Back to Journals » Therapeutics and Clinical Risk Management » Volume 12
Safety of long-term use of linezolid: results of an open-label study
Authors Vazquez JA, Arnold A, Swanson R, Biswas P, Bassetti M, Kenreigh C
Received 29 March 2016
Accepted for publication 21 June 2016
Published 1 September 2016 Volume 2016:12 Pages 1347—1354
DOI https://doi.org/10.2147/TCRM.S109444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Jose A Vazquez,1 Anthony C Arnold,2 Robert N Swanson,3 Pinaki Biswas,3 Matteo Bassetti4
1Section of Infectious Diseases, Medical College of Georgia, Georgia Regents University, Augusta, GA, USA; 2UCLA Department of Ophthalmology, Jules Stein Eye Institute, Los Angeles, CA, USA; 3Clinical Research, Global Innovative Pharmaceutical, Pfizer Inc., New York, NY, USA; 4Infectious Diseases Division, Santa Maria della Misericordia University Hospital, Udine, Italy
Objective: The objective of this study was to assess the long-term safety of linezolid in patients with chronic infections requiring treatment for ≥6 weeks. Enhanced monitoring for optic neuropathy was included to characterize the early development of this side effect and to identify ophthalmologic tests that might be valuable in early detection of this event.
Methods: This was a multicenter, open-label, pilot study of patients aged ≥18 years on long-term linezolid therapy. Matched control patients were included for baseline assessment comparison. Patients were assessed at study entry, monthly while on treatment, at the end of treatment, and 30 days following the last dose. Aggregate ocular safety data were reviewed. Response to treatment was reported.
Results: The study was terminated owing to slow enrollment. Twenty-four patients received linezolid; nine patients were included as matched controls. Linezolid was prescribed for a median of 80.5 days (range, 50–254 days). In patients with a reported clinical outcome, the majority were considered improved or cured. Common treatment-related adverse events (AEs) included anemia, peripheral neuropathy, polyneuropathy, vomiting, and asthenia, and were consistent with the known safety profile. Most AEs resolved or stabilized with discontinuation of treatment. Results of ophthalmologic tests in the one case adjudicated as probable linezolid-associated optic neuropathy revealed abnormal color vision, characteristic changes in the optic disk, and central scotomas in each eye.
Conclusion: In our small population, linezolid was generally well tolerated and AEs were consistent with the known safety profile. Extensive ophthalmologic testing of all 24 linezolid-treated patients identified one case adjudicated as probable, linezolid-associated optic neuropathy.
Keywords: linezolid, oxazolidinones, optic nerve diseases, peripheral nervous system diseases, safety
Introduction
Linezolid is a synthetic oxazolidinone antibiotic, active against gram-positive bacteria.1 The antibacterial effect of linezolid comes from the inhibition of the 50S subunit of the bacterial ribosome. In the US and Europe, linezolid is approved for the treatment of nosocomial pneumonia, community-acquired pneumonia, complicated skin, and skin structure infections caused by susceptible gram-positive bacteria, including methicillin-resistant Staphylococcus aureus.1,2 Additionally, US-only indications include uncomplicated skin and skin structure infections and vancomycin-resistant Enterococcus faecium infections.1 Recommended duration of treatment varies (10–28 days) with the type of infection being targeted. In clinical practice, linezolid has been used for a longer duration in difficult-to-treat infections.3–5 The safety and efficacy of linezolid, when given for >28 days, have not been evaluated in controlled clinical trials.
