Back to Journals » Drug, Healthcare and Patient Safety » Volume 14
Safety Aspects and Rational Use of Lanadelumab Injections in the Treatment of Hereditary Angioedema (HAE): Clinical Insights
Authors Petkova E, Yordanova V, Staevska M, Valerieva A
Received 6 September 2022
Accepted for publication 10 December 2022
Published 22 December 2022 Volume 2022:14 Pages 195—210
DOI https://doi.org/10.2147/DHPS.S345443
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rajender R Aparasu
Elena Petkova, Vanya Yordanova, Maria Staevska, Anna Valerieva
Department of Allergology, Medical University of Sofia, University Hospital “Alexandrovska”, Sofia, Bulgaria
Correspondence: Anna Valerieva, Email [email protected]
Abstract: Hereditary angioedema (HAE) is a rare genetic disorder characterized by recurrent episodes of skin/mucosal swelling, and/or attacks of severe abdominal pain when it affects the gastrointestinal tract. The disease might be unexpectedly fatal when the upper airways are compromised. HAE clinical presentation, disease course and prognosis are associated with significant disease burden and severely impaired quality of life. Lanadelumab is a breakthrough therapy for the prevention of attacks in HAE type 1 and 2 patients. This revolutionary approach to administer a single subcutaneous injection (once every two to four weeks) and achieve complete disease control has dramatically improved patient care resulting in significant change in the life of affected families. Current data support the drug’s tolerability in adult and adolescent patients without notable safety concerns in both clinical research and real-world settings. Rational use of prophylactic treatments of HAE searches for a socio-economic balance, taking into account the life-long course of the disease, the public health funds who pay the monetary price, and the patients who might need to receive the therapy for a period longer than investigated during the development program. In this review, we address the current evidence on lanadelumab’s tolerability, highlighting aspects of the drug’s rationale use in clinical practice. Further studies need to investigate whether this therapy might be appropriate in other forms of angioedema, such as idiopathic primary angioedema and HAE with normal C1 inhibitor. Future efforts must focus to improve modern drugs’ accessibility in more countries. Although modern prophylactic options lessen the risk of fatal laryngeal attacks, patients must be equipped with reliable on-demand therapies and be trained how to use them as such a risk cannot be fully diminished with potentially life-threatening attacks occurring even in subjects with successful and stable long-term prophylaxis. Notwithstanding, further studies are needed to identify early responders from non-responders and develop therapies for the latter.
Keywords: hereditary angioedema, C1 inhibitor, bradykinin, kallikrein, lanadelumab, drug safety
Introduction
Hereditary angioedema (HAE) is a rare genetic disorder characterized by recurrent episodes of skin/mucosal swelling (affecting the limbs/face/genitals), and/or attacks of severe abdominal pain when it affects the gastrointestinal tract. The disease might be unexpectedly fatal when the upper airways are compromised due to angioedema.1 There are two main forms of HAE. The most common form is associated with a defective C1 inhibitor (C1-INH) protein (HAE-C1-INH): due to either protein deficiency (type I) or dysfunction (type II). The other identified form is characterized by normal C1-INH plasma activity (HAE-nlC1-INH) and can be associated with various mutations within genes, affecting the kallikrein-kinin system or the vascular endothelium permeability.2,3 The reduction in C1-INH function results in uncontrolled activation of several biochemical systems including the bradykinin system and subsequent release of the potent vasodilator bradykinin. Bradykinin overproduction increases smooth muscle relaxation in the walls of blood vessels and leads to cutaneous swelling attacks, abdominal angioedema attacks, and in severe cases, life-threatening laryngeal attacks. HAE clinical presentation, disease course and prognosis are associated with significant disease burden and severely impaired quality of life. HAE has been described in all races, and no sex predominance has been reported in patients with types I and II, however HAE with normal C1-INH (HAE-nlC1-INH) is by far more predominant in females.4
The most common form of HAE is associated with mutations in the C1 inhibitor gene (SERPING1) and autosomal dominant inheritance. On the other hand, HAE-nlC1-INH is a heterogeneous entity with many genotypes, and underlying pathomechanisms.5
The onset of HAE symptoms is usually in the first or second decade of life. The disease is characterized by unpredictable course and a complex of clinical features that include debilitating, repetitive episodes of non-inflammatory, non-allergic, bradykinin-mediated angioedema.6 The swelling attacks are caused by a sudden transient increase of the vascular permeability, affecting different areas of subcutaneous or mucosal tissues. The most commonly affected body sites are the extremities and present with nonpitting, nonpruritic skin swellings. Abdominal attacks are also common among patients and present with severe, debilitating abdominal pain, nausea, vomiting and diarrhea, recurrent transient ascites.7,8 Upper airway edema is less common (approximately one out of 125 episodes) but potentially life-threatening presentation, because sudden and progressing laryngeal obstruction may lead to asphyxiation.9 Although less than 1% of all HAE attacks affect the larynx, it is estimated that about 50% of all patients will develop a laryngeal attack during their lifetime and more importantly, even the first occurrence of laryngeal edema could be fatal.
Pathophysiology of HAE
Understanding the underlying pathophysiological mechanisms is the key for accurate diagnosis and appropriate treatment of HAE patients.10 C1-INH is responsible for the fine regulation of the complement, coagulation, fibrinolytic, and contact system.11 Many different mutations in SERPING1 gene have been described that result in either decreased levels or functionally inert C1-INH leading to overactivation of the kallikrein-kinin cascade, hence to hyperproduction of bradykinin.12 Bradykinin activates the bradykinin B2 receptor, which results in increased vascular permeability with fluid extravasation and angioedema.13 Thus, bradykinin plays a key mediator of swelling in HAE and its elevated plasma levels are responsible for the clinical manifestations and natural course of HAE attacks.
The Role of Kallikrein in HAE
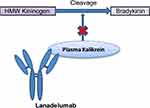
Kallikrein-kinin (contact) cascade, also known as the bradykinin-forming pathway, plays a critical role in the underlying mechanisms characterizing HAE attacks.14 The cascade is triggered by the activation of factor XII into factor XIIa upon contact with negatively charged surfaces. Factor XIIa subsequently converts prekallikrein to active plasma kallikrein, which cleaves high-molecular weight kininogen (HMWK) to release a small, short-living vasoactive peptide, bradykinin.15 Activation of plasma kallikrein is two-step process: (1) autoactivation and (2) activation of factor XII by kallikrein, creating a positive feedback loop. C1-INH controls both plasma kallikrein and factor XIIa activation. Thus, plasma level of active C1-INH below a critical threshold, leads to disequilibrium of bradykinin-forming enzymes and results in bradykinin overproduction (Figure 1).
