Back to Journals » Drug Design, Development and Therapy » Volume 14
Safety and Feasibility of Low-Dose Apatinib Combined with S-1 as the Second-Line Therapy or Beyond in Chinese Patients with Pulmonary and Hepatic Metastasis of Nasopharyngeal Carcinoma
Authors Zhou L, Lin J, Wu G, Chen J , Huang X , Zhang S
Received 29 December 2019
Accepted for publication 18 March 2020
Published 30 March 2020 Volume 2020:14 Pages 1257—1262
DOI https://doi.org/10.2147/DDDT.S244102
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Liya Zhou,* Jie Lin,* Gang Wu, Jiawei Chen, Xiaopeng Huang, Shuai Zhang
Department of Radiation Oncology, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, Hainan Province 570311, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaopeng Huang
Department of Radiation Oncology, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, Hainan Province 570311, People’s Republic of China
Tel + 86-18976772979
Email [email protected]
Shuai Zhang
Department of Radiation Oncology, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, Hainan Province 570311, People’s Republic of China
Tel + 86-13876428968
Email [email protected]
Introduction: The purpose of this study was to analyze the safety and feasibility of low-dose apatinib combined with S-1 as a second-line therapy or beyond in Chinese patients with pulmonary and/or hepatic metastases of nasopharyngeal carcinoma (NPC).
Methods: Forty-one Chinese NPC patients with pulmonary and hepatic metastases were treated with low-dose apatinib plus S-1. The S-1 dose was determined according to each patient’s body surface area (BSA): 40 mg twice a day for BSA < 1.25 m2; 50 mg twice a day for 1.25 m2≤BSA < 1.5 m2; and 60 mg twice a day for BSA ≥ 1.5 m2. S-1 was received for 14 days, after stopping for 7 days, given 3 weeks apart. Apatinib, 125 mg was orally administered daily on days 1 through 28 of each 4-week cycle. If the toxicity was not tolerable, the dose of apatinib was reduced to 125 mg every other day.
Results: Treatment efficacy was evaluated in all 41 patients after four courses of chemotherapy. The objective response rate was 34.1%, and the disease control rate was 80.4%. The median progression-free survival was 9.7 months (95% confidence interval, 6.2– 13.8 months), and the median overall survival was 22.1 months (95% confidence interval, 15.1– 28.9 months). The 2-year survival rate was 41.5%. The most common toxicities included loss of appetite in 39.0% of patients, dyslipidemia in 34.1%, hypertension in 31.7%, myelosuppression in 24.4%, fatigue in 21.9%, and hand-foot syndrome in 17.1%. Seven patients received dose adjustment of apatinib due to side effects.
Conclusion: In patients with pulmonary and/or hepatic metastases of NPC, low-dose apatinib plus S-1 yielded an excellent survival benefit, and the toxicities were mild and tolerable.
Keywords: Nasopharyngeal carcinoma, NPC, metastasis, apatinib, S-1, prognosis
Introduction
Nasopharyngeal carcinoma (NPC) is a common head and neck malignancy in eastern and southern China and Southeast Asia. The most common pathological type is non-keratinized. With continuous advancements in radiotherapy technology, specifically intensity-modulated radiation therapy (IMRT) and volume of rotating intensity-modulated radiation therapy (VMRT), the 5-year survival rate of NPC patients has reached over 80%, and the local and regional control rate is over 90%.1 At present, distant metastasis is the most frequent cause of treatment failure, and the lung and liver are the main sites of distant metastasis of NPC. Treatments for pulmonary and hepatic metastatic NPC include chemotherapy, radiotherapy, radioactive seed implantation, radiofrequency ablation, targeted drug delivery and immunotherapy. However, the 2-year survival rates have ranged from only 15.0–34.4%, with median overall survival (OS) times of only 9.0–15.6 months, and the various treatments have yet to yield better results.2 Use of the monoclonal antibody of immune check point PD-1 has resulted in an objective response rate (ORR) to treatment of 20–30% in recurrent or metastatic NPC.3 Thus, the effective rate of immunotherapy alone has remained low, and such treatment is not only expensive but also associated with major adverse reactions. To date, no effective markers have been identified for screening of metastatic NPC cases most likely to response to immunotherapy.
