Back to Journals » Clinical Ophthalmology » Volume 15
Safety of Once-Daily Oxymetazoline HCl Ophthalmic Solution, 0.1% in Patients with Acquired Blepharoptosis: Results from Four Randomized, Double-Masked Clinical Trials
Authors Wirta DL, Korenfeld MS , Foster S , Smyth-Medina R, Bacharach J, Kannarr SR, Jaros MJ, Slonim CB
Received 17 July 2021
Accepted for publication 13 September 2021
Published 8 October 2021 Volume 2021:15 Pages 4035—4048
DOI https://doi.org/10.2147/OPTH.S322326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by David Wirta.
Views: 219
David L Wirta,1 Michael S Korenfeld,2 Shane Foster,3 Robert Smyth-Medina,4 Jason Bacharach,5 Shane R Kannarr,6 Mark J Jaros,7 Charles B Slonim8
1Aesthetic Eye Care Institute & Eye Research Foundation, Newport Beach, CA, USA; 2Comprehensive Eye Care, Ltd., Washington, MO, USA; 3Athens Eye Care, Athens, OH, USA; 4North Valley Eye Medical Group, Mission Hills, CA, USA; 5North Bay Eye Associates, Petaluma, CA, USA; 6Kannarr Eye Care, Pittsburg, KS, USA; 7Summit Analytical, Denver, CO, USA; 8Department of Ophthalmology, University of South Florida Morsani College of Medicine, Tampa, FL, USA
Correspondence: David L Wirta
Aesthetic Eye Care Institute & Eye Research Foundation, 520 Superior Avenue, #235, Newport Beach, CA, 92663, USA
Tel +1 949-650-1863
Email [email protected]
Purpose: An oxymetazoline 0.1% ophthalmic solution was recently approved for treatment of acquired blepharoptosis in adults. This study’s objective was to evaluate the safety profile of oxymetazoline 0.1% when administered once daily for 14– 84 days.
Patients and Methods: Pooled analysis examined safety outcomes from four randomized, double-masked, placebo-controlled clinical trials conducted at 6, 16, 27, and 35 sites, respectively, in the United States. In total, 568 participants with acquired blepharoptosis were evaluated. Median age was 66 years and 74.8% of participants were female. Overall, 375 participants self-administered oxymetazoline 0.1% to both eyes once/day and 193 self-administered placebo (vehicle) daily. Treatment-emergent adverse event (TEAE) rates, severity, and causality were evaluated in the overall population and within participant subgroups defined based on age, race, and ethnicity. Vital signs and ophthalmic findings were evaluated at predefined study visits. Patient-reported treatment tolerability was recorded at study end.
Results: TEAE incidence was similar among participants using oxymetazoline 0.1% (31.2%) or vehicle (30.6%). Nearly all TEAEs were mild-to-moderate, and most were not suspected of being treatment related. Serious TEAEs occurred in four participants receiving oxymetazoline 0.1% and one participant receiving vehicle. Nine and two participants in the oxymetazoline 0.1% and vehicle groups, respectively, discontinued due to a TEAE. Ocular TEAEs occurring in ≥ 2% of participants receiving oxymetazoline 0.1% were punctate keratitis, conjunctival hyperemia, dry eye, blurred vision, instillation site pain, and corneal vital dye staining, with none occurring in > 3.5% of participants. TEAE rates were similar across subgroups based on age, race, and ethnicity. No clinically significant mean changes in vital signs or ophthalmologic findings occurred, and > 98% of participants rated oxymetazoline 0.1% as causing no/mild discomfort.
Conclusion: Once-daily oxymetazoline 0.1% was safe and well tolerated in participants with acquired blepharoptosis when used for 14– 84 days. Safety did not appear to differ based on age, race, or ethnicity.
Keywords: adrenergic agonist, adverse event, eye drop, intraocular pressure, Müller’s muscle, pupil, topical
Plain Language Summary
Acquired blepharoptosis is a common condition of the upper eyelid. It is characterized by drooping of one or both eyelids, which affects the appearance of the eyes and can also impair the superior (upper) visual field and negatively impact daily activities. A solution of oxymetazoline 0.1%, used as a once-daily eye drop, recently became available for the treatment of acquired blepharoptosis in adults, making it the first drug approved for this condition (previously, surgery was the only effective treatment option). The efficacy and safety of oxymetazoline 0.1% have been studied in four clinical trials ranging in duration from 14 to 84 days. This analysis examined data from all four studies to provide a comprehensive evaluation of the safety of oxymetazoline 0.1% when used once daily in both eyes. In total, we evaluated 375 participants who used oxymetazoline 0.1% and 193 participants who used placebo (vehicle solution). Overall rates of unwanted adverse events were similar between the comparator groups. In addition, most events reported were mild and unrelated to treatment, and serious or severe events were rare. Adverse event rates were also found to be similar across participant groups defined based on age, race, and ethnicity. Oxymetazoline 0.1% did not cause any meaningful changes in ocular measures such as intraocular pressure and pupil diameter, and finally, the vast majority of participants indicated that oxymetazoline 0.1% use was associated with either no or only mild discomfort. These results provide important insights about oxymetazoline 0.1% and support a favorable safety profile.