Burden of HAE
Although HAE is a rare disease, with prevalence around 1:50,000 when appropriate diagnosis is available, the diagnosis significantly influences both physical and emotional functioning of patients, families, and caregivers.16 HAE is associated not only with an immediate threat to patients due to potentially fatal laryngeal involvement, but also seriously impacts their long-term well-being which is thought to depend more on psychological and social factors than on physical status. Disease characteristics and risk factors that are associated with worsened quality of life in HAE patients are:
- Most attacks are unpredictable, in terms of onset, severity, course and outcomes causing patients live in constant fear.17
- Lifelong management and follow-up due to the chronic nature of HAE (availability of drugs with rapid onset of action, proven efficacy, and a well-established safety profile).
- Lack of adequate treatment, both for on-demand and prophylaxis in the past, often with a history of tragic incidents among close relatives.
- Difficulties associated with intravenous drug delivery.
- Concerns about adverse events, both short-term associated with regular use of on-demand therapy, and long-term side effects of continuous prophylactic treatment.
- Delayed diagnosis which could lead to unnecessary surgical procedures (in patients with abdominal attacks)8 and additionally, unrecognized HAE could have lethal consequences.18
- Avoidance of identified triggers, including trauma, stress, specific interventions, certain medications, could limit daily activities, practicing sports or hobbies, travelling and social life, and might challenge the management of existing concomitant conditions.19
- Fear of passing HAE to children.
All these features result in increased prevalence of mental health disorders, including depression and anxiety.20 In addition to psychological distress, HAE is also associated with significant economic burden due to higher direct medical costs and indirect costs related to work/school absenteeism which increases with attack severity and frequency, and results in lost productivity.21
Therapeutic Strategies
In line with the huge scientific progress resulting in the development of new therapeutic strategies based on the emerging knowledge of HAE pathophysiology, the goals of HAE treatment should be to achieve complete disease control (no angioedema attacks) and to improve patients’ quality of life.3 An individualized management plan, involving medical team, patients, and family members or caregivers should be established, based on attacks severity, and frequency and designed to meet the specific patient’s needs. They should address the diverse factors influencing disease characteristics, course, and outcomes and should cover both acute angioedema episodes (on-demand treatment) and asymptomatic periods between HAE attacks (prophylactic treatment). Treatment plans should be discussed regularly and modified based on patient’s current clinical condition and future needs. HAE management plans should include detailed information, written in clear, easy-to-understand language about: (a) on-demand medications that address patient’s specific needs, based on disease course, self-administration skills, access to medical care (b) use of short-term prophylactic medications before medical interventions or exposure to other triggers known to precipitate HAE attacks, and (c) eligibility for long-term prophylaxis to reduce their attack frequency and severity.22 The approach should be balanced and in line with current available options, taking into account disparities among countries.23
On-Demand Treatment (ODT)
Current treatment guidelines state that ODT should be considered for all acute HAE attacks and is required for all attacks with potential laryngeal involvement.24 Whenever treatment is considered indicated, it should be initiated as soon as possible because early treatment is known to shorten the time to resolution of symptoms as well as total attack duration independently of attack location or severity.25 It is recommended that physicians should ensure that all HAE patients have adequate supply of on-demand medications for treatment of at least two acute attacks and always carry their on-demand medication and are educated how to administer it.26 Currently approved on-demand drugs include replacement therapy with plasma-derived human C1-INH concentrate (pdC1INH), and recombinant human C1-INH (rhC1INH), both for intravenous (IV) administration, a bradykinin B2 receptor antagonist (icatibant) for subcutaneous (SC) administration, and a subcutaneous plasma kallikrein inhibitor (ecallantide). All of these have demonstrated efficacy and safety in randomized, controlled studies, and were later supported by real-world evidence.27–29
Approaches in Prophylactic Treatment of HAE
Short-Term Prophylaxis (STP)
STP administered prior to identified angioedema-triggering events, including medical, surgical, or dental procedures, or exposure to known psychological attack-inducing factors, such as school/work related stress aims to minimize the risk of developing angioedema associated with these situations.30 Therefore, in line with current guidelines, preprocedural prophylaxis is recommended before patients undergo medical interventions (especially those associated with mechanical impact to the upper aerodigestive tract) and STP should be considered before exposure to other attack-inducing events.3 Intravenous C1-INH is recommended as first-line option for STP and it should be administered as close as possible to the start of the intervention.31,32
Long-Term Prophylaxis (LTP)
Modern LTP therapies represent an important milestone in the treatment of HAE altering the natural course of this disabling chronic disease and improving patients’ quality of life. Just a few years ago the main aim of HAE treatment was to decrease the duration and severity of HAE episodes and to prevent mortality from acute laryngeal attacks, while nowadays therapies go beyond to concentrate on reduction of the overall burden of the disease on patients, families and caregivers. Consequently, the latest update of the International World Allergy Organization/European Academy of Allergy and Clinical Immunology (WAO/EAACI) guidelines for the management of HAE postulates that ultimate goals of treatment in HAE should be achieving complete disease control and normalizing patients’ lives.3 Currently, this can be accomplished only with LTP treatment which has the potential to modify disease evolution, minimize the burden of HAE, and drastically improve quality of life. LTP with androgens/gestagens is not considered first-line therapy in countries with access to modern therapies, while these agents remain the only possibility in a majority of countries outside Europe and North America.23
Recently, several highly effective and safe drugs for LTP, including IV and SC C1-INH and the kallikrein inhibitors lanadelumab (SC) and berotralstat (PO), have been developed and approved and real-world evidence has been already emerging to prove this novel LTP strategy could become a life changing treatment for patients living with HAE.33 Finally, it is important to emphasize that ODT and LTP should not be considered mutually exclusive, but LTP should rather be adopted as an additional option to be used in the same patient. Additionally, all patients, including those with good response to LTP must be provided with medications for ODT to use in case of breakthrough attacks.
Guidelines Recommendations for LTP
The development of novel, disease-specific treatment options for LTP with high efficacy and favorable safety profile, have further led to the improvement of the HAE management guidelines. Thus, recently updated WAO/EAACI guidelines reflect the advancement in HAE understanding in terms of setting new goals of achieving total disease control and normalizing quality of life and include recommendations for the use of different drug classes in LTP (Table 1).
 |
Table 1 Drugs for Long-Term Prophylaxis of Hereditary Angioedema |
Lanadelumab
Lanadelumab is a first-in-class fully human IgG1 monoclonal antibody made in recombinant Chinese Hamster Ovary cells that binds plasma kallikrein with high affinity and selectivity (Figure 2). 34 Subcutaneous lanadelumab has been approved for the prevention of HAE attacks in patients with HAE type 1 and 2, aged 12 years and above.35 The drug’s efficacy was demonstrated in clinical trials and further showed sustained effectiveness with long-term use in selected HAE patients.36–38 Use of lanadelumab also resulted in significant improvement in HAE-associated quality of life.39 The drug is associated with a good tolerability profile with most adverse reactions being mild, transient, and reversible, as well as low immunogenicity rate. The US FDA granted this application orphan drug designation, priority review and breakthrough therapy designation. The European Medicinal Agency (EMA) granted lanadelumab orphan designation and Accelerated assessment.
