Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Safety and Performance of Etermis 3 and 4 in Wrinkles/Folds Treatment and Facial Volume Enhancement: A Prospective, Evaluator-Blinded, Open-Label Study
Authors Sattler G, Kerscher M , Noah EM , Prager W, Fischer TC, Ogilvie P , Hofmann M, Dersch H , Odena G
Received 3 January 2020
Accepted for publication 4 August 2020
Published 19 August 2020 Volume 2020:13 Pages 591—599
DOI https://doi.org/10.2147/CCID.S244598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Gerhard Sattler,1 Martina Kerscher,2 Ernst Magnus Noah,3 Welf Prager,4 Tanja C Fischer,5 Patricia Ogilvie,6 Matthias Hofmann,7 Hanna Dersch,7 Gemma Odena8
1Rosenparkklinik GmbH, Darmstadt, Germany; 2Division of Biochemistry and Molecular Biology, Cosmetic Science, University of Hamburg, Hamburg, Germany; 3Department of Plastic, Reconstructive, Aesthetic and Hand Surgery, Kassel, Germany; 4Prager & Partner Dermatologische Praxis, Hamburg, Germany; 5Haut- Und Lasercentrum Berlin-Potsdam, Potsdam, Germany; 6SkinConcept, Munich, Germany; 7Merz Pharmaceuticals GmbH, Frankfurt Am Main, Germany; 8Merz North America, Inc., Raleigh, NC, USA
Correspondence: Gerhard Sattler Email [email protected]
Background: Facial aging is characterized by increased prominence of nasolabial folds (NLFs), marionette lines, and thinning of the lips. Cross-linked hyaluronic acid injection is a very effective method for the temporary correction of these areas.
Objective: To confirm the clinical performance and the safety of Etermis 3 (ET3) and/or Etermis 4 (ET4) in the treatment of moderate and severe wrinkles/folds, as well as lip volume enhancement.
Methods: Subjects were treated in at least two facial areas (NLFs, marionette lines, lips). ET3 was used in facial moderate wrinkles while ET4 was used in severe facial skin volume loss. An optional touch-up 1 month after treatment was possible. A blinded investigator assessed improvement on Merz Aesthetic Scales (MAS). Subjects were followed-up for 12 months after the last treatment.
Results: In total, 154 healthy subjects were enrolled. The proportion of subjects achieving ≥ 1 score improvement in MAS after treatment was above 60% for ET4 (Month 6/7: NLFs 94.9% and marionette lines 81.4%, p≤ 0.0004; Month 3/4: lips 63.0%, p=0.39) and ET3 (Month 6/7: marionette lines 79.4%, p=0.0005; Month 3/4: lips 65.5%, p=0.31). Facial improvement was still visible at Month 12/13 for ET4 (NLFs ≥ 76.6%, marionette lines ≥ 61%, lips ≥ 36%) and ET3 (marionette lines ≥ 50% and lips ≥ 21.9%). No treatment-related serious AEs occurred. The most frequent AEs were injection-site reactions.
Conclusion: Etermis 3 and Etermis 4 demonstrated good clinical performance and safety for NLFs and marionette lines volume enhancement for up to 12 months. Both products can also be used safely to treat lips for volume augmentation.
Keywords: hyaluronic acid, wrinkles, facial volume enhancement
Introduction
The skin aging process is a complex interplay of intrinsic and extrinsic factors across multiple layers of the face.1 Changes in the skin are caused by decreased synthesis of collagen and elastin as well as their increased degradation, decreased synthesis of Hyaluronic Acid (HA), reduced proliferative capacity of fibroblasts and perturbations in the organization of elastic fiber network.2 Facial aging is characterized by transverse forehead lines which become prominent and can be accompanied by lowering of the eyebrows, increase prominence of the nasolabial folds (NLFs), vertical rhytides in the perioral area, ptosis of the oral commissures, thinning of the lips and flattening of the upper lip. Marionette lines may develop in the form of a labiomandibular grove and a prejowl depression (marionette lines).3
Injectable implants are medical device implants that are employed for helping to create a smoother and/or fuller appearance in the face, when injected notably in NLFs, cheeks and lips, and other facial areas.4–8 Among these, cross-linked HA fillers have been used successfully for many years for the temporary correction of deeper lines running from the corner of the nose to the corner of the mouth (nasolabial folds, NLFs), for lines running from the corner of the mouth to the chin (marionette lines), radial upper lip wrinkles or to augment the lips or enhance lip fullness.9–15 Because of their identity to the body’s own HA, commercially available HA fillers rarely cause allergic and immunological reactions.16
Etermis fillers are sterile non-pyrogenic physiological gels made of reticulated HA of non-animal origin, that use butanediol diglycidyl ether (BDDE) as a cross-linking agent and contain mannitol. Etermis products are indicated for filling of facial NLFs and marionette lines as well as for an increase of lip volume but differ on their HA concentration presented in a pre-filled, graduated single-use syringe. While Etermis 3 (ET3; 23 mg/mL HA) is indicated for filling moderate wrinkles, Etermis 4 (ET4; 24 mg/mL HA) is indicated for filling deep and severe wrinkles.