Introduction
Blepharoptosis is a common condition of the upper eyelid, for which treatment options have been relatively limited. In addition to affecting the appearance of the eyes, drooping of the eyelids can impair the superior visual field.1–4 The eyelid is raised primarily by the levator palpebrae superioris (levator), which receives input from the oculomotor nerve and inserts, via its aponeurosis, onto the anterior surface of the superior tarsal plate. Remaining lift is provided by the superior tarsal (Müller’s) muscle, which receives sympathetic innervation from the superior ganglionic chain and inserts onto the superior tarsal plate.5–9
Acquired forms of blepharoptosis are typically classified based on underlying cause.5,9–12 In a series of 251 surgical patients, aponeurotic blepharoptosis (due to stretching, dehiscence, or detachment of the levator muscle complex that is typically age-related) was the most common form of the condition,10 a finding consistent with evidence showing increasing blepharoptosis prevalence with age.13–15
The standard of care for acquired blepharoptosis is surgical intervention targeting the levator, Müller’s muscle, and levator aponeurosis.5,16,17 Surgery is effective in improving eyelid elevation, superior visual field function, and quality-of-life measures,16,18–20 but it also presents complication risks. These range from short-term concerns (bleeding, swelling, infection) that typically heal in the weeks post-procedure, to more persistent complications (lagophthalmos, exposure keratopathy) requiring further intervention.5 Surgery can also have variable cosmetic outcomes, resulting in asymmetry, eyelid crease abnormalities, or over- or under-correction.5 In a series of 1519 surgical patients, revision was required in 8.7% of cases, with over/under-correction identified as the leading causes for revision.21
The use of pharmacologic agents targeting α-adrenergic receptors, which are expressed on Müller’s muscle,22–24 has been described, with some evidence of eyelid elevation with topical phenylephrine, apraclonidine, brimonidine, or naphazoline.25–33 The evidence for these agents is limited to short-term use, however, and their practical applications are limited, given their side effect profiles. For example, side effects of chronic apraclonidine use can include decreased visual acuity, allergic dermatitis, and dry mouth.34–36
Oxymetazoline HCl ophthalmic solution, 0.1% (oxymetazoline [1 mg/mL, equivalent to 0.9 mg oxymetazoline base/mL]; Upneeq®, RVL Pharmaceuticals, Inc., Bridgewater, NJ) was recently approved for the treatment of acquired blepharoptosis. The active chemical entity is the α-adrenergic agonist oxymetazoline, which has been used as a topical treatment for nasal decongestion (0.05% solution)37,38 and reduction of ocular hyperemia (0.025% solution).39,40 Application of oxymetazoline 0.1% to the eye is believed to stimulate α-adrenergic receptors on Müller’s muscle,22–24 resulting in contraction and eyelid elevation.
The efficacy of once-daily oxymetazoline 0.1% administration was examined in two randomized, double-masked, placebo-controlled, 6-week Phase 3 clinical trials in individuals with acquired blepharoptosis. These studies demonstrated a significant effect of oxymetazoline 0.1%, with improvement of superior visual field deficits and upper eyelid elevation at predefined time points (treatment days 1 and 14).41 Safety was also evaluated over the 42-day treatment period in these studies, revealing similar treatment-emergent adverse event (TEAE) rates with oxymetazoline 0.1% and vehicle, and few treatment-related ocular TEAEs.41
Oxymetazoline 0.1% has been evaluated in two additional randomized, double-masked, placebo-controlled trials: a 2-week Phase 1/2a proof-of-concept study and a 12-week safety study. This analysis provides an in-depth evaluation of the safety of once-daily oxymetazoline 0.1% administration for 14–84 days by combining results from all four oxymetazoline 0.1% efficacy and safety trials, with an emphasis on TEAEs in the overall population and participant subgroups defined based on age, race, and ethnicity, as well as ophthalmic assessments and tolerability.
Materials and Methods
Studies
All studies had a randomized, double-masked, placebo-controlled design, and were conducted in compliance with the principles of the Declaration of Helsinki and Good Clinical Practice and International Council for Harmonisation guidelines. Protocols and informed consent forms were approved by a central Institutional Review Board (Alpha IRB, San Clemente, CA) prior to initiation. All participants completed written informed consent. Participant information and data were handled per Health Insurance Portability and Accountability Act provisions. The studies enrolled n=46 (6 sites [NCT01848041]), n=140 (16 sites [NCT02436759]), n=164 (27 sites [NCT03565887]), and n=234 (35 sites [NCT03536949]) participants, respectively. Data were pooled given consistency in study design, inclusion/exclusion criteria, treatment, and safety endpoints. Rationale, methodology, results, and conclusions are reported in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines.
In all studies, participants were randomized 2:1 to self-administer oxymetazoline 0.1% or placebo (vehicle) daily to both eyes, for the entire study period (Table 1). Randomization schemes were created by an independent biostatistician using a block design. Participants, investigators, staff, and study management personnel were masked to the identity of treatment until after final database lock.
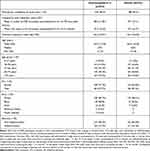 |
Table 1 Participant Disposition, Demographics, Treatment Exposure, and Compliance, by Treatment Group |
Participants
Key inclusion criteria were age ≥9 (studies RVL-1201-202, RVL-1201-203) or ≥18 years (studies RVL-1201-001, RVL-1201-201), presence of acquired blepharoptosis, defined by Marginal Reflex Distance 1 (MRD-1) ≤2.5 mm (study RVL-1201-001) or ≤2.0 mm (phase 3 studies), and superior visual field deficit (assessed via Humphrey Visual Field Test in study RVL-1201-001 and Leicester Peripheral Field Test (LPFT) in studies RVL-1201-201 and RVL-1201-202) in at least one eye. There was no superior visual field criterion in the 12-week study (RVL-1201-203). Individuals were excluded if they had pseudoptosis, congenital blepharoptosis, Marcus Gunn jaw-winking syndrome, Horner syndrome, mechanical blepharoptosis, myasthenia gravis, substantial dermatochalasis, or history of blepharoptosis surgery or periocular neurotoxin injection <3 months pre-enrollment. Potential participants were also excluded if, at screening, they had resting heart rate (HR) outside of the normal range (defined as 60–100 beats per minute [bpm] in studies RVL-1201-001 and RVL-1201-201, and 50–110 bpm in studies RVL-1201-202 and RVL-1201-203) or hypertension (defined as diastolic blood pressure (DBP) >105 mmHg in studies RVL-1201-001 and RVL-1201-201, and DBP > 105 mm Hg or systolic blood pressure (SBP) >220 mmHg in studies RVL-1201-202 and RVL-1201-203). The phase 1/2a study (RVL-1201-001) excluded individuals with advanced arteriosclerotic disease, history of myocardial infarction, angina, arrhythmia, or irregular pulse, as well as individuals using a beta blocker within 14 days preceding screening. The remaining studies excluded individuals with advanced arteriosclerotic disease and history of cerebrovascular accident. Notably, hypertension was the most common non-ocular medical history finding in the pooled population (50.4% and 48.2% of participants in the oxymetazoline 0.1% and vehicle groups, respectively).