Safety Aspects
As most drugs with Orphan designation, lanadelumab is a subject of additional monitoring which aims to enhance reporting of suspected adverse drug reactions for medicines for which the clinical evidence base is less well developed due to the limited experience during the drug’s development program. The manufacturer is obliged to collect information as early as possible to further inform the safe and effective use of the medicine and support the benefit-risk profile when used in everyday medical practice. It does not mean that the medicine is unsafe.
Summarized below are the safety findings as available by manuscript preparation.
Pivotal Studies
Safety profile of lanadelumab has been extensively studied during its clinical development program. No significant safety issues were reported. Phase Ia study was a randomized, double-blind, and single-center study to evaluate the SC administration of different doses of lanadelumab in 32 healthy subjects (24 on lanadelumab, eight on placebo).34 Serious AEs or deaths were not reported and clinically significant changes in vital signs, electrocardiogram and laboratory findings were not observed. Additionally, antidrug antibodies were not detected in patients given lanadelumab. The most frequently reported AEs in the lanadelumab group were headache (25%) which was considered not severe, and upper respiratory tract infection (12.5%) and did not result in study discontinuation.
Phase Ib study was a randomized, multicenter, double-blind, placebo-controlled, multiple ascending dose study in 37 patients with C1-INH-HAE (24 on lanadelumab, 13 on placebo).38 No AE-related deaths or study withdrawal, serious AEs, or clinically significant changes in vital signs, physical state or laboratory parameters were observed. Most commonly reported AEs in the lanadelumab groups were injection-site pain (25%) and headache (8%). Additionally, antidrug antibodies developed in two patients but were non-neutralizing and did not interfere with the PK/PD parameters of lanadelumab.
Approval was based on data from a phase III HELP study: a multicenter, randomized, double-blind, placebo-controlled, parallel-group study in 125 patients with HAE. Patients who received lanadelumab had clinically meaningful and statistically significant reductions in the rate of investigator-confirmed HAE attacks compared to placebo over a 6-month treatment period.37 Authors reported no deaths or related serious AEs. The most commonly observed treatment-emergent adverse events in patients on lanadelumab included injection site pain (42.9%), viral upper respiratory tract infection (23.8%), headache (20.2%), injection site erythema (9.5%), injection site bruising (7.1%), and dizziness (6.0%). Most of these (98.5%) were considered mild to moderate in severity. Two patients from the placebo group discontinued the study due to tension headache and HAE attack, whereas one patient in the lanadelumab group withdrew due to transient elevation of liver enzymes. However, this patient had metabolic syndrome, hepatic steatosis and concomitantly received multiple drugs. One patient in the lanadelumab group reported two hypersensitivity reactions presenting with mouth tingling and pruritus, which were considered mild and moderately severe, transient, and recovered without treatment. A low titer of antidrug antibodies was detected in 11.9% patients on lanadelumab, and 4.9% taking placebo. In three patients from the lanadelumab group low preexisting antibody titers were observed and in two patients transient neutralizing antibodies were detected. In summary total incidence of AEs was comparable to the group receiving placebo.
Phase 3 HELP Open-Label Extension Study (HELP-OLE)
HELP-OLE Study is a phase 3 open-label extension to evaluate long-term safety and efficacy of lanadelumab. The study included patients who completed HELP study (rollover patients, n=109) and newly enrolled patients who did not take part in the double-blind study (non-rollover patients n=113).36 Rollover patients were administered with a single dose of lanadelumab 300 mg and were followed until the first documented HAE attack and then treatment with lanadelumab 300 mg every two weeks was initiated. Non-rollover patients were assigned to treatment with lanadelumab 300 mg every two weeks. The study’s primary objective was to investigate the long-term safety of repeated SC administrations of lanadelumab in HAE type 1 or 2 patients. The secondary objectives of the study were to assess the long-term efficacy of lanadelumab for prevention of HAE attacks. The collected safety data from the HELP OLE was consistent with the collected safety data from the HELP Study.35,36 For the duration of treatment most of the patients (97.2%) reported ≥1 treatment-emergent adverse event (TEAE) except for HAE attacks (~30 months). The most frequent TEAEs included injection site pain (47.2%), viral upper respiratory tract infection (42.0%), upper respiratory tract infection (25.9%), and headache (24.5%). Most of those events were classified as mild to moderate in severity. Most common reactions were injection site pain, resolving within one hour (70.2%) or one day (92.6%). No serious TEAEs were reported including anaphylaxis, anaphylactoid reactions, and deaths. Six patients (2.8%) discontinued their participation in the study due to AEs.
Real World Evidence Studies
There are two ongoing real world evidence studies conducted by Takeda, EMPOWER
(US and Canada based) study and ENABLE (a European-based study).40,41 Interim analysis were recently announced.41–44
EMPOWER
This study (NCT03845400) is a non-interventional prospective, currently conducted in Canada and the United States assessing the real-world effectiveness of lanadelumab in type 1 or type 2 HAE patients.40 The planned enrollment period is 18-months with lanadelumab naïve population (≥70%) or patients who have received <4 doses of lanadelumab at enrollment (new users). The rest of the patient population consists of lanadelumab users (patients who have received ≥4 lanadelumab doses prior to enrollment). Subjects will be followed for 36 months not dependent from the received treatment during the study.
The primary objective of the study is evaluation of real-world effectiveness of lanadelumab. The secondary objectives of the study include description of the utilization of lanadelumab in the real- world clinical practice by describing the health care resource utilization (HCRU) necessary for HAE attacks before and after treatment with lanadelumab, reviewing patient caregiver reports (when available), PROs and comparing the HAE control before and after treatment with lanadelumab. The exploratory endpoints include other measures of effectiveness related to attack management during lanadelumab treatment and the use of concomitant treatments (C1-INH).
Recently, an interim analysis was reported by Johnston et al, which included 93 enrolled patients, 15 of which were new users and 78 were established users.42 The mean duration of drug exposure for the patients was 470.8 (DS=191.6) days. The mean attack rate per month for the established users during the 490.9 (SD183.6) days was 0.2 (SD=0, 50). For the new users, the mean attack rate was 1.2 (SD=1.4) attack per month before treatment with lanadelumab and 0.2 (SD=0, 21) after start of treatment with lanadelumab, therefore there was an 83, 3% reduction in the attacks per month. During the study 7.8% of the established users discontinued treatment. The reported AEs during the treatment were 24.5% of which most frequent were infections (23.6%); two percent of the AEs were serious.