The use of both products in different indications can be further explained by their distinct elastic modulus G′ value ranges, 70–140 Pa for ET3 and 140–260 Pa for ET4. The elastic modulus is a measure of firmness of injectable implants, describing their resistance to deformation.13 It is known that intermediate G′ products are a more effective solution for NLFs and marionette lines.17 High G’ products may be better suited for corrective needs where deep, targeted product deposition and less distribution are necessary to achieve lift and projection such as in moderate to severe NLFs.13,17
Both Etermis fillers are intended to be injected into the mid to deep dermis of the face or into the lips.
Studies analyzing the long-term safety and performance of dermal fillers are scarce and the clinical data supporting the use of ET3 and ET4 in NLFs, lips, and marionette lines are limited. This open-label, multicenter, evaluator-blinded, post-market clinical follow-up clinical study was performed to confirm the clinical performance and the safety of ET3 and/or ET4 in the treatment of moderate and severe wrinkles/folds, as well as lip volume enhancement, during a 12-month period.
Methods
Study Design
The study was a 14-month, open-label, observational, multi-center, post-market clinical follow-up trial, conducted in compliance with the International Conference on Harmonization (ICH), EN ISO 14,155, and Good Clinical Practice (GCP) principles. The trial was approved by the ethical committee of the Landesärztekammer Hessen and complied with local regulatory authority requirements. Following the guidelines of the relevant German Medical Device law §23b, it was only necessary to receive an ethics approval for the study. The study was also registered at http://www.clinicaltrals.gov (NCT04210258).
Participants
Subjects were recruited at six sites in Germany. In order to be included in the study, subjects must have been healthy male or non-pregnant female ≥18 years old. Written informed consent was obtained from subjects who wished to receive a HA dermal filler treatment to correct at least two of the following regions: NLFs, marionette lines, upper and lower lip fullness. Specific inclusion criteria are described in Table 1.
 |
Table 1 Specific Inclusion Criteria |
Exclusion criteria included the receipt of any kind of dermal filler or botulinum toxin injection or surgery in the region (mid and lower face) to be treated prior and during the study. Subjects who wished to be treated with laser treatment, chemical peeling or dermabrasion in immediate association with the study procedures were also excluded. Subjects who wished to receive treatment with ET3 on the NLFs were excluded from this study as the performance of ET3 in this indication has been shown in previous studies.
Treatments Administered
All subjects received a single treatment on two to three facial sites with needle injections (primarily 27G 1/2 for ET3 and 27 G 1/2 and/or 25 G 1/2 for ET4) in the mid to deep dermis of the NLFs, marionette lines, and/or upper/lower lips. Subjects could receive a single optional touch-up 1 month after treatment for one or all indications if agreed upon the subject and the investigator. The choice of injection technique was at left at the discretion of the investigators, and included serial puncture, linear threading, parallel lines, cross-hatching and fanning techniques. Prior to the injection’s subjects were allowed to receive topical anesthetics agents, eg topical lidocaine and prilocaine cream, at the discretion of the investigators. After treatment and a potential touch-up visit, study subjects returned to the investigator’s site for five follow-up visits to evaluate long-term safety and durability of the treatment. The study visit schedule was as follows: Day 0 (baseline + treatment), Month 1 ± 7 days (M1), Month 3 ± 14 days (M3), Month 6 ± 14 days (M6), Month 9 ± 14 days (M9), and Month 12 ± 28 days (M12).
In case of an optional touch-up during the M1 visit, subjects were required to present for an additional follow-up visit at M2 and all subsequent follow-up visits were shifted by 1 month, as follows: Day 0 (baseline + treatment), M1 ± 7 days (M1 follow-up visit and optional touch-up treatment), Month 2 ± 7 days (M2), Month 4 ± 14 days (M4), Month 7 ± 14 days (M7), Month 10 ± 14 days (M10), and Month 13 ± 28 days (M13).