Safety
Efficacy endpoints evaluated in the 6-week studies have been previously described.41 In all studies, TEAEs were recorded from screening to completion and classified per the Medical Dictionary for Regulatory Activities (MedDRA). TEAE severity, causality, and relationship to discontinuation, were assessed by investigators. Serious TEAEs were defined as TEAEs that were life-threatening, medically significant, or resulted in death, persistent or significant disability/incapacity, or inpatient hospitalization/prolongation of hospitalization. Investigators provided detailed narratives for any serious TEAE or TEAE leading to discontinuation.
Vital signs, intraocular pressure (IOP), pupil diameter, Snellen visual acuity (VA), corneal fluorescein staining, slit lamp exam, and ophthalmoscopy and fundus exam were monitored in all studies, at predefined study visits (Figure 1). Common post-instillation time points across at least two studies and included in the pooled safety analysis were as follows: HR and blood pressure (treatment days 1 [2 and 8 hours], 14 [0, 2, and 8 hours], and 42); IOP (treatment day 42); Snellen VA (treatment days 1 [8 hours], 14 [0 and 8 hours], and 42); pupil diameter (treatment days 1 [2 hours], 14 [2 hours], and 42); corneal fluorescein staining (treatment days 1 [8 hours], 14 [8 hours], and 42); slit lamp exam (treatment days 1 [8 hours], 14 [0 and 8 hours], and 42); ophthalmoscopy and fundus exam (treatment day 42). Patient-reported treatment tolerability was recorded at the end of each study (final visit/early termination), using a 4-point scale.
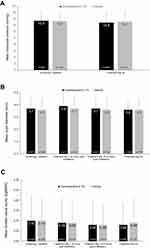 |
Figure 1 Mean ± standard deviation (A) intraocular pressure (IOP), (B) pupil diameter, and (C) Snellen visual acuity (VA) at selected time points. Results shown are for OD. Data from N=568 participants enrolled in four oxymetazoline 0.1% clinical trials ranging in duration from 14 to 84 days, except IOP, which presents data from N=538 participants enrolled in three phase 3 trials ranging in duration from 42 to 84 days. Includes data from participants from two phase 3 efficacy studies 42 days in duration with data previously reported by Slonim et al 2020.41 In the phase 1/2a study, pupil diameter and Snellen VA were evaluated at screening and on treatment days 1 and 14, and IOP was evaluated at screening and on treatment days 7 and 14. As previously reported, in the phase 3 efficacy trials, pupil diameter and Snellen VA were evaluated at all study visits, and IOP was evaluated at screening and on treatment day 42. In the phase 3 safety trial, pupil diameter and Snellen VA were evaluated at all study visits, and IOP was evaluated at baseline/screening and on treatment days 42 and 84. |
Participants randomized to once-daily oxymetazoline 0.1% treatment or vehicle were included in the safety analysis, with grouping determined on an as-treated basis. In addition to the total population, TEAEs were examined in subgroups defined post hoc, based on data collected at screening. Subgroups were defined by age (9–17, 18–50, 51–64, 65–75, >75 years), race (white, non-white), and ethnicity (Hispanic/Latino, Not Hispanic/Latino). TEAE incidences are presented as number of participants and percentages within each treatment and participant subgroup. Continuous variables are presented as mean (SD). Statistical testing was not performed given that the pooled population was not powered to reliably detect statistical differences in safety signals between treatment groups.
Results
Population
Safety analysis included 375 participants treated with oxymetazoline 0.1% once daily in both eyes, and 193 participants who received vehicle (Table 1). Overall, 94.9% and 97.4% of participants receiving oxymetazoline 0.1% and vehicle, respectively, completed all study visits. Most participants in both treatment groups were >50 years old (oxymetazoline 0.1%: 85.1%; vehicle: 82.4%) and the majority were female (oxymetazoline 0.1%: 77.3%; vehicle: 69.9%). Similarly, most participants were white (oxymetazoline 0.1%: 87.7%; vehicle: 88.1%) and identified their ethnicity as Not Hispanic/Latino (oxymetazoline 0.1%: 84.5%; vehicle: 83.9%). Treatment compliance was >97% in both treatment groups, across phase 1/2a and phase 3 studies. Mean treatment exposure was 56.4 days in both groups.
Adverse Events
Overall TEAE incidences were similar for the participants treated with oxymetazoline 0.1% (31.2% (n=117 participants)) and vehicle (30.6% (n=59 participants)) (Table 2). There were no apparent differences in TEAE rate with oxymetazoline 0.1% in participant subgroups defined based on age, race, or ethnicity. TEAE incidence ranged from 29.6% to 34.0% in the age groups examined (excluding the 9–17 years group, which included 2 participants/treatment group). Similarly, TEAE rates in the oxymetazoline 0.1% group were 30.4% among white participants, 37.0% among non-white participants, 20.7% among Hispanic/Latino participants, and 33.1% among non-Hispanic/Latino participants (Table 2).
