrich, USA) at concentrations of 50 mM and 100 mM, respectively, and the solutions were stored at −20°C. The final concentration of DMSO was less than 0.1%. Antibodies against PI3K (1:1000 dilution), Akt (1:1000 dilution), phosphorylated Akt (Ser473) (1:1000 dilution), p53 (1:1000 dilution), cleaved poly ADP-ribose polymerase (PARP) (1:1000 dilution), and β-actin (1:1000 dilution) as well as the PI3K inhibitor LY294002 were purchased from Cell Signaling Technology. Antibodies against Bcl-2 (1:100 dilution) and Bax (1:100 dilution) were obtained from Santa Cruz Biotechnology.
Cell Viability Assay
Cell viability was determined by using the 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Caco-2 and CX-1 cells were trypsinized, seeded in a 96-well plate (5×103 cells/well) and allowed to adhere overnight. The cells were treated with different concentrations either SAL (0, 2.5, 5, 10, 20, 40, 80 μM) or SFN (0, 5, 10, 20, 40, 60, 80, 100 μM) alone for 24, 48 and 72 h. Some cells were treated with a mixture of both compounds for 24 and 48 h at concentrations detailed in Figure 1G. Various concentrations of DMSO were added as a negative vehicle control. Then, 20 μL of MTT (thiazolyl blue tetrazolium bromide, 5 mg/mL, M2128, Sigma-Aldrich, USA) was added to each well, and the plates were incubated for another 4 h at 37°C. After removal of the MTT-containing medium, the formazan crystals were dissolved in 100 μL of DMSO per well, and the plates were agitated at 37°C for 10 min before the absorbance of the colored solution was measured at 550 nm using a microplate reader. The half maximal inhibitory concentration (IC50) values were calculated using GraphPad 8.0.1. The viability rate was calculated as follows: (ODtest–ODblank)/(ODcontrol–ODblank)×100%. The effect of SAL and SFN was analyzed by the combination index (CI) obtained using Calcusyn software based on Chou-Talalay’s method. An Fa-CI plot (Fa, fraction affected) and isobologram determined whether there were interactions between the two drugs when combined; the Fa-CI plot is effect-oriented, while the isobologram is dose-oriented.21–23
EdU Assay
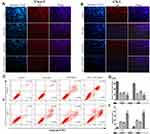
A 5-ethynyl-20-deoxyuridine (EdU) DNA Proliferation in vitro Detection kit (C10310-1, Guangzhou Ribobio, China) was used according to the manufacturer’s instructions. Caco-2 and CX-1 cells (1×104 cells/well) were seeded in 96-well plates and incubated overnight. Then, the cells were treated with DMSO (the control group was treated with an equal volume of DMSO), 5 μM SAL, 10 μM SFN or a combination of SAL and SFN for 48 h. Next, the cells were incubated with 100 μL of medium containing 50 μM EdU for 4 h, fixed, permeabilized and stained. Finally, cell nuclei were stained with 1× Hoechst 33342 nuclear dye for 30 min before fluorescence microscopy images were captured.
Apoptosis Assessment by Flow Cytometry (FCM)
Caco-2 and CX-1 cells (1×106 cells/dish) were seeded in 60 mm dishes and treated for 48 h with drugs as previously described. Apoptosis was detected with a FITC Annexin V Apoptosis Detection Kit I (556547, BD Biosciences, USA) following the manufacturer’s instructions. Cells were collected, incubated with 5 μL of Annexin V-FITC and 5 μL of propidium iodide (PI) for 15 min at room temperature and analyzed on a FACSCalibur flow cytometer (BD Biosciences, USA) within 1 h of staining. Data were analyzed using CellQuest Pro software.