The treatment outcomes in HAE patients type 1 and 2 that are naïve to lanadelumab treatment and switch to lanadelumab from ODT or other LTP was reported by Lumry et al by presenting data from interim analysis.43 The study had three periods: pre-enrolment, pre-lanadelumab treatment and post-lanadelumab treatment. The patients were followed-up for 36 months, every 6±2 months and data were collected by mobile application. Before the enrollment the number of attacks was as follows: 28 (27.2%) mild, 42 (40.8%) moderate, 27 (26.2%) severe, 3 (2.9%) life-threatening in all patients (N=93). The new lanadelumab users were 15 out of 93 of which 12/15 (80.0%) with HAE type 1 and a mean <1 severe attack per month. Prior to enrollment in the study those patients were distributed as follows: (6/15) were using ODT without LTP, (4/15) were using ODT and LTP, (3/15) were using LTP without ODT. The patients that administered ODT without LTP (6/15, 40.0%) and the patients that administered LTP (7/15, 46.7%) continued their previous treatment for an average 440–349 days. The reduction of the mean monthly attack rate was as follows (95% CI) of 85% (56, 95%) for the patients using only ODT and 68% (28, 86) in the patients on LTP. The reported treatment-emergent AE rate was 24.5% with the majority (93%) assessed as mild to moderate. This data is consistent with the data collected during the pivotal studies.
ENABLE
The ENABLE study is a non-interventional, three-year, multicenter, prospective study, ongoing in Europe. The study is designed to evaluate the long-term effectiveness of lanadelumab in a real-world practice.41 The target patient population includes HAE patients type 1 and 2 that are planned to start treatment with lanadelumab as per the product labeling. The primary study objective is to evaluate the effectiveness of lanadelumab over the frequency of HAE attacks in the real-world clinical practice, and secondary objectives including additional assessments for lanadelumab effectiveness based on PROs and caregiver-reported outcomes, utilization patterns of lanadelumab as well as tolerability and safety.
Interim analysis of this real-life data reported for sixteen HAE patients which switched to prophylactic treatment with lanadelumab as of Jan 2020.41 Prophylaxis was initiated with SC lanadelumab 300 mg every two weeks and the treatment intervals were extended to 2.5 or three weekly intervals in case the patient was attack-free for more than three months. The patients were followed at intervals of three months. Medical history was collected including previous treatments for HAE attacks for twelve months period before start of lanadelumab treatment. The patients were distributed as follows: HAE type 1 n=13, HAE type 2 n=1, acquired AE n=2, age between 13.5 and 77.1 years (10 female, 6 male; 1 male/1 female Acquired AE). Patients initiated prophylaxis with lanadelumab in February 2019 and included a mean period of four to eleven months per patient. The frequency of the treated HAE attacks prior to lanadelumab start was 5–12 attacks per month and decreased to 0–4 attack per month in the beginning of treatment and in the first three months of treatment. No life-threatening attacks, including laryngeal attacks were registered during the follow-up. The drug was also well tolerated, and patients adhered to the prophylaxis regimen.
The ENABLE study recent interim analysis included data for 54 patients (mean age [range] 41.9 [14–73] years; 68.5% female; 98.1% White) from Germany, Austria, Switzerland and Israel.41 The mean treatment observation was 325 days, and the mean (SE) baseline attack rate was 5.07 (0.10, vs 0.55 (0.24) on treatment (incidence rate ratio [95% CI] 0.11 [0.08, 0.16]). Of all 54 patients, 47 (94.0%) reached a reduction in the attack rate ≥70% during treatment in comparison to baseline. The median (95% CI) time to first attack was 54 (11–324) days. Twelve (22.2%) patients reported HAE attacks in the area of upper airways at baseline versus 0 for the patients on treatment with lanadelumab. At baseline 24 attacks were reported (44.4%) vs 12 (22.2%) for the patients on treatment. There were two discontinuations, but neither was due to an AE. Treatment-emergent AEs (TEAEs) were reported 38/54 (70.4%) of the patients. There was one serious TEAE (abdominal pain) in one patient which was assessed as not related to treatment (1.9%). The most reported TEAEs were injection site reactions (7 [13.0%] patients, 8 TEAEs) most of them assessed as mild (41.7%) to moderate (33%). No deaths due to TEAEs were reported.
Patient reported outcomes (PROs) were collected throughout the study from all 54 patients and were included in the interim analysis.45 They included Angioedema Control Test (AECT), Angioedema Quality of Life Questionnaire (AE-QoL), the Hospital Anxiety and Depression Scale (HADS) and the Treatment Satisfaction Questionnaire for Medication (TSQM-9; adult patients). The results for AECT mean (SD) were increased from 7.6 (3.8) at baseline to 14.8 (2.2) at month 12 showing improved disease control. The results for AE-QoL showed improvement of the score from a mean (SD) of 43.9 (17.0) at baseline to 15.5 (13.4) at month 12. The results for HADS mean (SD) scores decreased from baseline (anxiety: 7.6 [4.4]; depression: 5.0 [3, 6]) to month 12 (anxiety: 3.6 [3.1]; depression: 2.2 [3.1]. The results for TSQM-9 mean (SD) global score showed increase from 53.4 (13.7) at baseline to 74.2 (6.6) at month 12 demonstrating treatment satisfaction. The interim data for the treatment patterns of lanadelumab in HAE type 1 or 2 patients were analyzed by Recke et al and included data for patients from Germany, Austria, Switzerland and Israel who started treatment with lanadelumab as per the product labeling.44 The analysis included data from all patients enrolled that received ≥1 dose of lanadelumab and had ≥1 post-enrollment assessment of effectiveness (cut-off May 2021). The mean duration of treatment with lanadelumab in the study was 325 (108–525) days. The treatment was started with 300 mg every two weeks and dosing frequency was changed for 21/54 patients (38.9%); 34/52 (65.4%) stayed on the initial frequency every two weeks (three patients switched to “other” frequency and then again to every two weeks), three (5.8%) were on every four weeks and 15 (28.8%) were on “other” frequency. For patients who changed the dosing regimen: a total of 21 patients, a mean of two (one) doses were administered before the change. Concerning administration: 92.7% of patients self-administered treatment vs 3.6% where “other” or healthcare specialist administered lanadelumab. Two patients (3.7%) discontinued treatment (one patient administered two doses and the other administered three doses of lanadelumab). Discontinuations were not due to an AE. Six patients (11.1%) used LTP with C1-INH and two (3.7%) used androgens. For the duration of the study seven (13.0%) patients used IV C1-INH for STP.