Outcomes
The primary endpoints of the study were to determine the responder rate based on a live blinded investigator’s assessment at each site, after treatment from Day 0 pre-injection to: M3/4 visit for the lips, M6/7 visit for marionette lines, and M6/7 visit for NLFs, depending on touch-up. Each marionette line (left, right), NLF (left, right) and lip (upper and lower) were rated independently. For each indication and product, subjects with ≥1-point improvement on the Merz Aesthetics Scales (MAS) from Day 0 pre-injection to each evaluated visit were considered as responders. For each indication, the worse rating of the two evaluated sides (left or right; upper or lower) was used for classification. MAS used in this study were “Nasolabial folds – At Rest”, “Marionette lines – At Rest” and “Upper and Lower lip fullness – At Rest”. The blinded evaluator at each site had no access to any study data and assessed all three MAS used in the study regardless of the treatment administered to ensure blinding was maintained.
Secondary endpoints, from Day 0 pre-injection to all other visits, included: changes in live blinded investigator’s assessment per treated indication and per product according to the appropriate MAS for each indication; subject’s satisfaction on the Global Impression of Change scale (GICS); and blinded investigator’s satisfaction on the Global Aesthetic Improvement Scale (GAIS). Investigator’s satisfaction was evaluated through an investigator’s satisfaction questionnaire administered to each site after all injections were completed.
Safety Assessments
Subject’s major/relevant medical history, previous cosmetic procedures on the face, and concomitant treatments to assess any potential interactions were collected. Occurrence of adverse events (serious or not), adverse device effects, and/or incidents potentially related or not to the injection/use of injectable devices were assessed at each study visit.
Sample Size and Statistical Analysis
Forty-five subjects per treatment and per indication were required to show a significant result, with a power of 80% and a significance level (α) of 2.5%. Because subjects were to be treated in at least two different indications, a minimum of 70 subjects for ET3 and 100 subjects for ET4 were required.
Safety analyses were performed on the safety evaluation set (SES; all subjects who received at least one injection). AEs were coded according to the Medical Dictionary for Regulatory Activities version 20.1. Only treatment emergent AEs were analyzed, ie, AEs with onset/worsening after the first injection up to and including the end of study visit.
Performance analyses were based on the full analysis set (FAS; all subjects who have at least one primary performance assessment at M3/4).
The aim of primary variable analyses were to show that there were significantly more than 60% of responders for each primary variable on the respective MAS, tested in an exploratory fashion, using the one-sided exact binomial test on a significance level of 2.5% separately for product and indication.
Results
Subject Demographics
The study was conducted at six sites in Germany and 154 subjects were treated analyzed for safety (Table 2). Mean age (standard deviation, SD) of study subjects was 52.3 (9.8) years. Most subjects were female (97.4%) and identified their race as “White” (99.4%), as described in Table 3.
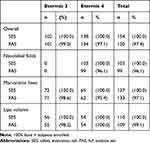 |
Table 2 Disposition of Subjects |
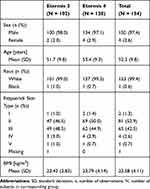 |
Table 3 Baseline Demographic Characteristics for All Subjects Receiving Study Treatment (Safety Evaluation Set, N = 154) |
Performance was analyzed in 150 subjects, of which 101 were treated with ET3 and 134 received ET4 (Table 2). NLFs were only treated with ET4 (n = 99). Lips and marionette lines were treated either with ET3 or ET4. A total of 109 subjects were treated in the lips – 55 subjects received ET3 and 54 subjects received ET4. For the marionette lines indication 133 subjects received treatment, whereof 71 subjects were treated with ET3 and 62 with ET4.