 |
Table 2 Summary of Treatment-Emergent Adverse Events (TEAEs) |
Nine participants receiving oxymetazoline 0.1% and two participants receiving vehicle had a TEAE that led to discontinuation from the study. In the oxymetazoline 0.1% group, TEAEs resulting in study withdrawal and suspected of being treatment-related were single instances of mild eyelid edema, mild instillation site pain and headache, mild ocular discomfort, mild allergic blepharitis, moderate conjunctival hyperemia and dry eye, and mild eye irritation. Other, non-treatment-related TEAEs leading to discontinuation in the oxymetazoline 0.1% group were single instances of mild glare and moderate migraine, moderate upper limb fracture, and moderate eye irritation and ocular hyperemia. In the vehicle group, withdrawal from the study was reported for one participant with mild iritis and a second participant with moderate lower gastrointestinal hemorrhage (neither suspected of being treatment-related). Given the infrequency of discontinuation due to a TEAE, it is difficult to discern any potential difference across patient subgroups, however the data suggest similar rates across participants based on age and race (Table 2). There were no deaths during any study, and serious TEAEs were reported in four participants (1.1%) treated with oxymetazoline 0.1% and one participant (0.5%) receiving vehicle. All serious TEAEs were non-ocular, not suspected of being treatment-related by the site investigator, and were resolved (brief narratives in Table 3).
 |
Table 3 Summary of Serious Treatment-Emergent Adverse Events (TEAEs) |
The most common TEAEs, regardless of severity and causality, are summarized in Table 4. No TEAE occurred in >3.5% of participants in either treatment group. TEAEs in the Eye Disorders System Organ Class (SOC) occurred in 74 (19.7%) and 26 (13.5%) participants in the oxymetazoline 0.1% and vehicle groups, respectively. TEAEs reported for ≥2.0% of participants in either treatment group were: punctate keratitis (oxymetazoline 0.1%: n=13 (3.5%); vehicle: n=4 (2.1%)), conjunctival hyperemia (oxymetazoline 0.1%: n=11 (2.9%); vehicle: n=1 (0.5%)), dry eye (oxymetazoline 0.1%: n=9 (2.4%); vehicle: n=1 (0.5%)), blurred vision (oxymetazoline 0.1%: n=8 (2.1%); vehicle: n=0), instillation site pain (oxymetazoline 0.1%: n=8 (2.1%); vehicle: n=0), corneal vital dye staining (oxymetazoline 0.1%: n=8 (2.1%); vehicle: n=4 (2.1%)), and headache (oxymetazoline 0.1%: n=8 (2.1%); vehicle: n=2 (1.0%)).
 |
Table 4 Most Common Treatment-Emergent Adverse Events (TEAEs; Occurring in >1% of Patients in Either Treatment Arm) |
Cardiovascular TEAEs were uncommon. Among the 375 participants in the oxymetazoline 0.1% treatment group, there was one event each (0.5%) of bradycardia and tachycardia, both of which were mild in severity and judged to be unrelated to treatment. A TEAE of hypertension was reported for 3/375 participants (0.8%) in the oxymetazoline 0.1% group, as well as 2/193 participants (1.0%) in the vehicle group. This TEAE was judged to be unrelated to treatment in all 3 participants in the oxymetazoline 0.1% group and 1 of 2 participants in the vehicle group. Hypertension was mild in 2 participants in the oxymetazoline 0.1% group and severe in the other (a 56-year-old female with a history of hypertension), and mild and moderate in one participant each in the vehicle group. A TEAE of increased DBP that was mild, and judged to be unrelated to treatment, occurred in one participant (0.3%) receiving oxymetazoline 0.1%, and one participant in the vehicle group had a TEAE of atrial fibrillation (moderate in severity, unrelated to treatment).
Among participants with ≥1 reported TEAE, 95.7% in the oxymetazoline 0.1% group and 100% in the vehicle group had a maximum TEAE intensity of mild or moderate (Table 5). Across all participant subgroups, 20.7% to 24.6% of participants receiving oxymetazoline 0.1% had a maximum TEAE intensity of mild (23.5% overall). In the oxymetazoline 0.1% group, the proportion of participants with a TEAE of moderate intensity ranged from 5.8% to 7.4% across age subgroups (excluding the 9–17 group). A TEAE of moderate intensity occurred in 5.5% (n=18/329) of white participants and 13.0% (n=6/46) of non-white participants receiving oxymetazoline 0.1%. No Hispanic/Latino participant had a moderate TEAE.
 |
Table 5 Relationship to Treatment and Severity of Treatment-Emergent Adverse Events (TEAEs) |
Five participants (1.3%) in the oxymetazoline 0.1% group and none in the vehicle group had a TEAE that was judged to be severe (Table 5). All severe TEAEs were non-ocular, and included the incidences of hyperparathyroidism, arthralgia, and nephrolithiasis noted in Table 3. Additionally, a 53-year-old white female had TEAEs of viral infection and secondary dehydration, and a 56-year-old white female had a TEAE of hypertension, judged to be severe. The latter individual had a medical history of hypertension. On day 14, the participant’s SBP and DBP increased from baseline (151/92 mmHg) to 170/105 mmHg. The TEAE was resolved, and at end of study, SBP and DBP were 156 and 97 mmHg, respectively. HR did not change from baseline. No severe TEAE was suspected of being treatment-related by the site investigator.