Transwell Assay
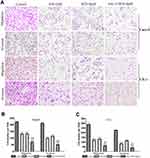
The migration and invasion abilities were assessed with 24-well transwell chambers (8.0 μm pore size, 3422, Corning Costar, USA). For the invasion assay, the surface of the upper chambers was coated with 60 μL of diluted Matrigel matrix (1:9 dilution in serum-free medium, 356234, Corning Costar, USA) and incubated for 1 h at 37°C. After treatment with the respective treatments for 48 h, Caco-2 and CX-1 cells were digested and suspended in FBS-free medium. Then, 20×104 cells in 200 μL of FBS-free medium were added to the upper chamber, and the lower chamber was filled with 750 μL of medium containing 10% FBS. After the transwells were incubated for 24 h, noninvading cells in the upper chamber were removed with a cotton swab, whereas the cells that invaded the membrane were fixed with methanol for 5 min and stained with crystal violet for 30 min. Images of five random fields of the stained cells were acquired through a Nikon Ti-E inverted microscope. For the migration assay, 10×104 cells in 200 μL of FBS-free medium were added to the upper chamber, which were not surface-coated with Matrigel matrix; all other aspects of the procedures were the same.24
Western Blotting
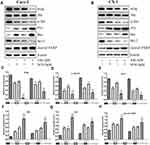
Total protein was isolated from the samples after 48 h of treatment as previously described. For the LY294002 + combination treatment group, cells were pretreated with 10 μM LY294002 for 90 min, and then SAL and SFN were added to the medium of the CRC cells.25 The cells were lysed with RIPA buffer containing protease inhibitor cocktail, and protein concentrations were measured using a BCA protein assay kit. Equal amounts (30 μg) of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) through 6–12% gels and electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA). The membranes were blocked with 5% skimmed milk in TBS-Tween (0.1%) for 2 h and then incubated with the primary antibodies overnight at 4°C. Then, the blots were washed three times and incubated with specific secondary antibodies.26 The bands were visualized using enhanced chemiluminescence (ECL) detection reagent and quantified by ImageJ software. The β-actin band served as a control.
Real-Time Reverse Transcription Quantitative Polymerase Chain Reaction (Real-Time RT-qPCR)
Total RNA from CRC cells cultured with drugs for 48 h was extracted using TRIzol reagent (Invitrogen, Thermo Fisher Scientific) and subsequently transcribed into cDNA using Reverse Transcriptase (Invitrogen, Thermo Fisher Scientific). Quantitative RT-PCR (10-μL reaction volume) was performed using SYBR Premix Ex Taq (TaKaRa, Shiga, Japan).24 β-Actin expression was used as an internal control. The gene-specific primers (BioSune, Shanghai, China) used in this study are listed in Table 1.
 |
Table 1 Real-Time PCR Primer Sequences |
Immunofluorescence Assay
CRC cells (4×104 cells/well) were seeded in 24-well plates and divided into four groups: control group, 5 μM SAL group, 10 μM SFN group and combination (SAL and SFN) group. After 48 h of treatment, the cells were fixed with cold methanol for 15 min and washed three times with cold phosphate-buffered saline (PBS). Cells were permeabilized, washed, blocked with 3% bovine serum albumin (BSA), and incubated with primary antibody (anti-p-Akt, 1:200 dilution) at 4°C overnight. Then, they were washed and incubated with Cy3-conjugated goat anti-rabbit IgG for 1 h. Finally, cell nuclei were stained with DAPI staining solution for 3 min, and the fluorescence was detected by fluorescence microscopy.27
Xenografted Colorectal Cancer Cells in Nude Mice
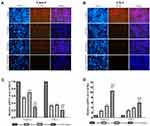
Athymic BALB/c nude mice (male, 4–5 weeks old) were obtained from Beijing Sipeifu Biotechnology Co., Ltd. and housed in SPF breeding units. A Caco-2 cell suspension (1×107 cells in 100 μL) was subcutaneously implanted into the right flanks of each mouse. After the transplanted tumors became palpable (~14 days), 20 successfully generated model mice were randomly divided into four groups (five mice per group): control group, SAL group (5 mg/kg), SFN group (5 mg/kg) and combination (5 mg/kg SAL and 5 mg/kg SFN) group. The drugs were administered to mice via intraperitoneal injection every two days a total of eight times. Tumor volume was monitored every four days using calipers and calculated with the formula width2×length/2.28
Statistical Analysis
All the data are expressed as the means ± standard deviation, and the results were statistically significant at p<0.05. One-way analysis of variance (ANOVA) was used to compare the statistical significance between the control and experimental groups. Comparisons between two groups were performed using unpaired Student’s t-test. Drug synergism and the CI were calculated using CompuSyn software (by Ting-Chao Chou and Nick Martin Biosoft, Cambridge, UK). The rest of the data were analyzed using GraphPad Prism version 8.0.1. The results from all studies were confirmed in at least three independent experiments.