Extent of Exposure
Injection volumes are provided in Table 4. During treatment, the mean injected volume of ET3 was 1.47 mL in marionette lines (right and left), and 1.20 mL in lips (upper and lower). The mean injected volume of ET4 was 1.59 mL in marionette lines (right and left) and 1.02 mL in lips (lower and upper). NLFs (left and right) were only treated with ET4, where the mean injected volume was 1.84 mL. A single touch-up was allowed for all indications. A total of 22 (30.9%) subjects and 16 (29.1%) received a touch-up with ET3 in marionette lines and lips, respectively. Similarly, 33 (33.5%) subjects, 18 (29.0%) subjects and 14 (25.9%) subjects received a touch-up with ET4 in NLFs, marionette lines and lips, respectively.
 |
Table 4 Injection Data (Volume in mL) |
Clinical Performance
The responder rate (≥1-point improvement on the MAS) at M6/M7 for the treatment of NLFs with ET4 was 94.9%, which was significantly above 60% (p<0.0001; Figure 1). Blinded investigators assessments showed that facial improvement was still visible at M9/M10 visits (83.2% right and left NLFs) and at M12/M13 visits (77.9% right and 76.6% left NLF, respectively), as shown in Figure 2.
The responder rate at M6/M7 visit for treatment of marionette lines with ET3 was 79.4%, which was significantly above 60% (p=0.0005; Figure 1). Also, the responder rate of 81.4% in the same indication treated with ET4 was significantly above 60% (p=0.0004; Figure 1). Improvement was still visible at M12/13 visit for subjects treated with ET3 (50.0% left and 57.8% right marionette lines, respectively) and with ET4 (66.1% left and 61.0% right marionette lines, respectively), as shown in blinded investigators assessments (Figure 3).
The responder rate at M3/M4 visit for lips treatment was above 60% with 65.5% for ET3 and with 63.0% for ET4 (Figure 1). However, these results were not statistically significant (p= 0.3111 and p= 0.3834 for ET3 and ET4, respectively). ET4 effect was better sustained in time at M12/13 (45.9% upper lips and 36% lower lips) than ET3 (21.95 upper lips and 28% lower lips), as shown in blinded investigators assessments (Figure 4).
Mean changes (Mean, SD) from baseline on MAS “Marionette lines – At Rest” were highest at M2 for both ET3 (left line −1.5, 0.6; right line −1.4, 0.7) and ET4 (left line −1.8, 0.7; left line −1.8, 0.8). For MAS “Upper and Lower lip fullness – At Rest”, mean changes from baseline after treatment were highest at M3/4 for ET3 (lower lip: 0.8; 0.6; upper lip: 0.9; 0.6) and at M2 for ET4 (lower lip: 1.1, 0.6; upper lip: 1.0, 0.7). Mean changes from baseline in MAS “Nasolabial – At Rest” were highest at M2 for ET4 (left fold: −1.9, 0.7; left fold: −1.9, 0.6).
Clinical performance of ET3 and ET4 was also evaluated based on GAIS assessment by a blinded investigator´s live rating. At the M1 visit, 98% of subjects were rated as “Improved” to “Very much improved” (Figure 5). Up to 10 months after the initial treatment, blinded investigators continued to rate most subjects as having at least an “Improved” appearance according to GAIS.
Subject-rated satisfaction with their overall appearance on the GICS scale (rating of “Improved” to “Very much improved”) was 98.0% at the M1 visit and 96.6% at the M2 visit. These values indicated a high satisfaction with treatment results and following a similar trend to the investigator GAIS results. Beginning with the M3/M4 visit, subject’s satisfaction with their appearance slightly decreased over time (Figure 5).
All investigators (N = 6) in this study reported that ET3 and ET4 were easy, fairly easy, or very easy to inject, and all investigators were willing to use Etermis for future treatment of subjects (Figure 6).
Safety
Overall, 121 subjects (78.6%) experienced a total of 294 AEs. None of the AEs led to study discontinuation, nor were any fatal AEs reported. A total of three subjects suffered four serious AEs (Muscle strain; Radius fracture; Tibia fracture; and Papillary cystadenoma lymphomatosum), none of which were related to treatment.
Regarding adverse device effects, 95 subjects (61.7%) experienced 181 AEs related to treatment. Nearly all related AEs were injection-site reactions (Table 5). For all ET3 indications, 64 subjects (62.7%) experienced 119 AEs related to treatment. The most frequent AEs related to ET3 treatment were injection-site hematomas and injection-site swelling (Table 6). For all ET4 indications, 90 subjects (65.2%) experienced 174 AEs related to treatment. The most frequent AEs related to ET4 treatment were injection-site hematomas, injection-site swelling, and injection-site bruising (Table 7). The overall worst AE intensity was reported as mild or moderate for all AEs related to treatment.
 |
Table 5 Summary of Adverse Events Related to Device or Injection (Safety Evaluation Set, N = 154) |
 |
Table 6 Summary of Adverse Events Related to Etermis 3 (Safety Evaluation Set, N = 102) |
 |
Table 7 Summary of Adverse Events Related to Etermis 4 (Safety Evaluation Set, N = 138) |
Discussion
Overall, the results of the study show that treatment with ET3 for moderate wrinkles and/or ET4 for severe wrinkles within the defined indications has a positive effect on facial appearance for marionette lines and NLFs and is well tolerated by subjects.