Most TEAEs were not suspected of being treatment-related. Among the 117 participants receiving oxymetazoline 0.1% and reporting a TEAE, 47/117 (40.2%) had ≥1 event suspected of being treatment-related (vs 15/59 participants (25.4%) receiving vehicle). Treatment-related TEAE rates were similar across age, race, and ethnicity subgroups, though rates were low in the relatively small Hispanic/Latino ethnicity group (Table 5). Overall, 28 (7.5%) participants in the oxymetazoline 0.1% group and 8 (4.1%) participants in the vehicle group had a TEAE in the Eye Disorder SOC that was judged to be treatment-related. The TEAEs in this category most commonly judged to be treatment-related in the oxymetazoline 0.1% group were conjunctival hyperemia and dry eye (both n=6 (1.6%)), and punctate keratitis, blurred vision, and eye irritation (all n=3 (0.8%)). Among these TEAEs in the vehicle group, n=1 incidence of dry eye (0.5%) was treatment-related. No other TEAE in this category was treatment-related in >0.3% of participants receiving oxymetazoline 0.1%. In the vehicle group, the only TEAE in this category judged to be related to treatment in ≥1.0 of participants was increased lacrimation (n=2 (1.0%)). Other treatment-related TEAEs occurring in ≥1.0% of participants in either group were instillation site pain (oxymetazoline 0.1%: n=8 (2.1%); vehicle: n=0), instillation site complication (oxymetazoline 0.1%: n=1 (0.3%); vehicle: n=3 (1.6%)), and corneal vital dye staining (oxymetazoline 0.1%: n=6 (1.6%); vehicle: n=3 (1.6%)).
Vital Signs and Ophthalmic Endpoints
There were no clinically significant mean post-baseline changes in HR, SBP, or DBP in either treatment group. Mean change from baseline HR on day 42 in the oxymetazoline 0.1% and vehicle groups was 0.6 (8.26) bpm and 0.7 (7.36) bpm, respectively. Mean change from baseline SBP on day 42 in the oxymetazoline 0.1% and vehicle groups was −0.9 (12.43) mmHg and −2.2 (14.29) mmHg, respectively, and mean change in DBP at the same time point was −0.5 (8.18) mmHg in the oxymetazoline 0.1% group and −1.4 (8.42) mmHg in the vehicle group.
No clinically significant shifts from baseline were noted for IOP, pupil diameter, or Snellen VA in either treatment group (Figure 1). Evaluating phase 3 study participants, mean OD IOP was 15.4 (3.03) mmHg at screening and 14.8 (3.10) mmHg on day 42 in the oxymetazoline 0.1% group, and 15.3 (2.93) mmHg at screening and 15.1 (3.13) mmHg on day 42 with vehicle. Mean OD Snellen VA was 0.10 (0.125) LogMAR at baseline and 0.08 (0.107) LogMAR on day 42 in the oxymetazoline 0.1% group and 0.10 (0.111) LogMAR at baseline and 0.09 (0.120) LogMAR on day 42 with vehicle. Mean pupil diameter did not differ from baseline at any time point evaluated. Mean OD pupil diameter was 3.7 (0.89) mm at baseline and 3.6 (0.82) mm on day 42 in the oxymetazoline 0.1% group, and 3.6 (0.95) mm at baseline and 3.6 (0.88) on day 42 with vehicle. Results in OS were similar. Results for corneal fluorescein staining, slit lamp examination, and dilated ophthalmoscopy suggested no differences between treatment groups.
Tolerability
At the end of the 14-day study, all participants receiving once-daily oxymetazoline 0.1% or vehicle rated treatment as causing no discomfort. At the end of the 6-week studies, 95.5%, 3.0%, and 1.5% of participants receiving oxymetazoline 0.1% rated treatment as causing no discomfort, mild discomfort, and moderate discomfort, respectively. Among participants receiving vehicle, 99.0% and 1.0% rated treatment as causing no discomfort and mild discomfort, respectively. No participants rated either treatment as causing severe discomfort after 6 weeks’ use. At the end of the 12-week study, 92.0% and 8.0% of participants receiving oxymetazoline 0.1% rated treatment as causing no discomfort or mild discomfort, respectively. Corresponding numbers with vehicle were and 93.4% and 6.6%. No participant rated either treatment as causing moderate or severe discomfort after 12 weeks’ use.
Discussion
The data from four randomized, double-masked, placebo-controlled clinical studies support the safety of once-daily oxymetazoline 0.1% for 14–84 days. Further, the data reveal similar TEAE rates, severity, and relationship to treatment, across participant subgroups based on age, race, and ethnicity, though a larger patient sample is required to comprehensively evaluate safety with respect to these factors. While ocular TEAEs tended to be treatment-related more frequently among participants receiving oxymetazoline 0.1%, these were uncommon overall. Only instillation site pain (all events mild) was judged to be treatment-related in >2% of participants receiving oxymetazoline 0.1%. Further, the low incidences of severe (1.3%) or serious (1.1%) TEAEs, and TEAEs leading to discontinuation (2.4%) are encouraging. The effect of oxymetazoline 0.1% on ophthalmic measures was minimal, and participant evaluations revealed that once-daily oxymetazoline 0.1% use caused little or no discomfort.
Oxymetazoline is thought to act via α-adrenergic receptors on Müller’s muscle,22–24 resulting in muscle contraction and eyelid elevation. This muscle remains intact and functional in the most common form of acquired blepharoptosis (aponeurotic),9,10 and it is a common surgical target.16,42,43 Functional studies demonstrate that oxymetazoline is a full α2 agonist and a partial α1 agonist, with an approximately 5-fold greater affinity for α2.44,45 Within the α1 receptor subgroup, oxymetazoline has been shown to have a higher affinity for α1A vs α1B, and weak affinity for the α1D subtype.46 The in vivo pharmacology of oxymetazoline 0.1% remains to be fully elucidated, however it is possible that receptor selectivity may contribute to the observed safety profile. Tachyphylaxis is common with prolonged use of α1-selective or mixed α1/α2 agents, and the mechanism of this phenomenon is thought to be a reduced α1-adrenergic receptor response.45 Improvement in upper eyelid elevation has been shown with naphazoline, a mixed α1/α2 agonist,45 but so has tachyphylaxis with repeated daily dosing.32 Similarly, rebound effects of ocular decongestants are also thought to occur via an α1-dependent mechanism.45 Following administration of phenylephrine, which is α1-selective,45 patients can experience clinically significant pupil dilation.47 In comparison, there was a negligible effect on pupil diameter with oxymetazoline 0.1% and no reports of a TEAE of mydriasis in the present studies. Similarly, there were no documented cases of tachyphylaxis over 14–84 days of treatment with oxymetazoline 0.1%. Chronic use of oxymetazoline 0.05% nasal spray can cause tachyphylaxis and rebound congestion,48,49 thus making investigation of this question essential in future studies.