Results
SAL in Combination with SFN Synergistically Inhibited the Viability of Caco-2 and CX-1 Cells
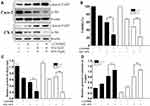
Our first goal was to examine the effects of SAL and SFN in colorectal cancer cells in monotherapy as well as in combination treatment by MTT assay. Initially, colorectal cancer cells were treated with different doses of the respective drug for 24 h, 48 h and 72 h. We found that SAL and SFN significantly inhibited the viability of Caco-2 cells (Figure 1A and C) and CX-1 cells (Figure 1B and D) in a dose- and time-dependent manner. We calculated the IC50 of each drug alone, as shown in Table 2.
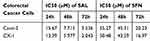 |
Table 2 Effects of SAL and SFN as Single Drugs in Colorectal Cancer Cells |
Next, doses for the combination treatment were chosen based on the cytotoxic effects of the drugs when added alone, and we selected concentrations lower than the IC50 at the 24-h point in the study. The twelve different combination ratios are shown in Figure 1G. SAL and SFN cotreatment in Caco-2 and CX-1 cells showed synergistic cytotoxic effects (the CI value for each combination ratio was <1, indicating drug synergism), which are represented by a CI plot (Figure 1E) and an isobologram (Figure 1F). Lower doses of SAL and SFN at synergistic combinations (CI<1) were selected for further studies. The combination of 5 μM SAL and 10 μM SFN effectively inhibited cell viability at 24 h (Figure 1H) and 48 h (Figure 1I). As illustrated in Figure 1H and I, the effect of the combination was significantly stronger than that of either 5 μM SAL or 10 μM SFN alone in the Caco-2 and CX-1 cells (p < 0.01).
SAL and SFN Synergistically Inhibited Colorectal Cancer Cell Proliferation and Induced Cell Apoptosis
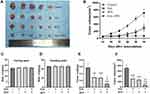
A similar result was obtained by EdU staining with the MTT assay. The combination of SAL and SFN produced a synergistic effect with decreased cell proliferation after 48 h of treatment in CRC cells compared to that of cells treated with control or either monotherapy (Figure 2A, B and D). As shown in Figure 2C and E, cotreatment effectively increased apoptotic cell populations.
The Combination of SAL and SFN Decreased Migration and Invasion
The migration and invasion abilities of Caco-2 and CX-1 cells were measured with transwell chambers after the respective treatments for 48 h (Figure 3A–C). Then, we used transwell chambers coated with Matrigel to detect cell invasion. We found that the number of invading cells in the cotreatment group was significantly decreased compared to that in the control and monotherapy groups. The number of migrated cells was also markedly decreased in the combination groups compared to the control and monotherapy groups.
The Combination of SAL and SFN Induced Apoptosis by Inhibiting the PI3K/Akt Pathway in Colorectal Cancer Cells
To determine the possible role of the PI3K/Akt pathway in apoptosis induced by the combined treatment, we detected the expression levels of phosphorylated Akt (p-Akt), PI3K, p53 (a tumor suppressor protein), Bax, Bcl-2 and cleaved PARP (Figure 4A and B). The expression levels of PI3K, p-Akt and Bcl-2 (Figure 4C–E) were decreased, whereas those of Bax, p53 and cleaved PARP (Figure 4F–H) were significantly increased in the cotreatment groups compared with the control and monotherapy groups. Using the immunofluorescence assay, we found that p-Akt preferentially accumulated in the cytoplasm in the combined treatment group compared to the other treatment groups (Figure 5A and B). In addition, we also detected the Bcl-2 and Bax mRNA expression levels (Figure 5C and D), which showed opposing trends. The results were similar to those of Western blotting.