Before this study, only limited clinical data supporting the use of ET3 and ET4 in NLFs and marionette lines were available.18,19 No previous clinical data supporting the use of ET3 and ET4 to increase lip volume were identified.
The results of the current study support and prove the good clinical performance and safety of ET3 and ET4 to fill NLFs and marionette lines and it adds first clinical data for ET3 and ET4 in the treatment of lip volume augmentation. Aesthetic outcomes were similar between ET3 and ET4. Clinical performance evaluations indicated that both were effective in treating marionette lines and NLFs. High responder rates were reported at M6/7 and remained substantial at M 12/13, which indicate long-term aesthetic improvement by both products. The study results point toward a longer effect duration of ET4, when compared to ET3, for marionette lines; this observation may be attributed to the higher content of cross-linked hyaluronic acid in ET4.
Furthermore, results indicate that the majority of subjects treated for lip volume augmentation benefitted from lip treatment while showing good tolerability, even if the performance results were not statistically significant. Moreover, lip volume improvement remained recognizable after 1 year of the initial treatment for several subjects.
GICS and GAIS analyses both showed similar levels of satisfaction and supported the above results, showing overall improved appearance and a long-lasting effect for the majority of subjects. Subjects and investigators were satisfied with treatment results for NLFs and marionette lines. In addition, investigators were satisfied with the ease of injection for ET3 and ET4 and were willing to use both products for future treatments.
The AEs observed during the study for ET3 and ET4 for all treated indications were similar to the expected safety and risk profiles of both products and comparator products.18–20 Although ET3 and ET4 are lidocaine-free, only 3.2% of treated subjects indicated injection-site pain as an adverse event. Also, only one event of injection-site-related erythema was reported. This shows that the use of a topical numbing agent prior dermal filler injection can enhance the subject comfort by reducing injection-site pain and injection-site redness. In lidocaine containing dermal fillers, the injected lidocaine, a vasodilator, may lead to additional redness due to the dilation of blood vessels.
Injection of both products was well tolerated by subjects, resulting in no unexpected or serious injection-site-related adverse events.
The study had an open-label design that allowed evaluating the performance and safety of ET3 and ET4 when used according to the currently approved instructions for use. Importantly, as neither of the products contains lidocaine, ET3 and ET4 are of high value for potential subjects with known lidocaine hypersensitivity who seek aesthetic treatment.
ET3 and ET4 demonstrated good clinical performance and safety for NLFs and marionette lines volume enhancement for up to 12 months. Both products can also be used safely to treat lips for volume augmentation, with lip volume augmentation still visible up to 12 months after treatment.
Abbreviations
AE, adverse event; ET3, Etermis 3; ET4, Etermis 4; FAS, full analysis set; GAIS, Global Aesthetic Improvement Scale; GICS, Global Impression of Change scale; GCP, Good Clinical Practice; HA, hyaluronic acid; ICH, International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use; IFU, instructions for use; MAS, Merz Aesthetic Scales; M, Month; NLFs, nasolabial folds; SAE, serious adverse event; SD, standard deviation; SES, safety evaluation set.
Data Sharing Statement
The data that support the findings of this study are available upon reasonable request. Any data the authors intend to share will be deidentified patient data. Please contact Dr. Matthias Hofmann ([email protected]) for data inquiries.