Cohort Studies
France
Bouillet et al reported data for the treatment of HAE in France under the temporary authorization of use (ATU).46 On 29 Aug 2018 the French national agency for medicine and health products safety granted ATU in a cohort (cATU) to Shire, a Takeda company for use of lanadelumab for prevention of attack in HAE type 1 and 2 patients ≥12 years for whom other medications for routine prevention of attacks were ineffective or unavailable. Patients from 16 centers in 14 cities were included of which (81%) were part of the French HAE-dedicated management Network. The patients were enrolled in the period 12 Oct 2018 to 13 Mar 2019 and follow-up was performed for a period of six months through 23 Sep 2019. History of HAE treatment was evaluated at treatment access request (TAR), type of attacks and AEs were assessed at TAR, treatment start and then at a 3-month follow-up visits. Patient reported outcomes were collected: Angioedema Quality of Life (AE-QoL: higher the scores - greater impairment), at initiation and then monthly; Angioedema Activity Score questionnaires: completed daily (lower scores: lower disease activity) and on follow-up visits. The effectiveness of lanadelumab was evaluated by the change of monthly attack rates. At study entry all patients were administered with lanadelumab 300 mg every two weeks. Results included 75 patients: age at time of TAR, mean (SD) was 41.7 (±15.6) years, 69% of the patients were female, and 89.3% had HAE type 1. The attack rate six months prior to TAR was a median (range) of 13.5 (1–99) attacks. Most of the patients (93.3%) had a previous LTP and 77.3% had ongoing LTP. Of all attacks that required treatment, 62.8% were severe. Sixty-seven patients had ≥1 visit after start of the treatment. The Mean (SD) exposure to lanadelumab was 234±61.6 days and the cumulative incidence of HAE attacks during this period was 33.6%. The mean (SD) frequency of monthly attacks decreased from 2.3 ±2.1 before start of treatment to 0.2±0.5 towards the end of the follow-up period. Ninety-six AEs were recorded in 46 patients, and they were not serious. The safety profile of lanadelumab was similar to the previously reported results. A total of five patients discontinued treatment. Median (range) AE-QoL scores were 37.1 (0.00–86.8) at the start of the study and 11.0 (0.00–90.6) after four months (p<0.0001), demonstrating decreased impairment over time. AAS28 scores=0 was 59.2% at the start of the treatment and 83.3% after six months of treatment indicating reduced burden over time. The conclusion of the authors was that lanadelumab demonstrated effectiveness (as sole LTP) and improved QoL in the real-world practice. No new safety signals were detected.
Germany
Effectiveness, safety and the possibility for extending the administration interval of lanadelumab were assessed in a cohort study of 34 patients with HAE conducted in Germany.47 In this study patients with HAE (n=24) and patients with acquired C1-inhibitor deficiency (n=4) were switched from previous treatment to treatment with lanadelumab. Six patients continued from the HELP OLE study.36,48
The treatment period was 29.9 weeks (range: 3.3–65.3). All patients reached adequate disease control: angioedema control test improved from average 7.5 (poorly controlled disease) to 14.9 (well-controlled disease). Dosage intervals were extended upon reaching symptom-free state.
No AEs were reported during the study. However, three patients reported itching, wheals and redness, two patients reported hematomas at the injection site, one patient reported local urticarial reaction and one patient reported dizziness after the injection.
Another prospective observational study in Germany aims to assess real-life LTP of lanadelumab in HAE type 1.49 A total of twelve patients were included, mean age 45.4 (±18.2) years 66.6% of which were female. Treatment with lanadelumab 300 mg/sc was administered every two weeks for at least six months. A total of 24 attacks were recorded for all patients before the start of lanadelumab treatment. The mean number of attacks per month decreased from 6.4 before start of lanadelumab treatment to 0.3 towards the end of the cohort study (P<0.0001). One patient reported a local erythema at the site of the injection and pain during the injection, both mild in intensity.
United Kingdom
A retrospective multicenter study assessing the efficacy of lanadelumab in HAE patients was conducted in the United Kingdom. The study evaluated the total number of attacks before the start of treatment and at the 1st, 3rd, 6th and 12th month after treatment initiation.50 A total of 57 patients were included with a mean age of 40.6 (±16.5 years), 40 were females. Fifty-three were on other prophylaxis before lanadelumab initiation. Thirty-two patients received lanadelumab every two weeks and 25 were administered every three weeks or more.
The total and the severe monthly attack frequency were significantly decreased at all time points vs baseline (P<0.01). At 6th month, there was a reduction of 90.7% and at 12th month there was a reduction of 96.5% compared to baseline. No adverse events were reported.
United States Food and Drug Administration (US FDA) Adverse Event Reporting System (FAERS) Public Dashboard
The US FDA Adverse Event Reporting System (FAERS) is a Public Dashboard organized as a highly interactive web-based tool with the intention to expand access of FAERS data to the general public to search for information related to human adverse events reported to the FDA by the pharmaceutical industry, healthcare providers and consumers. The database discloses that its list may contain duplicates and incomplete reports. Existence of a report does not establish causation and the reports were not verified which does not allow to establish rate of occurrence. As of date of manuscript preparation, there are 1740 adverse events with lanadelumab that have been reported to the system (database updated on 30 June 2022). Of the listed reports, 1095 were classified as serious adverse events and 60 resulted with death. Considering the limitations of this public reporting system: a majority of the serious adverse events with a death outcome can be associated with either life-threatening angioedema attacks, neurologic conditions, comorbidities such as infections (including SARS-COV2 infection), or acute cardiovascular events.51 Further, real-world clinical experience will notify whether these events are presented comparably among the general HAE population.
Rational Use of Lanadelumab
According to the World Health Organization (WHO), rational use of medications refers to their appropriate use in terms of drug selection, dose regimen, and duration of treatment which should be in line with current guidelines.52 Medicines should adequately meet patients’ needs at the lowest cost to the healthcare systems, society, and the patient, and should be dispensed correctly and taken properly. Furthermore, all these characteristics must be regularly monitored, preferably at each clinic visit, and therapeutic modifications made to meet current patients’ needs and to ensure rational use of medications.
Drug Initiation
LTP should be considered in all patients, however, the decision whether to initiate it and which LTP drug to choose should be strongly personalized and relies on the expertise and experience of the treating physician and eligibility of the patient.33 Factors that can positively influence this decision include:
- Disease-related factors: frequency, localization, duration, and severity of attacks; history of life-threatening attacks in the past.