To further confirm that the PI3K/Akt pathway was involved in apoptosis induced by the combined treatment, cells were pretreated with 10 μM LY294002 (a PI3K inhibitor). The protein levels and cell viability were detected by Western blot analysis and MTT assay, respectively (Figure 6A and B). Pretreatment of cells with LY294002 obviously enhanced the apoptosis induced by the combined treatment, which was associated with growth inhibition, inactivation of p-Akt and PARP cleavage (Figure 6B–D). These results indicated that the combined effects of SAL and SFN on inducing apoptosis and inhibiting growth were influenced by the PI3K/Akt pathway.
Combination Therapy with SAL and SFN Caused a Strong Cytotoxic Effect in vivo
To evaluate the antitumor effects of SAL, SFN and the combined treatment in vivo, we subcutaneously inoculated Caco-2 cells into the right flanks of nude mice and initiated treatment when the tumors (mean diameter of 2–4 mm) were palpable (~14 days). Administration of SAL or SFN alone reduced tumor growth, but the combined treatment of SAL and SFN completely blocked tumor growth (Figure 7B) and significantly reduced both tumor volume (Figure 7A, B and F) and weight (Figure 7E) compared to those of the control and monotherapy groups. There was no significant change in the body weight (Figure 7C and D) of nude mice in the different treatment groups, which indicated that there was no toxicity of the combination treatment.
Discussion
The isothiocyanate SFN has been identified as an effective chemotherapeutic agent and may prevent or treat CRC. Various studies have demonstrated via many molecular mechanisms that SFN induces apoptosis and inhibits proliferation and migration in human colon cancer.29–32 SFN can also activate the Nrf2 signaling pathway and protect against H2O2-mediated oxidative damage in normal colonic cells.33 SAL exhibits antitumor, antibacterial, antifungal, antiparasitic, antiviral and anti-inflammatory activities.34 Several studies have demonstrated that SAL inhibits the migratory and invasive potential of cancer cells,35,36 and the anticancer activity of SAL has been shown in multiple cancers. Various mechanisms of action and molecular targets have been reported in which SAL exerts its antitumorigenic effects, including causing DNA damage, inducing autophagy, targeting molecular pathways (Wnt signaling pathway and PI3K/Akt pathway), inhibiting mitochondrial function and acting as an inhibitor of the ATP-binding cassette (ABC) transporter. SAL plays a crucial role in sensitizing cancer cells to many chemotherapeutic agents; thus, the combination of SAL and SFN might be an effective therapeutic strategy against human CRC while exhibiting lower toxicity. In the present study, we demonstrated that cotreatment with SAL and SFN synergistically enhanced the induction of apoptosis and the inhibition of proliferation in CRC cells both in vitro and in vivo.
First, we found that these two drugs had dose- and time-dependent effects on Caco-2 and CX-1 cells. Chou-Talalay analysis showed that the two drugs acted synergistically (CI<1). We selected 5 μM SAL and 10 μM SFN for subsequent experiments. Then, we found that the combination treatment resulted in enhanced apoptosis and inhibited proliferation compared with those of the other groups. The transwell assay showed that cotreatment with SAL and SFN synergistically decreased the migration and invasion of CRC cells. Indeed, this effect seems especially important because more than 90% of cancer-related mortality comes from cancer invasion and metastasis.
Next, we investigated the possible mechanism of the synergistic cytotoxic activity of SAL and SFN. The PI3K/Akt pathway is a classical signaling pathway, and its activation leads to cell growth, EMT and tumor metastasis. Using Western blotting, we determined the expression levels of PI3K, Akt and p-Akt (Ser473) and found that the PI3K/Akt pathway was inhibited by the cotreatment. The p53 protein is an important downstream site of the PI3K/Akt signaling pathway;37,38 activation of Akt can inhibit p53 activation through MDM2 and therefore inhibit mitochondrial p53-dependent apoptosis.39 Inhibition of Akt phosphorylation by the combination of SAL and SFN in the present study resulted in increased p53 protein levels. It has been demonstrated that inactivation of the p53 protein is associated with an increase in the antiapoptotic protein Bcl-2.40 In this study, we observed the same result: p53 protein expression was increased, while Bcl-2 expression was decreased.