In clinical studies, the most common adverse reactions reported in patients treated with linezolid include diarrhea, vomiting, headache, nausea, and anemia. Myelosuppression, optic neuropathy, and peripheral neuropathy have also been reported. In general, spontaneous reports of optic and peripheral neuropathies in patients receiving linezolid have occurred when patients received treatment extending beyond the approved maximum 28 days of treatment,1,2,6–8 yet occurrences associated with shorter term use have also been reported.8,9 The clinical presentation of optic neuropathy is similar to that seen with toxic, metabolic, drug-related, Cuban epidemic, and hereditary optic neuropathies; these syndromes have been attributed to abnormalities in mitochondrial transport and defective mitochondrial placement along the neuron.10,11 Optic and peripheral neuropathies described with linezolid have been reported as symmetrical and progressive.8,9,12,13 The neuropathies may become permanent, with optic neuropathy resulting in a loss of vision with continued therapy. Some visual recovery is reported following treatment discontinuation.13
A study was undertaken to prospectively assess the overall long-term safety of linezolid in patients with chronic infections who required treatment with 600 mg every 12 hours for ≥6 weeks. The study included enhanced monitoring for optic neuropathy in an attempt to characterize the early development of this side effect and to identify ophthalmologic tests that might be valuable in early detection of this event.
Patients were closely monitored for development of peripheral and optic neuropathies as well as for bone marrow suppression and lactic acidosis.
Materials and methods
This multicenter, open-label, pilot study was conducted at six recruiting centers in the US and three centers in Europe between 17 November, 2008, and 27 December 2013. ClinicalTrials.gov: NCT00359632. The study, and protocol and informed consent documentation were reviewed and approved by the following: Henry Ford Health Systems Institutional Review Board (Detroit, MI, USA), Regionala Etikprövningsnämnden i Stockholm (Stockholm, Sweden), Western Institutional Review Board (Olympia, WA, USA), St Bernards Medical Center Institutional Review Board (Jonesboro, AR, USA), Ochsner Clinic Foundation Institutional Review Board (New Orleans, LA, USA), Conitato Etico Azienda Ospedaliero Universitaria San Martino (Genova, Italy), and the Research Subjects Protection Program (Minneapolis, MN, USA). Written informed consent was provided by all patients prior to participation in the study. This study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all the International Conference on Harmonization (ICH) Good Clinical Practice (GCP) Guidelines and all local regulatory requirements.
Study patients
Patients considered for study enrollment were ≥18 years old, required long-term antibiotic therapy, and had received linezolid 600 mg every 12 hours for 6 weeks or longer and were currently on drug (or had received linezolid for 6 weeks or longer and discontinued use within 7 days of baseline evaluation). Use of any medication, including antibiotics, with a known potential to produce ocular or neurologic toxicity indistinguishable from linezolid-associated toxicity, was not permitted. Control patients individually matched to linezolid-treated patients were included if they had been treated with antibiotics other than linezolid for ≥6 weeks and had similar infection diagnoses (based on anatomical site and chronicity), duration of treatment, and demographics (age and sex). Data collected from control patients were limited to baseline assessments. This was not a parallel-group study.
Duration of linezolid therapy while on the study was at the investigator’s discretion based on the benefit and risks of continued therapy. Patients of childbearing potential were to use a highly effective method of contraception throughout the study and for at least 28 days after the last dose of therapy. Patients were excluded from the study if they were pregnant or nursing; had known optic nerve damage or significant peripheral nerve damage unrelated to linezolid; had a pre-existing ophthalmologic condition that would affect study testing (eg, dense cataracts, macular degeneration, congenital color vision deficiency, nystagmus, high myopia, and retinitis pigmentosa); had significant exposure or anticipated exposure to medications known to cause optic neuropathy or peripheral neuropathy; had deficiency states which may cause optic neuropathy; or had lactic acidosis, or a condition, or were on a medication, that causes lactic acidosis. Patients with an active communicable disease, severe liver disease, or other severe acute or chronic conditions were not permitted in the study.
Treatment
Once enrolled, patients continued receiving linezolid 600 mg orally or intravenously every 12 hours if considered medically necessary. Treatment could be discontinued at any point in the study (some patients may have discontinued therapy within the 7 days prior to baseline assessment). The decision to continue or discontinue linezolid treatment, if there was a clinical suspicion or evidence of linezolid-associated toxicity, was at the discretion of the treating physician. Patients were permitted to remain on study for a maximum of 1 year. The study was terminated after 5 years due to slow enrollment. No patient received linezolid for >5 months.