The molecular targets of oxymetazoline, α-adrenergic receptors, are widely expressed in smooth muscle and blood vessels of the eye, in structures including the conjunctiva, iris-ciliary structures, and aqueous outflow tract,50 which may in part explain the occurrence of ocular TEAEs such as punctate keratitis, conjunctival hyperemia, and dry eye in participants using oxymetazoline 0.1%. It is also noteworthy that dry eye, punctate keratitis, and conjunctival hyperemia were among the most commonly reported ocular history findings in the pooled population (reported for 43.8%, 5.8%, and 5.3% of participants, respectively), suggesting general susceptibility to corneal irritation or sensitivity. A 0.026% topical oxymetazoline solution has been shown to transiently reduce tear volume and flow,51 and while these tear parameters were not evaluated in the oxymetazoline 0.1% clinical trials, it is possible that some transient effects on tear volume may have contributed to the observed ocular TEAEs.
Occurrences of blurred vision and instillation site pain with oxymetazoline 0.1% were transient and mild. A single event of mild transient instillation site pain led to discontinuation of a 77-year-old participant with a history of dry eye. This event had resolved without intervention on the day of discontinuation. Sympathomimetic agents such as oxymetazoline can be associated with transient mydriasis and acute angle closure glaucoma (of which blurred vision is a sign) in patients with narrow angle glaucoma. The oxymetazoline 0.1% trials, however, excluded individuals with a history of closed/narrow angle glaucoma (unless patent peripheral iridotomy had been performed >3 months prior to enrollment). Further, as shown in Figure 1, there were no significant shifts from baseline in pupil diameter observed with oxymetazoline 0.1% use. Thus, any potential mechanism of transient blurring of vision requires further investigation. It is possible that some instances of transient blurred vision with oxymetazoline 0.1% may have been related to the presence of hypromellose, a viscoelastic polymer that is commonly included in ophthalmic solutions.
Conclusions
While limited with respect to duration of oxymetazoline 0.1% use (14–84 days), these findings further support the potential clinical utility of this non-surgical therapeutic agent. Longer-duration studies including larger numbers of patients will be needed to evaluate the ocular and systemic safety of oxymetazoline 0.1% in clinical practice, and future studies into the efficacy and safety of this agent in pediatric or congenital blepharoptosis patients may provide further insight into its broader utility. While no direct comparison has been made between oxymetazoline 0.1% and surgery, the low rates of treatment-related ocular TEAEs suggest that for some patients with acquired blepharoptosis, particularly those with mild or moderate eyelid droop, this pharmacologic option may, in addition to being efficacious,41 offer a desirable safety profile.
Data Sharing Statement
The authors do not intend to share individual deidentified study participant data.
Acknowledgments
Studies reported were funded by RVL Pharmaceuticals, Inc., an affiliate of Osmotica Pharmaceuticals plc (Bridgewater, NJ, USA). Editorial and administrative support was provided by BioScience Communications (New York, NY, USA) through funding provided by Osmotica Pharmaceuticals. Portions of the data reported in this manuscript were presented at the American Academy of Optometry and 3rd World Congress of Optometry, October 23–27, 2019, in Orlando, Fl and American Academy of Aesthetic Medicine Congress, November 8–10, 2019, in Las Vegas, NV.
Disclosure
DL Wirta reports research support from RVL Pharmaceuticals, Inc., and Osmotica Pharmaceuticals. MS Korenfeld reports consultant fees from Osmotica Pharmaceuticals and RVL Pharmaceuticals, Inc. S Foster reports consultant and speaker fees from Osmotica Pharmaceuticals and RVL Pharmaceuticals, Inc. He also reports non-financial support from BioScience Communications. R Smyth-Medina reports personal fees from Sun Pharmaceutical Industries, Inc.; research support from Aerie Pharmaceuticals, Alcon, Allergan, Aurinia, Auven, Bausch & Lomb, Eleven, Encore, Evidera, Eyegate, Hi Tech Pharmacal, Inotek, Inspire, Ista, Kala, Novartis, Oculos Clinical Research LLC, OmegaD, Ono, OTX, RVL Pharmaceuticals, Inc., Santen, SARcode, Shire, Silk, Senju, Valeant, and Xigen. J Bacharach reports speaker fees from Aerie Pharmaceuticals, Alcon, Allergan, Bausch & Lomb, Glaukos, New World Medical, Sun Pharmaceutical Industries, Inc., and RVL Pharmaceuticals, Inc.; consultant fees from Aerie Pharmaceuticals, Alcon, Allergan, Bausch & Lomb, Injectsense, New World Medical, Optovue, and Osmotica Pharmaceuticals; personal fees from Sun Pharmaceutical Industries, Inc.; research support from Aerie Pharmaceuticals, Allergan, Novartis, Glaukos, Optovue, and Ocular Therapeutix. SR Kannarr reports personal fees from Allergan, Alcon, Bausch & Lomb, Essilor, Johnson & Johnson, Novartis, Oculos, Optovue, and Osmotica Pharmaceuticals. MJ Jaros reports consultant fees from Osmotica Pharmaceuticals. CB Slonim reports research support and consultant fees from RVL Pharmaceuticals, Inc. and Osmotica Pharmaceuticals. He also reports a contingent earn-out tied to product sales related to a corporate sale transaction completed in 2018 from Pointguard Partners LLC. The authors report no other conflicts of interest in this work.