The proapoptotic protein Bax is an important transcriptional target of p53. p53 activates monomeric Bax by inducing its homo-oligomerization, which is followed by the translocation of Bax to the mitochondria.41 In addition, activation of Akt led to phosphorylation of Bax at Ser184, which inhibits its proapoptotic abilities.42 In our study, we demonstrated using Western blotting and RT-qPCR assays that Bax expression was increased in the cotreatment group compared with the control and monotherapy groups. The concomitant increase in Bax and decrease in Bcl-2 led to a change in the Bax/Bcl-2 ratio. This results in Bax translocation from the cytosol to mitochondria, leading to the efflux of cytochrome c from mitochondria, assembly of the apoptosome and caspase-9 activation, followed by the activation of effector caspases. Cell death induced through the p53 pathway is executed by caspase proteinases, which, by cleaving their substrates, lead to the characteristic apoptotic phenotype.43 Caspase-3 cleaves the 116-kDa PARP into 85-kDa fragments (cleaved PARP). This process during apoptosis facilitates cellular disassembly and ensures the completion and irreversibility of the process; cleaved PARP has been used extensively as a marker of apoptosis.44 In the present study, we performed Western blotting and detected increased cleaved PARP levels after cotreatment.
Moreover, inactivation of the PI3K/Akt pathway by the PI3K-specific inhibitor LY294002 enhanced the effects of the cotreatment on apoptosis and PARP cleavage. These results indicated that SAL and SFN induced apoptosis by inhibiting the PI3K/Akt pathway.
Finally, to validate our in vitro results, we also investigated the effects of combining SAL and SFN in vivo using nude mice. Consistent with our in vitro findings, our in vivo data showed that cotreatment was superior to the control and both monotherapy treatments.
Taken together, the data of our in vitro and in vivo experiments indicate that the combined treatment of SAL and SFN synergistically inhibited CRC proliferation and promoted apoptosis; therefore, this novel combination could be a promising potential therapeutic approach for CRC treatment.
Ethics Approval and Consent to participate
All of the procedures and protocols used in the animal experiments were performed in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council). This project was approved by the laboratory animal ethical and welfare committee of Shandong University Cheeloo College of Medicine (Approval number: 19072).
Funding
The present study was funded by the National Natural Science Foundation of China (grant no.81372681).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018:1–31.
2. Gaona-Gaona L, Molina-Jijón E, Tapia E, et al. Protective effect of sulforaphane pretreatment against cisplatin-induced liver and mitochondrial oxidant damage in rats. Toxicology. 2011;286(1–3):20–27. doi:10.1016/j.tox.2011.04.014
3. Negrette-Guzmán M, Huerta-Yepez S, Vega M, et al. Sulforaphane induces differential modulation of TED MANUSCRIPT mitochondrial biogenesis and dynamics in normal cells and tumor cells. Food Chem Toxicol. 2017;100–107.
4. Choi S, Lew KL, Xiao H, et al. D,L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis. 2007;28(1):151–162. doi:10.1093/carcin/bgl144
5. Gamet-Payrastre L, Li P, Lumeau S, et al. Sulforaphane,a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res 2000; 60(5):1426–1433.
6. Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6(3):1013–1021. doi:10.1158/1535-7163.MCT-06-0494
7. Wang M, Chen S, Wang S, et al. Effects of phytochemicals sulforaphane on uridine diphosphate-glucuronosyltransferase expression as well as cell-cycle arrest and apoptosis in human colon cancer caco-2 cells. Chin J Physiol. 2012;55(2):134–144. doi:10.4077/CJP.2012.BAA085
8. Wang X, Govind S, Sajankila SP, et al. Phenethyl isothiocyanate sensitizes human cervical cancer cells to apoptosis induced by cisplatin. Mol Nutr Food Res. 2011;55(10):1572–1581. doi:10.1002/mnfr.201000560
9. Kerr C, Adhikary G, Grun D, et al. Combination cisplatin and sulforaphane treatment reduces proliferation, invasion, and tumor formation in epidermal squamous cell carcinoma. Mol Carcinog. 2018;57(1):3–11. doi:10.1002/mc.22714
10. Kallifatidis G, Labsch S, Rausch V, et al. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther. 2011;19(1):188–195. doi:10.1038/mt.2010.216
11. Miyazaki Y, Shibuya M, Sugawara H, et al. Salinomycin,a new polyether antibiotic. J Antibiot. 1974;27(11):814–821. doi:10.7164/antibiotics.27.814
12. Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–659. doi:10.1016/j.cell.2009.06.034
13. Zhou J, Li P, Xue X, et al. Salinomycin induces apoptosis in cisplatin-resistant colorectal cancer cells by accumulation of reactive oxygen species. Toxicol Lett. 2013;222(2):139–145. doi:10.1016/j.toxlet.2013.07.022
14. Ketola K, Hilvo M, Hyotylainen T, et al. Salinomycin inhibits prostate cancer growth and migration via induction of oxidative stress. Br J Cancer. 2012;106(1):99–106. doi:10.1038/bjc.2011.530
15. Kaplan F, Teksen F. Apoptotic effects of salinomycin on human ovarian cancer cell line (ovcar-3). Tumour Biol. 2016;37(3):3897–3903. doi:10.1007/s13277-015-4212-6
16. Arafat K, Iratni R, Takahashi T, et al. Inhibitory effects of salinomycin on cell survival, colony growth, migration and invasion of human non-small cell lung cancer a549 and lnm35: involvement of nag-1. PLoS One. 2013;8(6):e66931. doi:10.1371/journal.pone.0066931
17. Al Dhaheri Y, Attoub S, Arafat K, et al. Salinomycin induces apoptosis and senescence in breast cancer: upregulation of p21, downregulation of survivin and histone H3 and H4 hyperacetylation. Biochim Biophys Acta. 2013;1830(4):3121–3135. doi:10.1016/j.bbagen.2013.01.010
18. Parajuli B, GyoLee H, HoonKwon S, et al. Salinomycin inhibits Akt/NF-kB and induces apoptosis in cisplatin resistant ovarian cancer cells. Cancer Epidemiol. 2013;37(4):512–517. doi:10.1016/j.canep.2013.02.008
19. Zhang GL, Liang Y, Zhou LJ, et al. Combination of salinomycin and gemcitabine eliminates pancreatic cancer cells. Cancer Lett. 2011;313(3):137–144. doi:10.1016/j.canlet.2011.05.030
20. Zhang Y, Zhang Q, Sun J, et al. The combination therapy of salinomycin and gefitinib using poly (d,l-lactic-co-glycolic acid)-poly (ethylene glycol) nanoparticles for targeting both lung cancer stem cells and cancer cells. Onco Targets Ther. 2017;10:5653–5666. doi:10.2147/OTT.S141083
21. Chou TC, Martin N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values. Paramus, NJ: ComboSyn; 2005. Available from: http://www. combosyn. com/for video demonstration.
22. Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi:10.1124/pr.58.3.10
23. Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi:10.1158/0008-5472.CAN-09-1947
24. Wang Q, Lu W, Yin T, et al. Calycosin suppresses TGF-β-induced epithelial-to-mesenchymal transition and migration by upregulating BATF2 to target PAI-1 via the Wnt and PI3K/Akt signaling pathways in colorectal cancer cells. J Exp Clin Cancer Res. 2019;38(1):240–252. doi:10.1186/s13046-019-1243-7
25. Kim KY, Park KI, Kim SH, et al. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int J Mol Sci. 2017;18(5):1088–1101. doi:10.3390/ijms18051088
26. Han H, Chen W, Yang J, et al. Inhibition of cell proliferation and migration through nucleobase-modified polyamidoamine-mediated p53 delivery. Int J Nanomedicine. 2018;13:1297–1311. doi:10.2147/IJN.S146917
27. Feng M, Feng J, Chen W, et al. Lipocalin2 suppresses metastasis of colorectal cancer by attenuating NF-κB-dependent activation of snail and epithelial mesenchymal transition. Mol Cancer. 2016;15(1):77–95. doi:10.1186/s12943-016-0564-9
28. Zhou Q, Li W, Kong D, et al. DACH1 suppresses epithelial to mesenchymal transition (EMT) through notch1 pathway and reverses progestin resistance in endometrial carcinoma. Cancer Med. 2019;8(9):4380–4388. doi:10.1002/cam4.2317
29. Shusuke Y, Mano H, Toshiyuki S. Sulforaphane enhances apoptosis induced by Lactobacillus pentosus strain S-PT84 via the TNFα pathway in human colon cancer cells. Oncol Lett. 2019;18(4):4253–4261. doi:10.3892/ol.2019.10739
30. Dominic B, Gabriele D, Martina B, Behrens J. Sulforaphane inhibits growth and blocks Wnt/β-catenin signaling of colorectal cancer cells. Oncotarget. 2018;9(74):33982–33994. doi:10.18632/oncotarget.26125
31. Liu K, Shih T, Kuo C, et al. Sulforaphane induces cell death through G2/M phase arrest and triggers apoptosis in HCT116 human colon cancer cells. Am J Chin Med. 2016;44(06):1289–1310. doi:10.1142/S0192415X16500725
32. Pereira L, Silva P, Duarte M, et al. Targeting colorectal cancer proliferation, stemness and metastatic potential using Brassicaceae extracts enriched in isothiocyanates: A 3D cell model-based study. Nutrients. 2017;9(4):368–394. doi:10.3390/nu9040368
33. Wang Y, Dacosta C, Wang W, et al. Synergy between sulforaphane and selenium in protection against oxidative damage in colonic CCD841 cells. ScienceDirect. 2015;35:610–617.
34. Huczynski A, Janczak J, Antoszczak M, et al. Antiproliferative activity of salinomycin and its derivatives. Bioorg Med Chem Lett. 2012;22(23):7146–7150. doi:10.1016/j.bmcl.2012.09.068
35. Koeck S, Amann A, Huber JM, et al. The impact of metformin and salinomycin on transforming growth factor β-induced epithelial-to-mesenchymal transition in non-small cell lung cancer cell lines. Oncol Lett. 2016;11(4):2946–2952. doi:10.3892/ol.2016.4323
36. Li R, Dong TT, Hu C, et al. Salinomycin repressed the epithelial–mesenchymal transition of epithelial ovarian cancer cells via downregulating Wnt/β-catenin pathway. Onco Targets Ther. 2017;10:1317–1325. doi:10.2147/OTT.S126463
37. Ning H, Sun Z, Liu Y, et al. Insulin protects hepatic lipotoxicity by regulating ER stress through the PI3K/Akt/p53 involved pathway independently of autophagy inhibition. Nutrients. 2016;8(4):227.
38. Qiu W, Leibowitz B, Zhang L, et al. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene. 2010;29(11):1622–1632. doi:10.1038/onc.2009.451
39. Fenouille N, Puissant A, Tichet M, et al. SPARC functions as an anti-stress factor by inactivating p53 through Akt-mediated MDM2 phosphorylation to promote melanoma cell survival. Oncogene. 2011;30(49):4887–4900. doi:10.1038/onc.2011.198
40. Schmitt CA, Lowe SW. Bcl-2 mediates chemoresistance in matched pairs of primary E(mu)-myc lymphomas in vivo. Blood Cells Mol Dis. 2001;27(1):206–216. doi:10.1006/bcmd.2000.0372
41. Sonja W, Susan E, Gustavo P, et al. p53’s mitochondrial translocation and MOMP action is independent of puma and bax and severely disrupts mitochondrial membrane integrity. Cell Res. 2008;18(7):733–744. doi:10.1038/cr.2008.62
42. Shyra J, David A, Steve K, et al. ×Phosphorylation of Bax Ser 184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279(20):21085–21095. doi:10.1074/jbc.M400063200
43. Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001;29(6):684–688. doi:10.1042/bst0290684
44. Javier F, Guadalupe R, Ve´ronique R, et al. Importance of poly (ADP-ribose) polymerase and its cleavage in apoptosis. J Biol Chem. 1998;273(50):33533–33539. doi:10.1074/jbc.273.50.33533
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.