Study visits
Study visits for the treatment group occurred at study entry (screening/baseline), monthly for the duration of therapy, at the end of treatment (within 7 days of last dose) and at the end of study (30 days after the last dose). At each visit, general medical history, vital sign measurement, neurological examination, clinical laboratory testing, ophthalmologic screening, and adverse event (AE) monitoring were conducted. Patients entering the study who had discontinued linezolid-use within 7 days of baseline were assessed at a 1-month follow-up evaluation to ensure capture of any additional AEs occurring after discontinuation. Patients in the control group were assessed and tested a single time for baseline data to identify the presence of background abnormalities.
Assessments
Clinical outcome
Clinical outcome of the underlying infection was evaluated as a global assessment by the investigator. Response was categorized as cure (resolution of infection with no need for additional antimicrobials), improvement (improvement in two or more, but not all clinical signs and symptoms of disease with no additional treatment needed), failure (persistence or progression of signs and symptoms of infection or development of new finding consistent with active infection), or unknown (inability to assess response).
Safety
Clinical laboratory testing included assessment of blood chemistry, electrolytes, hematology, vitamin B6, vitamin B12, and folate levels. If abnormalities related to hematologic changes or peripheral neuropathy were identified during the treatment period, patients were studied periodically after discontinuation of linezolid treatment to determine the natural history (resolution, progression, or persistence) of identified events.
A battery of ophthalmologic tests designed to identify and characterize early development of optic neuropathy were selected by an independent Ocular Safety Expert Committee (OSEC) consisting of ophthalmologists and neuro-ophthalmologists. These included Snellen best-corrected visual acuity, intraocular pressure (IOP), relative afferent pupillary defect, color test plates (Ishihara – 14 plate series), color vision (Farnsworth D-28 Hue Test), contrast sensitivity (Pelli-Robson); Amsler grid (2- and 3-dimensional), Humphrey visual field (24-2 SITA standard and test of foveal sensitivity), slit lamp examination, dilated funduscopic examination, nerve fiber layer thickness (optical coherence tomography-3), and stereo optic nerve head photograph (baseline only, secondary test for subsequent visits). If abnormalities suggestive of optic nerve toxicity were identified during screening testing, comprehensive neuro-ophthalmologic testing was performed to determine the presence of optic neuropathy. The OSEC reviewed the aggregate ocular safety data on an ongoing basis. In addition, aggregate data were reviewed by the OSEC to correlate ophthalmologic screening test results with confirmed optic nerve toxicity.
Additional data were gathered to identify potential risk factors for development of ophthalmic and neuropathic target toxicities, including patient demographics, underlying comorbidities, concomitant medications, environmental factors, and mitochondrial genetics. The treating physician received all test results including ocular tests and made all decisions regarding treatment.
Clinical laboratory assessments were performed by Covance Clinical Laboratory Services, Inc., Indianapolis, IN, USA (for US centers), and Covance Central Laboratories, Geneva-CH, Switzerland (for centers in Europe).
Adverse events
Investigators recorded all observed and volunteered AEs and their opinion of the relationship to linezolid or matched control. Adverse events included serious AEs (an AE that resulted in death, was life-threatening, required hospitalization, resulted in persistent or significant disability/incapacity, or lack of efficacy for an approved indication), abnormal test findings, and clinically significant changes in physical examination findings.
Statistical analysis
A sample size of 30 patients per group (treated and control) was considered adequate to assess the study objectives (not based on statistical considerations). Descriptive statistics were used to summarize safety parameters and demographics. All enrolled patients were included in the safety analysis.
To aid in enrollment, the protocol was amended to reduce the minimum duration of prior linezolid treatment for study entry, from 8 weeks to 6 weeks.
Results
Patient disposition and characteristics
The study was prematurely terminated after 5 years owing to slow enrollment; study results were limited by the small sample size.