- Patient-related factors: comorbidities that are known to worsen HAE course; psychiatric comorbidities; concomitant medication, interfering with HAE therapy course; unavoidable triggers; not willing or able to use IV drugs (ODT); potential complications of on-demand drug use; patient’s preference.
- Impact on quality of life: absenteeism from work or school; living in chronic anxiety and fear; interference with everyday activities; burden of constant life event planning;
- healthcare system-related factors: access to emergency services; availability and reimbursement of LTP therapies; benefit–cost ratio of available LTP options.
Current WAO/EAACI guidelines recommend lanadelumab as one of the first-line LTP options for the prevention of HAE attacks in patients aged 12 years and older due to its efficacy, safety, and subcutaneous route of administration.
Dose Regimens
The recommended starting dose is 300 mg lanadelumab every two weeks. However, in patients who are well controlled and attack-free on treatment, a dose reduction of 300 mg lanadelumab every four weeks may be considered.35
Several studies have been conducted to establish the real-life efficacy of the extended dosing regimen in comparison to the standard dosing. Buttgereit et al conducted a real-life study in 34 patients with HAE who were either switched to lanadelumab or continued on lanadelumab from the HELP-OLE study to examine the effect of gradual injection intervals extension on disease control and quality of life in symptom-free patients on lanadelumab.47 AECT and AEQoL questionnaires were used to monitor patients’ status and proved that all participants remained adequately controlled and reported improved quality of life, respectively. Furthermore, authors presented a specific protocol for extending injection intervals by increasing the two-week interval by three days at a time, depending on the currently achieved response to lanadelumab and reported that most of their patients reached a treatment interval of 30 days. Therefore, they suggested this method could be used to guide physicians in adapting the therapy to individual patients. Importantly, this strategy has been implemented in the current WAO/EAACI guidelines to reflect the newest evidence and minimize the burden of therapy. Further studies are needed to identify if patients can benefit from further gradual treatment interval extension and whether it could be worthy to retry this strategy of extending dose intervals after once control has been lost and regained. Additionally, future studies could provide information whether STP should be organized differently in patients with a complete response to LTP, as well as whether there are specific biomarkers that could be used to predict the type of response to lanadelumab and by identifying the best responders in advance to ensure the implementation of precision medicine and warrant the rational use of lanadelumab.
More recently, Abuzakouk et al compared the efficacy of standard and extended dosing regimens of lanadelumab in nine patients in a single-center retrospective study and found the effect of lanadelumab in terms of number of angioedema attacks was significant in both extended and standard group at each time point (Months 1, 2, 3 and 9).53 Additionally, they found statistically significant improvement in the number of attacks requiring emergency care and all patients reported a significant quality of life improvement. Therefore, authors concluded that lanadelumab extended dosing regimen was equally effective compared to the standard one and could improve the rational use of the drug.
Duration of Treatment
Currently, there is no clear recommendation on whether patients initiated on LTP should continue the treatment life-long. Larger studies of longer lanadelumab use are ongoing to explore the duration of efficacy and long-term tolerability profile and to define its most appropriate use.
Thus, based on clinical trial evidence and real-life data, use of lanadelumab has been implemented in current guidelines to ensure achievement of complete control and normalized quality of life of HAE patients. Furthermore, recently adopted option of extended four-week regimen could potentially reduce costs for healthcare systems without losing clinical efficacy or raising safety issues.
Expert Commentary and Limits of Knowledge
The evolution of HAE therapies improved survival of HAE patients, but the development of modern LTP options allows patients to live not only longer, but much better by improving their quality of life. This process started the current revolution in HAE management. However, there are still some patient populations that cannot benefit from LTP and some areas of unmet needs that can be further improved.
LTP in Children
Currently, there is no approved LTP option in children younger than six years. The interim results of the Phase 3 SPRING study (NCT04070326) were announced at the European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress 2022, demonstrating positive clinical outcomes with lanadelumab for preventing HAE attacks in patients 2 to <12 years of age.54 The primary endpoint of this open-label, multicenter study was to evaluate the safety and pharmacokinetics of lanadelumab in patients aged 2 to <12 years, whereas prevention of HAE attacks was measured as a secondary endpoint. Results are consistent with earlier studies with adult and adolescent patients and final analyses are highly awaited.
LTP in Pregnant and Lactating Women
Currently data on the use of lanadelumab during pregnancy are not available. Lanadelumab has not been studied in breastfeeding women. The drug represents a large immunoglobulin molecule (molecular weight of approximately 146 000); hence it is unlikely to be secreted through the milk, and even if so: it would probably get destroyed in the infant’s gastrointestinal tract.
LTP in Women of Childbearing Potential
The physiological effects of plasma kallikrein are poorly understood during conception, pregnancy, placentogenesis and organogenesis. The long plasma half-life of the drug (approximately 21 days for 300 mg/sc injection) raises the question should women of childbearing potential (and planning to get pregnant) receive LTP with lanadelumab.
LTP in Comorbid Conditions
As clinical experience grows, the theoretical concern of treating polymorbid patients with lanadelumab stands back. Yet, constant plasma kallikrein inhibition has to demonstrate favorable long-term tolerability, given the attention that this therapy might be considered life-long for a number of patients.
Non-Responders
Currently, there are no established biomarkers available to predict the response to lanadelumab and both primary non-responders and patients who would develop secondary resistance to the drug over time cannot be defined.
Dosage Intervals
The recommended starting dose of lanadelumab is 300 mg every two weeks. However, attack-free patients can extend the dosage intervals and maintain disease control. Further studies must answer how to best tailor treatment regimens as to balance between angioedema control and stakeholders’ public funds. Furthermore, no reports have addressed the possibility to narrow dosage intervals as to achieve disease control in partial responders while maintaining favorable tolerability.
Duration of Therapy
No data is available on how long LTP with lanadelumab should continue. This question has both a socio-economic and a safety aspect. Further studies must be planned as to address it and bring clinical-decision strategies into practice.
Cost-Effectiveness
The cost of lanadelumab is associated with substantial burden on healthcare systems, insurance companies and patients, and could potentially limit its use.
Drugs Accessibility
As modern HAE therapies are particularly expensive, we can address this question as “the elephant in the room”. Novel drugs for prophylaxis of HAE are not available in the majority of countries outside Europe and North America. Future efforts must focus on bringing therapies to more countries and encouraging their accessibility for patients in need.
LTP in the Post-Covid-19 Era
Finally, it is of greatest importance that HAE patients remain well controlled during Covid-19 pandemic as it can pose additional challenges to disease course, treatment and outcomes of both HAE and Covid-19. The pandemic highlighted the importance of maintaining stable disease control and minimizing the need for emergency visits due to angioedema.