References
1. Alniemi ST, Pang NK, Woog JJ, Bradley EA. Comparison of automated and manual perimetry in patients with blepharoptosis. Ophthal Plast Reconstr Surg. 2013;29:361–363. doi:10.1097/IOP.0b013e31829a7288
2. Cahill KV, Burns JA, Weber PA. The effect of blepharoptosis on the field of vision. Ophthal Plast Reconstr Surg. 1987;3:121–125. doi:10.1097/00002341-198703030-00001
3. McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP; Los Angeles Latino Eye Study Group. Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143:1013–1023. doi:10.1016/j.ajo.2007.02.022
4. Meyer DR, Stern JH, Jarvis JM, Lininger LL. Evaluating the visual field effects of blepharoptosis using automated static perimetry. Ophthalmology. 1993;100:651–658. doi:10.1016/S0161-6420(93)31593-9
5. Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003;27:193–204. doi:10.1007/s00266-003-0127-5
6. Beard C. Muller’s superior tarsal muscle: anatomy, physiology, and clinical significance. Ann Plast Surg. 1985;14:324–333. doi:10.1097/00000637-198504000-00005
7. Freddo TF, Chaum E, eds. Anatomy of the Eye and Orbit: The Clinical Essentials.
8. Hamedani AG, Gold DR. Eyelid dysfunction in neurodegenerative, neurogenetic, and neurometabolic disease. Front Neurol. 2017;8:329. doi:10.3389/fneur.2017.00329
9. Latting MW, Huggins AB, Marx DP, Giacometti JN. Clinical evaluation of blepharoptosis: distinguishing age-related ptosis from masquerade conditions. Semin Plast Surg. 2017;31:5–16. doi:10.1055/s-0037-1598188
10. Lim JM, Hou JH, Singa RM, Aakalu VK, Setabutr P. Relative incidence of blepharoptosis subtypes in an oculoplastics practice at a tertiary care center. Orbit. 2013;32:231–234. doi:10.3109/01676830.2013.788673
11. Sudhakar P, Vu Q, Kosoko-Lasaki O, Palmer M. Upper eyelid ptosis revisited. Am J Clin Med. 2009;6:5–14.
12. Bacharach J, Lee WW, Harrison AR, Freddo TF. A review of acquired blepharoptosis: prevalence, diagnosis, and current treatment options. Eye (Lond). 2021;35:2468–2481. doi:10.1038/s41433-021-01547-5
13. Forman WM, Leatherbarrow B, Sridharan GV, Tallis RC. A community survey of ptosis of the eyelid and pupil size of elderly people. Age Ageing. 1995;24:21–24. doi:10.1093/ageing/24.1.21
14. Hashemi H, Khabazkhoob M, Emamian MH, et al. The prevalence of ptosis in an Iranian adult population. J Curr Ophthalmol. 2016;28:142–145. doi:10.1016/j.joco.2016.04.005
15. Kim MH, Cho J, Zhao D, et al. Prevalence and associated factors of blepharoptosis in Korean adult population: the Korea National Health and Nutrition Examination Survey. Eye (Lond). 2017;31:940–946. doi:10.1038/eye.2017.43
16. Cahill KV, Bradley EA, Meyer DR, et al. Functional indications for upper eyelid ptosis and blepharoplasty surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118(12):2510–2517. doi:10.1016/j.ophtha.2011.09.029
17. Pauly M, Sruthi R. Ptosis: evaluation and management. Kerala J Ophthalmol. 2019;31:11–16. doi:10.4103/kjo.kjo_2_19
18. Battu VK, Meyer DR, Wobig JL. Improvement in subjective visual function and quality of life outcome measures after blepharoptosis surgery. Am J Ophthalmol. 1996;121:677–686. doi:10.1016/S0002-9394(14)70634-8
19. Federici TJ, Meyer DR, Lininger LL. Correlation of the vision-related functional impairment associated with blepharoptosis and the impact of blepharoptosis surgery. Ophthalmology. 1999;106:1705–1712. doi:10.1016/S0161-6420(99)90354-8
20. Ho SF, Morawski A, Sampath R, Burns J. Modified visual field test for ptosis surgery (Leicester Peripheral Field Test). Eye (Lond). 2011;25:365–369. doi:10.1038/eye.2010.210
21. Chou E, Liu J, Seaworth C, et al. Comparison of revision rates on anterior- and posterior-approach ptosis surgery: a retrospective review of 1519 cases. Ophthal Plast Reconstr Surg. 2018;34:246–253. doi:10.1097/IOP.0000000000000938
22. Esmaeli-Gutstein B, Hewlett BR, Pashby RC, Oestreicher J, Harvey JT. Distribution of adrenergic receptor subtypes in the retractor muscles of the upper eyelid. Ophthal Plast Reconstr Surg. 1999;15:92–99. doi:10.1097/00002341-199903000-00005
23. Park SJ, Jang SY, Baek JS, Chin S, Jang JW. Distribution of adrenergic receptor subtypes and responses to topical 0.5% apraclonidine in patients with blepharoptosis. Ophthalmic Plast Reconstr Surg. 2018;34:547–551. doi:10.1097/IOP.0000000000001095
24. Skibell BC, Harvey JH, Oestreicher JH, et al. Adrenergic receptors in the ptotic human eyelid: correlation with phenylephrine testing and surgical success in ptosis repair. Ophthalmic Plast Reconstr Surg. 2007;23:367–371. doi:10.1097/IOP.0b013e3181462a2e
25. Hauck MJ, Steele EA, Perry CB. Predictability of the phenylephrine test with regard to eyelid skin appearanc ein patients who undergo Muller muscle-conjunctival resection without blepharoplasty. Ophthal Plast Reconstr Surg. 2019;36:191–193. doi:10.1097/IOP.0000000000001510
26. Garibaldi DC, Hindman HB, Grant MP, Iliff NT, Merbs SL. Effect of 0.5% apraclonidine on ptosis in Horner syndrome. Ophthal Plast Reconstr Surg. 2006;22:53–55. doi:10.1097/01.iop.0000196322.05586.6a
27. Kirkpatrick CA, Shriver EM, Clark TE, Kardon RH. Upper eyelid response to topical 0.5% apraclonidine. Ophthal Plast Reconstr Surg. 2018;34:13–19. doi:10.1097/IOP.0000000000000843
28. Lee GN, Lin LW, Mehta S, Freitag SK. Response to phenylephrine testing in upper eyelids with ptosis. Digit J Ophthalmol. 2015;21:1–12.