Thirty-four patients were enrolled in the study; 24 patients received linezolid (16 were treated while on study and eight were treated within 7 days prior to screening); one patient did not receive treatment and was not included in the safety analyses; and nine patients were identified as matched controls and completed the baseline assessment. Twenty of the linezolid patients (20/24, 83.3%) completed the study. The nine control patients were well-matched to their respective linezolid counterparts; 1:1 matching of controls was not possible for all linezolid-treated patients with the early study termination. Two deaths were reported in patients treated with linezolid, neither death was considered related to treatment. One death was related to sepsis and the other to heart transplant rejection. Five patients (20.8%) permanently discontinued linezolid due to AEs considered related to treatment (anemia, n=2; peripheral neuropathy, n=1; peripheral neuropathy and retinal nerve fiber layer thickening, n=1; and polyneuropathy, n=1). With the exception of the mild retinal thickening, these AEs were considered moderate in severity.
Baseline characteristics for the linezolid and the nine matched control patients are presented in Table 1. The majority of patients were male and white with a mean age of 53.4 years. The most common primary diagnoses in the linezolid group were device-related infections (n=11) and osteomyelitis (n=4). Bacterial arthritis (n=2), device-related infections (n=2), and osteomyelitis (n=3) were the most common primary infection in the nine matched control patients. The median duration of total linezolid treatment (pre- and post-study) was 80.5 days (range, 50–254 days). The median pre-enrollment treatment for the control group was 227 days (range, 60–1,877 days). Antibiotics used in the matched control patients included fluoroquinolones, sulfamethoxazole/trimethoprim, daptomycin, and vancomycin. A list of the antibiotics with treatment durations and infection type for the control group is shown in Table 2. Linezolid infection type and treatment durations are listed in Table 3.
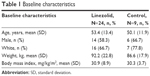  | Table 1 Baseline characteristics |
  | Table 2 Control patient antibiotics ≤6 weeks of study entrya |
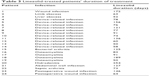  | Table 3 Linezolid-treated patients’ duration of treatment |
Clinical outcome
Successful long-term treatment with linezolid was reported. Clinical outcome was reported in 15 of the linezolid treated patients. At the end of treatment, most patients were considered improved (9/15, 60%). Additional outcomes reported included cure (1/15, 6.7%), improved with some symptoms (2/15, 13.3%), possibly cured (1/15, 6.7%), and failure (2/15, 13.3%). At the end of study, treatment was considered successful with majority of infections cured or improved (19/21, 90.5%). The small sample size prevented meaningful assessments comparing response rates across demographic or baseline characteristics.
General safety findings
As the control group patients were only included for baseline assessment, a direct comparison between the safety findings reported with linezolid over the entire study period is not appropriate. The linezolid safety population included 24 patients receiving linezolid for a median of 80.5 days.
Treatment-emergent AEs
All treatment-emergent AEs are reported in Table 4. The most common AEs reported that were considered related to treatment included anemia (n=5, 20.8%), peripheral neuropathy (n=3, 12.5%), polyneuropathy (n=3, 12.5%), vomiting (n=2, 8.3%), and asthenia (n=2, 8.3%). The remaining AEs occurred in no more than one patient (4.2%). Several AEs (pyrexia, muscular weakness, diarrhea, paresthesia, increased lactic acid, leukopenia, and neutropenia) were reported by a single patient. No cases of drug-induced liver injury were reported with linezolid.
  | Table 4 Treatment-emergent AEs reported with linezolida |
In our population, anemia was reported between days 44 and 136 of treatment; similarly, peripheral and polyneuropathy were reported between days 47 and 91 and days 47 and 146, respectively. Anemia resolved in the two patients who received blood transfusions and was still present at the end of study in those receiving no treatment beyond the discontinuation of linezolid. The cases of polyneuropathy and peripheral neuropathy were considered mild to moderate in severity. Additional treatment for these symptoms was given to some but not all patients, treatments included gabapentin, pregabalin, amitriptyline, and acetaminophen.