Conclusion
Lanadelumab is a breakthrough therapy for the prevention of attacks in HAE type 1 and 2. This revolutionary approach to administer a single subcutaneous injection (once every two to four weeks) and achieve complete disease control has dramatically improved patient care resulting in significant change in the life of affected families. Current data support the drug’s tolerability in adult and adolescent patients without notable safety concerns in both clinical research and real-world settings. Further studies have to investigate whether this therapy might be appropriate also in other forms of angioedema such as idiopathic primary angioedema and HAE with normal C1 inhibitor. Future efforts must focus to improve drug accessibility in more countries and bring modern therapies to patients in need for effective and well-tolerated treatments of HAE.
Although modern prophylactic options lessen the risk of fatal laryngeal attacks, patients must always be equipped with reliable on-demand therapies and be trained how to use them as such a risk cannot be fully diminished with potentially life-threatening attacks occurring even in subjects with successful and stable long-term prophylaxis. Notwithstanding, further studies are needed to identify early responders from non-responders and develop therapies for the latter.
Rational use of prophylactic treatments of HAE searches for a socio-economic balance, taking into account the life-long course of the disease, the public health funds who pay the monetary price, and the patients who might need to receive the therapy for a period longer than investigated during the development program.
Disclosure
Authors declare no conflict of interest in relation to this manuscript preparation. EP has received speaker honoraria/meeting sponsorship from Pharming Group NV and Takeda/Shire. VY have received speaker honoraria/meeting sponsorship from Pharming Group NV. AV has received consultancy/speaker honoraria/meeting sponsorship from, or collaborated in research with, Pharming Group NV, Takeda/Shire, Sobi, CSL Behring, and Pharvaris. MTS has received consultancy/speaker honoraria/meeting sponsorship from, or collaborated in research with, Pharming Group NV, Takeda/Shire, Sobi, CSL Behring, Kalvista and Pharvaris.
References
1. Cicardi M, Aberer W, Banerji A, et al. Classification, diagnosis, and approach to treatment for angioedema: consensus report from the hereditary angioedema international working group. Allergy. 2014;69:602–616. doi:10.1111/all.12380
2. Bork K, Machnig T, Wulff K, Witzke G, Prusty S, Hardt J. Clinical features of genetically characterized types of hereditary angioedema with normal C1 inhibitor: a systematic review of qualitative evidence. Orphanet J Rare Dis. 2020;15(1):289. doi:10.1186/s13023-020-01570-x
3. Maurer M, Magerl M, Betschel S, et al. The international WAO/EAACI guideline for the management of hereditary angioedema - The 2021 revision and update. World Allergy Organ J. 2022;15(3):100627. doi:10.1016/j.waojou.2022.100627
4. Bork K. Diagnosis and treatment of hereditary angioedema with normal C1 inhibitor. Allergy Asthma Clin Immunol. 2010;6(1):15. doi:10.1186/1710-1492-6-15
5. Germenis AE, Margaglione M, Pesquero JB, et al. International consensus on the use of genetics in the management of hereditary angioedema. J Allergy Clin Immunol Pract. 2020;8(3):901–911. doi:10.1016/j.jaip.2019.10.004
6. Bernstein JA. Severity of hereditary angioedema, prevalence, and diagnostic considerations. Am J Manag Care. 2018;24(14 Suppl):S292–S298.
7. Bork K, Davis-Lorton M. Overview of hereditary angioedema caused by C1-inhibitor deficiency: assessment and clinical management. Eur Ann Allergy Clin Immunol. 2013;45:7–16.
8. Gábos G, Dobru D, Mihály E, et al. Recurrent ascites: a need to evaluate for hereditary angio-oedema. Lancet. 2017;390(10107):2119–2120.
9. Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1-INH deficiency. J Allergy Clin Immunol. 2012;130(3):692–697. doi:10.1016/j.jaci.2012.05.055
10. Zuraw BL. The pathophysiology of hereditary angioedema. World Allergy Organ J. 2010;3(9 Suppl):S25–8. doi:10.1097/WOX.0b013e3181f3f21c
11. Kajdácsi E, Jandrasics Z, Veszeli N, et al. Patterns of C1-inhibitor/plasma serine protease complexes in healthy humans and in hereditary angioedema patients. Front Immunol. 2020;11:794. doi:10.3389/fimmu.2020.00794
12. Banday AZ, Kaur A, Jindal AK, Rawat A, Singh S. An update on the genetics and pathogenesis of hereditary angioedema. Genes Dis. 2020;7(1):75–83. doi:10.1016/j.gendis.2019.07.002
13. Zuraw BL, Christiansen SC. HAE pathophysiology and underlying mechanisms. Clin Rev Allergy Immunol. 2016;51(2):216–229. doi:10.1007/s12016-016-8561-8
14. Busse P, Kaplan A. Specific targeting of plasma kallikrein for treatment of hereditary angioedema: a revolutionary decade. J Allergy Clin Immunol Pract. 2022;10(3):716–722. doi:10.1016/j.jaip.2021.11.011
15. Kaplan AP, Joseph K. The bradykinin-forming cascade and its role in hereditary angioedema. Ann Allergy Asthma Immunol. 2010;104(3):193–204. doi:10.1016/j.anai.2010.01.007
16. Lumry WR, Settipane RA. Hereditary angioedema: epidemiology and burden of disease. Allergy Asthma Proc. 2020;41(Suppl 1):S08–S13. doi:10.2500/aap.2020.41.200050
17. Bygum A, Aygören-Pürsün E, Beusterien K, et al. Burden of illness in hereditary angioedema: a conceptual model. Acta Derm Venereol. 2015;95(6):706–710. doi:10.2340/00015555-2014
18. Moldovan D, Bara N, Nădășan V, Gábos G, Mihály E. Consequences of misdiagnosed and mismanaged hereditary angioedema laryngeal attacks: an overview of cases from the Romanian registry. Case Rep Emerg Med. 2018;2018:6363787. doi:10.1155/2018/6363787
19. Savarese L, Mormile I, Bova M, et al. Psychology and hereditary angioedema: a systematic review. Allergy Asthma Proc. 2021;42(1):e1–e7. doi:10.2500/aap.2021.42.200073
20. Fouche AS, Saunders EFH, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2014;112(4):371–375. doi:10.1016/j.anai.2013.05.028
21. Aygören-Pürsün E, Bygum A, Beusterien K, et al. Socioeconomic burden of hereditary angioedema: results from the hereditary angioedema burden of illness study in Europe. Orphanet J Rare Dis. 2014;9(1):99. doi:10.1186/1750-1172-9-99