Commonly used chemotherapeutic drugs for metastatic NPC include paclitaxel, docetaxel, albumin paclitaxel, gemcitabine combined with cisplatin, nedaplatin, lobaplatin, fluorouracil, and others. With all of these, tumor resistance eventually occurs. In fact, multidrug resistance (MDR) is the main cause of chemotherapy failure in NPC cases, and the most common cause of death in these patients.4 Therefore, there is an urgent need to develop a more economical and effective treatment with low toxicity.
Apatinib is a novel small molecule receptor tyrosine kinase inhibitor that selectively targets vascular endothelial growth factor receptor-2 (VEGFR-2). Recently, apatinib was show to have satisfactory efficacy against various types of cancer, such as gastric cancer, breast cancer, and NPC.5 At the same time, it was shown to have acceptable toxicities. S-1 is an oral anticancer drug and fluorouracil derivative that can be converted to 5-Fu in vivo, and the advantages of S-1 include its convenient delivery, effectiveness, and mild side effects.6 The aim of the present study was to investigate the safety and efficacy of low-dose apatinib combined with S-1 as second-line therapy or beyond for NPC with pulmonary and/or hepatic metastasis.
Materials and Methods
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, China. Written informed consent was obtained from each patient.
Patients
This retrospective analysis included 41 patients with pulmonary and/or hepatic metastases of NPC in whom first-line or later therapies failed in Hainan General Hospital from January 2015 to February 2017. The inclusion criteria were as follows: age ≥18 years, definite pathological diagnosis, Karnofsky performance score ≥80, absence of nasopharynx recurrence, life expectancy ≥3 months, and previous treatment with paclitaxel or gemcitabine in combination with platinum. The clinical characteristics of the patients included in the study are shown in Table 1.
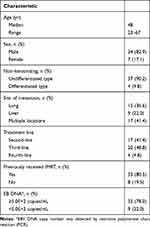 |
Table 1 Clinical Characteristics of NPC Patients with Pulmonary and/or Hepatic Metastasis |
Drug Administration
The therapeutic program for all patients consisted of S-1 plus apatinib. The dose of S-1 was determined according to each patient’s body surface area (BSA): 40 mg twice a day for BSA <1.25 m2; 50 mg twice a day for 1.25 m2 ≤BSA <1.5 m2; and 60 mg twice a day for BSA ≥1.5 m2. S1 were received for 14 days, after stopping for 7 days, given 3 weeks apart. Apatinib, 125 mg was administered orally daily on days 1 through 28 of each 4-week cycle. If the toxicity was not tolerable, the dose of apatinib was changed to 125 mg every other day. Liver and renal function tests as well as routine blood and serum tests were performed until disease progression was observed.
Efficacy and Safety Assessments
The primary end point of our study was progression-free survival (PFS), defined as the time from enrollment to documented tumor progression based on computed tomography (CT)/magnetic resonance imaging (MRI) scans or death as a result of any cause, whichever occurred first. The secondary end points were ORR = complete response (CR) + partial response (PR), the disease control rate (DCR) = CR + PR + stable disease (SD), and toxicity. Treatment efficacy was evaluated in accordance with the Response Evaluation Criteria in Solid Tumors and classified as CR, PR, SD, or progressive disease (PD). Adverse events were assessed and graded according to the National Cancer Institute Common Toxicity Criteria version 3.0.
Statistical Analysis
The data were processed using SPSS 21.0 software. OS and PFS were analyzed using the Kaplan–Meier model. Comparisons of OS and PFS between groups were performed using the log rank test. Comparisons of the frequency of data between groups were carried out using the χ2 test. P values <0.05 were considered statistically significant.
Results
Response Rates
All 41 patients completed at least four courses of S-1, and thus, all patients were included in the evaluation of treatment efficacy after 4 courses of chemotherapy. The treatment outcomes included PR in 14 cases, SD in 19 cases, and PD in 8 cases. The ORR was 34.1%, and the DCR was 80.4%. We analyzed the potential relationships between ORR and age, sex, pathological diagnosis, toxicities, location and number of tumor metastases, EB-DNA load, line of chemotherapy, IMRT, and nutritional status. This analysis identified trends of decreasing ORR and a shortened time to benefit with an increase in the line of chemotherapy.