Five ocular events were reported in the linezolid group: optic neuropathy (n=2), toxic optic neuropathy, retinal nerve fiber thickening, diabetic retinal edema, and visual impairment (n=1 for each); one patient from the control group reported narrow anterior chamber angle. The ocular event was identified at baseline after linezolid had been discontinued in two patients (optic neuropathy and optic nerve toxicity) and during study treatment in three patients. Three of the cases of visual abnormalities (optic neuropathy [n=12], toxic optic neuropathy, and retinal nerve fiber thickening) were considered related to linezolid treatment by the investigator. The OSEC used visual testing results in conjunction with patient history to identify optic toxicity. A single case was adjudicated by the OSEC as probable optic neuropathy related to linezolid, and one additional case was adjudicated as possible optic neuropathy unrelated to linezolid. These cases are described subsequently.
OSEC adjudications
Probable optic neuropathy related to linezolid
Diagnosed in a 39-year-old black female treated for a device-related (central line) infection; received 145 days of linezolid prestudy exposure and 11 days of study exposure predominantly at 1,200 mg/d. The patient died on study day 38, from multiple causes not considered related to study drug. Initial examination completed on study day 1 showed: visual acuity of 20/20 right eye and 20/30 left eye, IOP measured 17 mmHg in each eye, no relative afferent pupillary defect, and markedly abnormal color vision (two of 14 plates correctly identified in each eye). The optic disks showed prominent surface telangiectatic vessels in both eyes without clear optic atrophy, and visual fields showed cecocentral scotomas each eye. Testing was negative for the three common mutations associated with Leber hereditary optic neuropathy. The optic neuropathy was considered consistent with linezolid use.
Possible nonprogressive optic neuropathy and severe optic atrophy unrelated to linezolid
Diagnosed in a 57-year-old black male treated for a limb abscess and line infection; received 61 days of linezolid prestudy exposure and 2 days of study exposure at 1,200 mg/d. Concurrent diagnoses included cataract, diabetic retinopathy, and glaucoma. Concomitant treatment for primary open angle glaucoma included topical Combigan® eye drops and oral acetazolamide. Initial examination showed: visual acuity of 20/200 (right eye) and 20/15 (left eye), IOP measured 22 mmHg (right eye) and 12 mmHg (left eye), and a relative afferent pupillary defect was present in the right eye. The optic disks showed severe glaucomatous excavation with moderate atrophy right eye, less severe glaucomatous excavation, and atrophy left eye, and visual fields showed severe generalized depression right eye with equivocal nasal defect left eye. The examination remained unchanged through the end of study visit. While there was clear evidence of optic neuropathy right eye and equivocal evidence left eye, the damage was considered unrelated to linezolid.
Serious AEs
Six patients reported ten serious AEs: fever and sepsis (n=1), worsening hypertension (n=1), polyneuropathy (n=2), severe clinical impairment/general health deterioration with nausea, asthenia, weight loss, vomiting, increased lactate dehydrogenase (n=1), and increased lactate dehydrogenase (two separate events), sideroblastic anemia, and acute rejection of heart transplant (n=1). The investigators considered sideroblastic anemia, severe clinical impairment, and two cases of polyneuropathy related to linezolid. Recovery from polyneuropathy with continued peripheral neuropathy sequelae at 23 months postlinezolid exposure was reported in one patient with polyneuropathy; symptoms of bilateral foot neuropathy at 3 months postlinezolid discontinuation remained present in the second patient. Full recovery and resolution from sideroblastic anemia and severe clinical impairment were reported in the remaining two patients.
Discussion
To our knowledge, this is the first report of linezolid use in patients with infections requiring extended therapy that were closely followed up for safety outcomes in a clinical trial setting. This pilot study was designed to monitor AEs including optic and peripheral neuropathy associated with linezolid treatment for at least 6 weeks duration. In this study, the duration of linezolid treatment (median 80.5 days) was longer than the recommended durations in the approved indications (10–28 days) as reflected in the product labeling.1,2 Positive clinical outcomes were reported in the majority of patients, and linezolid was generally well tolerated for the prolonged duration of exposure. In general, AEs were considered reversible.