22. Busse PJ, Christiansen SC, Riedl MA, et al. US HAEA medical advisory board 2020 guidelines for the management of hereditary angioedema. J Allergy Clin Immunol Pract. 2021;9(1):132–150.e3. doi:10.1016/j.jaip.2020.08.046
23. Jindal AK, Reshef A, Longhurst H; GEHM workgroup (Global Equity in HAE Management). Mitigating disparity in health-care resources between countries for management of hereditary angioedema. Clin Rev Allergy Immunol. 2021;61(1):84–97. doi:10.1007/s12016-021-08854-5
24. Cicardi M, Bork K, Caballero T, et al. Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an international working group. Eur Ann Allergy Clin Immunol. 2012;67:147–157. doi:10.1111/j.1398-9995.2011.02751.x
25. Caballero T. Treatment of Hereditary Angioedema. J Investig Allergol Clin Immunol. 2021;31(1):1–16. doi:10.18176/jiaci.0653
26. Banerji A, Anderson J, Johnston DT. Optimal management of hereditary angioedema: shared decision-making. J Asthma Allergy. 2021;14:119–125. doi:10.2147/JAA.S284029
27. Cicardi M, Levy RJ, McNeil DL, et al. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med. 2010;363(6):523–531. doi:10.1056/NEJMoa0905079
28. Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 2010;363(6):513–522. doi:10.1056/NEJMoa0805538
29. Cicardi M, Banerji A, Bracho F, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010;363(6):532–541. doi:10.1056/NEJMoa0906393
30. Farkas H, Zotter Z, Csuka D, et al. Short-term prophylaxis in hereditary angioedema due to deficiency of the C1-inhibitor--a long-term survey. Allergy. 2012;67(12):1586–1593. doi:10.1111/all.12032
31. Magerl M, Frank M, Lumry W, et al. Short-term prophylactic use of C1-inhibitor concentrate in hereditary angioedema: findings from an international patient registry. Ann Allergy Asthma Immunol. 2017;118(1):110–112. doi:10.1016/j.anai.2016.10.006
32. Valerieva A, Staevska M, Jesenak M, et al. Recombinant human C1 esterase inhibitor as short-term prophylaxis in patients with hereditary angioedema. J Allergy Clin Immunol Pract. 2020;8(2):799–802. doi:10.1016/j.jaip.2019.08.011
33. Anderson J, Maina N. Reviewing clinical considerations and guideline recommendations of C1 inhibitor prophylaxis for hereditary angioedema. Clin Transl Allergy. 2022;12(1):e12092. doi:10.1002/clt2.12092
34. Chyung Y, Vince B, Iarrobino R, et al. A Phase 1 study investigating DX-2930 in healthy subjects. Ann Allergy Asthma Immunol. 2014;113(4):460–6.e2. doi:10.1016/j.anai.2014.05.028
35. Lanadelumab. Summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/takhzyro-epar-product-information_en.pdf.
36. Banerji A, Bernstein JA, Johnston DT, et al. Long-term prevention of hereditary angioedema attacks with lanadelumab: the HELP OLE Study. Allergy. 2022;77(3):979–990. doi:10.1111/all.15011
37. Banerji A, Riedl MA, Bernstein JA, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108–2121. doi:10.1001/jama.2018.16773
38. Banerji A, Busse P, Shennak M, et al. Inhibiting plasma kallikrein for hereditary angioedema prophylaxis. N Engl J Med. 2017;376(8):717–728. doi:10.1056/NEJMoa1605767
39. Lumry WR, Weller K, Magerl M, et al. Impact of lanadelumab on health-related quality of life in patients with hereditary angioedema in the HELP study. Allergy. 2021;76(4):1188–1198. doi:10.1111/all.14680
40. Giannattasio GBEBJ. Self-controlled study evaluation HAE attack rates with lanadelumab use in the US and Canada: EMPOWER study design; 2020.
41. Martinez-Saguer IKTMM. Evaluation of long-term effectiveness of lanadelumab for hereditary angioedema in real-world clinical practice: design of the ENABLE study; 2020.
42. Johnston D, Anderson J, Brouwer E, et al. Long-term effectiveness and safety of lanadelumab in the US and Canada: findings from the EMPOWER study. J Allergy Clin Immunol. 2022;149(2):AB165. doi:10.1016/j.jaci.2021.12.548
43. Lumry WAJSD Attack Rate Reduction After Switching to Lanadelumab Therapy in Patients with Hereditary Angioedema (HAE): interim Findings from EMPOWER; 2022.
44. Recke A, Spadaro GGJ. Lanadelumab treatment patterns among patients with HAE-C1-INH: interim analysis of the ENABLE study; 2022.
45. Martinez-Saguer IMMAT. Patient-reported outcomes (PROs) in patients with hereditary angioedema receiving lanadelumab: interim findings from the ENABLE study; 2020.
46. Bouillet L. Effectiveness of lanadelumab in the real-world setting: findings from a temporary authorization of use (ATU) in France for the treatment of hereditary angioedema type 1/2. Abstract. Allergy. 2020;75(S109):120–274. doi:10.1111/all.14506
47. Buttgereit T, Vera C, Weller K, et al. Lanadelumab efficacy, safety, and injection interval extension in HAE: a real-life study. J Allergy Clin Immunol Pract. 2021;9(10):3744–3751. doi:10.1016/j.jaip.2021.04.072
48. Riedl MA, Bernstein JA, Craig T, et al. An open-label study to evaluate the long-term safety and efficacy of lanadelumab for prevention of attacks in hereditary angioedema: design of the HELP study extension. Clin Transl Allergy. 2017;7(1):36. doi:10.1186/s13601-017-0172-9
49. Hahn J, Trainotti S, Wigand M, Schuler P, Hoffmann T, Greve J. Prospective analysis in patients with HAE under prophylaxis with lanadelumab: a real-life experience. J Drugs Dermatol. 2020;19(10):978–983. doi:10.36849/JDD.2020.5269
50. Dorr ADCCCT. Lanadelumab for the prevention of hereditary angio- oedema attacks: a real world UK perspective. Allergy. 2021;76(S110):638–661. doi:10.1111/all.15098
51. US FDA. Adverse event reporting system (FAERS) Public dashboard. Available from: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard.
52. Conference of Experts on the Rational Use of Drugs (1985: Nairobi) C. The rational use of drugs: report of the conference of experts, Nairobi, 25–29 November 1985; 1987: 329 p.
53. Abuzakouk M, Ghorab O, Al-Hameli H, Salvo F, Grandon D, Maurer M. Using an extended treatment regimen of lanadelumab in the prophylaxis of hereditary angioedema: a single-centre experience. World Allergy Org J. 2022;15(7):100664. doi:10.1016/j.waojou.2022.100664
54. Takeda press release. Available from: https://www.takeda.com/newsroom/newsreleases/2022/phase-3-spring-study-data-presented-at-eaaci/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.