29. Mendonça TB, Lummertz AP, Bocaccio FJ, Procianoy F. Effect of low-concentration, nonmydriatic selective alpha-adrenergic agonist eyedrops on upper eyelid position. Dermatol Surg. 2017;43:270–274. doi:10.1097/DSS.0000000000000967
30. Nagane Y, Utsugisawa K, Suzuki S, et al. Topical naphazoline in the treatment of myasthenic blepharoptosis. Muscle Nerve. 2011;44:41–44. doi:10.1002/mus.22002
31. Rehmani A, Mehta I, Smith E. Treatment of ptosis using brimonidine tartrate for anterior laminectomy-induced Horner syndrome. J Neuroophthalmol. 2020;40:95–96. doi:10.1097/WNO.0000000000000826
32. Uncini A, De Nicola G, Di Muzio A, et al. Topical naphazoline in treatment of myopathic ptosis. Acta Neurol Scand. 1993;87:322–324. doi:10.1111/j.1600-0404.1993.tb05516.x
33. Wijemanne S, Vijayakumar D, Jankovic J. Apraclonidine in the treatment of ptosis. J Neurol Sci. 2017;376:129–132. doi:10.1016/j.jns.2017.03.025
34. Araujo SV, Bond JB, Wilson RP, Moster MR, Schmidt CM
35. Robin AL, Ritch R, Shin D, Smythe B, Mundorf T, Lehmann RP. Topical apraclonidine hydrochloride in eyes with poorly controlled glaucoma. The Apraclonidine Maximum Tolerated Medical Therapy Study Group. Trans Am Ophthalmol Soc. 1995;93:421–438.
36. Stewart WC, Ritch R, Shin DH, Lehmann RP, Shrader CE, van Buskirk EM. The efficacy of apraclonidine as an adjunct to timolol therapy. Apraclonidine Adjunctive Therapy Study Group. Arch Ophthalmol. 1995;113:287–292. doi:10.1001/archopht.1995.01100030041019
37. Afrin® Original oxymetazoline decongestant nasal spray [package insert]. Whippany, NJ: Bayer Healthcare LLC; Revised February 2017.
38. Druce HM, Ramsey DL, Karnati S, Carr AN. Topical nasal decongestant oxymetazoline (0.05%) provides relief of nasal symptoms for 12 hours. Rhinology. 2018;56:343–350.
39. Duzman E, Anderson J, Vita JB, Lue JC, Chen CC, Leopold IH. Topically applied oxymetazoline. Ocular vasoconstrictive activity, pharmacokinetics, and metabolism. Arch Ophthalmol. 1983;101:1122–1126. doi:10.1001/archopht.1983.01040020124022
40. Visine L.R. (oxymetazoline hydrochloride 0.025%) [package insert]. New Brunswick, NJ: Johnson & Johnson Healthcare Products, Inc.; 2016.
41. Slonim CB, Foster S, Jaros M, et al. Association of oxymetazoline hydrochloride, 0.1%, solution administration with visual field in acquired ptosis: a pooled analysis of 2 randomized clinical trials. JAMA Ophthalmol. 2020;138:1168–1175. doi:10.1001/jamaophthalmol.2020.3812
42. Putterman AM, Fett DR. Müller’s muscle in the treatment of upper eyelid ptosis: a ten-year study. Ophthalmic Surg. 1986;17:354–360.
43. Putterman AM, Urist MJ. Muller muscle-conjunctiva resection. Technique for treatment of blepharoptosis. Arch Ophthalmol. 1975;93:619–623. doi:10.1001/archopht.1975.01010020595007
44. Haenisch B, Walstab J, Herberhold S, et al. Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam Clin Pharmacol. 2010;24:729–739. doi:10.1111/j.1472-8206.2009.00805.x
45. Hosten LO, Snyder C. Over-the-counter ocular decongestants in the United States - mechanisms of action and clinical utility for management of ocular redness. Clin Optom. 2020;12:95–105. doi:10.2147/OPTO.S259398
46. Sugden D, Anwar N, Klein D. Rat pineal α1-adrenoceptor subtypes: studies using radioligand binding and reverse transcription-polymerase chain reaction analysis. Br J Pharmacol. 1996;118:1246–1252. doi:10.1111/j.1476-5381.1996.tb15530.x
47. Barsegian A, Botwinick A, Reddy HS. The phenylephrine test revisited. Ophthalmic Plast Reconstr Surg. 2018;34:151–154. doi:10.1097/IOP.0000000000000903
48. Ramey JT, Bailen E, Lockey RF. Rhinitis medicamentosa. J Investig Allergol Clin Immunol. 2006;16:148–155.
49. Vaidyanathan S, Williamson P, Clearie K, Khan F, Lipworth B. Fluticasone reverses oxymetazoline-induced tachyphylaxis of response and rebound congestion. Am J Respir Crit Care Med. 2010;182:19–24. doi:10.1164/rccm.200911-1701OC
50. McAuliffe-Curtin D, Buckley C. Review of alpha adrenoceptor function in the eye. Eye (Lond). 1989;3:472–476. doi:10.1038/eye.1989.71
51. Gobbels MJ, Achten C, Spitznas M. Effect of topically applied oxymetazoline on tear volume and tear flow in humans. Graefes Arch Clin Exp Ophthalmol. 1991;229:147–149. doi:10.1007/BF00170547
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