Safety findings and AEs that were reported included neuropathies and bone marrow suppression that were consistent with the known safety profile of linezolid.1,2 Interference with mitochondrial function, specifically oxidative phosphorylation has been postulated as the mechanism responsible for neuropathy and bone marrow suppressions with long-term use of linezolid.2,13,14 Because bacterial ribosomes and mitochondrial ribosomes are homologous, drugs, such as linezolid, that inhibit bacterial protein synthesis often inhibit mitochondrial protein synthesis.10,11,15,16 The disruption of mitochondrial function may impede oxidative phosphorylation and adenosine triphosphate production.15
As part of the safety monitoring, a battery of ophthalmologic tests were used to screen for abnormalities, and potential cases of optic neuropathy were adjudicated by an expert panel. Of the limited cases reviewed, results of the visual field testing, color plate testing, optic photographs, and optical coherence tomography were valuable for identification of possible toxicities in linezolid-treated patients. The combination of dyschromatopsia, symmetric cecocentral visual field loss, and optic disk surface vascular abnormalities is consistent with a toxic/metabolic optic neuropathy. As previously discussed, mitochondrial dysfunction in retinal ganglion cells is the proposed mechanism of damage. As Leber hereditary optic neuropathy, an inherited mitochondrial disorder, produces similar, although more severe, visual loss, along with strikingly similar optic disk surface vascular telangiectasia, genetic testing helps differentiate the underlying cause.15,16 It should be noted, however, that nutritional deficiency optic neuropathy produces similar patterns of optic nerve dysfunction, and occasional cases have demonstrated optic disk surface vascular telangiectasia.17
Patients in the control group were treated with several different antibiotics on a long-term basis including fluoroquinolones, doxycycline, daptomycin, and sulfamethoxazole/trimethoprim. It is important to note that these agents, along with linezolid, are not without associated risks and their use in the treatment of chronic bacterial infections should be closely monitored. The decision to prescribe longer durations of therapy with these agents must be based on a careful assessment of benefit vs risk in each individual case.
The purpose of the study was to characterize safety in patients receiving ≥6 weeks of linezolid. AEs in patients receiving short-term treatment has been well studied and documented.1 Given that patients must have received and tolerated extended linezolid therapy for study inclusion, our study only reflects a population of patients able to tolerate linezolid for a period of ≥6 weeks. Early study termination and a smaller than desired sample size limits the interpretation of results. Insufficient data were collected as a result of the early study termination; data were insufficient to assess incidence of or to characterize in detail optic nerve toxicities or to relate their occurrence to the duration of treatment, comorbid conditions, or other epidemiologic factors. Due to the study design, the control group was only monitored for AEs and safety at baseline and no postbaseline data were captured, thus, a direct comparison between the control and linezolid treatment groups is not possible. Although the clinical outcomes of the infections were recorded, the study was not designed to draw conclusions regarding the efficacy.
In this close follow-up of a small number of patients receiving linezolid for extended periods of time, linezolid was well tolerated and the AEs were consistent with the known safety profile. The information gathered suggests that appropriate monitoring for the early detection of potential toxicities, such as use of appropriate ophthalmologic testing to identify optic neuropathy may be of value in the management of patients requiring treatment with linezolid that extends beyond the recommended duration. This is especially beneficial in cases of extreme medical need where therapeutic options are limited, and the benefits of continued therapy outweigh potential risks.