Disclosure
Dr. Hofmann and Ms. Dersch are employees of Merz Pharmaceuticals GmbH. Dr. Odena is an employee of Merz North America, Inc. Dr Fischer reports grants from MERZ Aesthetics, during the conduct of the study. Professor Kerscher reports grants from Merz Pharmaceuticals, Ipsen, and Galderma/Qmed, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Carruthers J, Burgess C, Day D, et al. Consensus recommendations for combined aesthetic interventions in the face using botulinum toxin, fillers, and energy-based devices. Dermatol Surg. 2016;42(5):586–597. doi:10.1097/DSS.0000000000000754
2. Baspeyras M, Rouvrais C, Liegard L, et al. Clinical and biometrological efficacy of a hyaluronic acid-based mesotherapy product: a randomised controlled study. Arch Dermatol Res. 2013;305(8):673–682. doi:10.1007/s00403-013-1360-7
3. Matarasso SL, Carruthers JD, Jewell ML. Consensus recommendations for soft-tissue augmentation with nonanimal stabilized hyaluronic acid (Restylane). Plast Reconstr Surg. 2006;117(3 Suppl):
4. Joseph JH. The case for synthetic injectables. Facial Plast Surg Clin North Am. 2015;23(4):433–445. doi:10.1016/j.fsc.2015.07.003
5. Bass LS. Injectable filler techniques for facial rejuvenation, volumization, and augmentation. Facial Plast Surg Clin North Am. 2015;23(4):479–488. doi:10.1016/j.fsc.2015.07.004
6. Rho NK, Chang YY, Chao YY, et al. Consensus recommendations for optimal augmentation of the asian face with hyaluronic acid and calcium hydroxylapatite fillers. Plast Reconstr Surg. 2015;136(5):940–956. doi:10.1097/PRS.0000000000001706
7. Mannino GN, Lipner SR. Current concepts in lip augmentation. Cutis. 2016;98(5):325–329.
8. Wu WT, Liew S, Chan HH, et al. Consensus on current injectable treatment strategies in the Asian face. Aesthetic Plast Surg. 2016;40(2):202–214. doi:10.1007/s00266-016-0608-y
9. Carruthers JD, Glogau RG, Blitzer A. Advances in facial rejuvenation: botulinum toxin type a, hyaluronic acid dermal fillers, and combination therapies–consensus recommendations. Plast Reconstr Surg. 2008;121(5 Suppl):
10. Cohen JL, Dayan SH, Brandt FS, et al. Systematic review of clinical trials of small- and large-gel-particle hyaluronic acid injectable fillers for aesthetic soft tissue augmentation. Dermatol Surg. 2013;39(2):205–231. doi:10.1111/dsu.12036
11. Hanke CW, Rohrich RJ, Busso M, et al. Facial soft-tissue fillers conference: assessing the state of the science. J Am Acad Dermatol. 2011;64(4Suppl):
12. Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23(4):423–432. doi:10.1016/j.fsc.2015.07.002
13. Gutowski KA. Hyaluronic acid fillers: science and clinical uses. Clin Plast Surg. 2016;43(3):489–496. doi:10.1016/j.cps.2016.03.016
14. Pascali M, Quarato D, Carinci F. Filling procedures for lip and perioral rejuvenation: a systematic review. Rejuvenation Res. 2018;21(6):553–559. doi:10.1089/rej.2017.1941
15. Sundaram H, Liew S, Signorini M, et al. Global aesthetics consensus: hyaluronic acid fillers and botulinum toxin type A-recommendations for combined treatment and optimizing outcomes in diverse patient populations. Plast Reconstr Surg. 2016;137(5):1410–1423. doi:10.1097/PRS.0000000000002119
16. Philipp-Dormston WG, Bergfeld D, Sommer BM, et al. Consensus statement on prevention and management of adverse effects following rejuvenation procedures with hyaluronic acid-based fillers. J Eur Acad Dermatol Venereol. 2017;31(7):1088–1095. doi:10.1111/jdv.14295
17. Fagien S, Bertucci V, von Grote E, Mashburn JH. Rheologic and physicochemical properties used to differentiate injectable hyaluronic acid filler products. Plast Reconstr Surg. 2019;143(4):707e–20e. doi:10.1097/PRS.0000000000005429
18. Kim BW, Moon IJ, Yun WJ, et al. A randomized, evaluator-blinded, split-face comparison study of the efficacy and safety of a novel mannitol containing monophasic hyaluronic acid dermal filler for the treatment of moderate to severe nasolabial folds. Ann Dermatol. 2016;28(3):297–303. doi:10.5021/ad.2016.28.3.297
19. Turlier V, Rouquier A, Black D, et al. Assessment of the clinical efficacy of a hyaluronic acid-based deep wrinkle filler using new instrumental methods. J Cosmet Laser Ther. 2010;12(4):195–202. doi:10.3109/14764172.2010.502461
20. Ramos ESM, Fonteles LA, Lagalhard CS, Fucci-da-Costa AP. STYLAGE(R): a range of hyaluronic acid dermal fillers containing mannitol. Physical properties and review of the literature. Clin Cosmet Investig Dermatol. 2013;6:257–261. doi:10.2147/CCID.S35251
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