Acknowledgments
The authors would like to acknowledge and thank the members of the Ocular Expert Safety Committee for their participation in the study. Committee members included Alfredo A Sadun MD, PhD, Doheny Eye Institute, University of Southern California; Anthony C Arnold, MD, Jules Stein Eye Institute, UCLA; Frederick Fraunfelder, MD, Casey Eye Institute, Oregon Health & Science University; Alan Laties, MD, University of Pennsylvania Medical School; Eberhart Zrenner, PhD, University Eye Hospital of Tuebingen; and Christopher Kennard, PhD, Imperial College London. Medical writing support was provided by Charlotte Kenreigh, PharmD, of Engage Scientific Solutions and funded by Pfizer Inc. This study was sponsored by Pfizer Inc.
Disclosure
Jose A Vazquez serves as a consultant and has participated in advisory boards for Actavis and has received funding for speaker honoraria for Pfizer Inc. and Actavis. Anthony Arnold has received remuneration from Pfizer for his services as a member of the Ocular Safety Expert Committee for this study. Matteo Bassetti serves on scientific advisory boards for Pfizer Inc. and has received funding for travel or speaker honoraria from Pfizer Inc. Pinaki Biswas is an employee of Pfizer Inc. Robert Swanson is a former employee of Pfizer Inc. The authors report no other conflicts of interest in this work.
References
Pfizer [webpage on the Internet]. Zyvox (linezolid) prescribing information; 2015. Available from: http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed April 21, 2015. | ||
European Medicines Agency [webpage on the Internet]. SPC Zyvox [linezolid] 600 mg Film-Coated Tablets; 2014. Available from: https://www.medicines.org.uk/emc/medicine/9857. Accessed June 2, 2015. | ||
Legout L, Valette M, Dezeque H, et al. Tolerability of prolonged linezolid therapy in bone and joint infection: protective effect of rifampicin on the occurrence of anaemia? J Antimicrob Chemother. 2010;65(10):2224–2230. | ||
Soriano A, Gomez J, Gomez L, et al. Efficacy and tolerability of prolonged linezolid therapy in the treatment of orthopedic implant infections. Eur J Clin Microbiol Infect Dis. 2007;26(5):353–356. | ||
Tascini C, Bongiorni MG, Doria R, et al. Linezolid for endocarditis: a case series of 14 patients. J Antimicrob Chemother. 2011;66(3):679–682. | ||
Corallo CE, Paull AE. Linezolid-induced neuropathy. Med J Aust. 2002;177(6):332. | ||
Narita M, Tsuji BT, Yu VL. Linezolid-associated peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome. Pharmacotherapy. 2007;27(8):1189–1197. | ||
Nambiar S, Rellosa N, Wassel RT, Borders-Hemphill V, Bradley JS. Linezolid-associated peripheral and optic neuropathy in children. Pediatrics. 2011;127(6):e1528–e1532. | ||
Joshi L, Taylor SR, Large O, Yacoub S, Lightman S. A case of optic neuropathy after short-term linezolid use in a patient with acute lymphocytic leukemia. Clin Infect Dis. 2009;48(7):e73–e74. | ||
Carelli V, Ross-Cisneros FN, Sadun AA. Optic nerve degeneration and mitochondrial dysfunction: genetic and acquired optic neuropathies. Neurochem Int. 2002;40(6):573–584. | ||
Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23(1):53–89. | ||
Li J, Tripathi RC, Tripathi BJ. Drug-induced ocular disorders. Drug Saf. 2008;31(2):127–141. | ||
Javaheri M, Khurana RN, O’Hearn TM, Lai MM, Sadun AA. Linezolid-induced optic neuropathy: a mitochondrial disorder? Br J Ophthalmol. 2007;91(1):111–115. | ||
De Vriese AS, Coster RV, Smet J, et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis. 2006;42(8):1111–1117. | ||
Sadun AA. Mitochondrial optic neuropathies. J Neurol Neurosurg Psychiatry. 2002;72(4):423–425. | ||
Sadun AA, Carelli V. Mitochondrial function and dysfunction within the optic nerve. Arch Ophthalmol. 2003;121(9):1342–1343. | ||
Frisen L. Fundus changes in acute malnutritional optic neuropathy. Arch Ophthalmol. 1983;101(4):577–579. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