Toxicities
The most common toxicities included loss of appetite in 39.0% (16/41) of patients, dyslipidemia in 34.1% (14/41), hypertension in 31.7% (13/41), myelosuppression in 24.4% (10/41), fatigue in 21.9% (9/41), hand-foot syndrome in 17.1% (7/41), and mucositis in 12.2% (5/41; Table 2). Seven patients required dose adjustment of apatinib due to side effects. The dose was reduced in 5 patients due to fatigue, whereas treatment was suspended for 4 weeks in 1 patient due to rupture of the appendix and for 6 weeks in 1 patient due to cerebral infarction.
 |
Table 2 Most Common Toxicities Observed During Treatment |
Survival Analysis
Patients were monitored for 8–35 months, with a median follow-up duration of 28 months. The median PFS was 9.7 months (95% confidence interval, 6.2–13.8 months; Figure 1), the median OS was 22.1 months (95% confidence interval, 15.1–28.9 months; Figure 2), and the 2-year survival rate was 41.5%. Causes of death included tumor progression, cachexia, multiple organ failure, and others. For EB-DNA–positive patients, we divided the EB-DNA level into high expression (>1E+5 copies/mL) and low expression (<1E+5 copies/mL) groups, and no differences in the OS and PFS were observed between the two groups (all P>0.05). These patients were also divided into symptomatic and asymptomatic groups according to the presence or absence of clinical symptoms, and no differences in PFS and OS were observed between the two groups (all P>0.05).
|
Figure 1 Kaplan–Meier estimate of PFS for Chinese NPC patients with pulmonary and/or hepatic metastasis treated with low-dose apatinib plus S-1. |
|
Figure 2 Kaplan–Meier estimate of OS for Chinese NPC patients with pulmonary and/or hepatic metastasis treated with low-dose apatinib plus S-1. |
Discussion
NPC has unique regional characteristics, with high incidence areas mainly distributed in southern China, Hong Kong, Southeast Asia, North Africa, the Middle East and Alaska. According to data from the International Agency for Cancer (IARC), 40% of NPC cases in the world occur in China. Within China, the Pearl River Delta region in Guangdong has the highest incidence rate of 20–30/100,000 people.7 According to the literature, in the era of two-dimensional conventional radiotherapy, the 5-year OS rate for NPC was 59–69%, and the LCR was 60.8–79%.8,9 Since the development of IMRT technology in the 1980s, the efficacy of NPC treatment has significantly improved, and the 5-year LCR and OS rates reaching 86.0–91.8% and 77.1–84.7%, respectively.10,11 The main cause of treatment failure is still distant metastasis, accounting for 70% of NPC disease-specific deaths.10 Therefore, the current challenges for the treatment of metastatic NPC are improving efficacy and reducing side effects. In the selection of chemotherapy regimens, docetaxel and cisplatin combined with fluorouracil have been shown to prolong OS in cases of locoregionally advanced NPC,12 and gemcitabine combined with cisplatin has been identified as the first choice for patients with recurrent or metastatic NPC.13
Although first-line chemotherapy has achieved very good results, progression is inevitable as tumor cells become resistant to chemotherapy drugs. S-1 is a combination of tegafur and gimeracil (CDHP) and oltiracetam (OXO) in a molar ratio of 1:0.4:1. Among these drugs, Tegafur is a prodrug of 5-Fu. After entering the body, it is converted into 5-Fu by the liver P450 enzyme. CDHP and OXO are included to enhance the efficacy and reduce the toxicity of tegafur, respectively. Previous research demonstrated that S-1 can provide significant additive effects for improving the survival rates among patients with recurrent or metastatic NPC.14 Apatinib is a newly developed oral small molecule tyrosine kinase inhibitor that targets VEGFR-2. Blocking the downstream signal transmission after VEGF binds to its receptor results in inhibition of tumor blood vessel formation, thereby inhibiting tumor growth. MDR involves not only resistance of tumor cells to multiple anti-tumor drugs but also cross-resistance to other anti-tumor drugs with different structures and mechanisms. Overexpression of adenosine triphosphate (ATP)-binding cassette (ABC) transporter is an important cause of MDR. The ABC transporter binds to ATP and acts to eliminate cytotoxic drugs from the cell. Apatinib competitively binds to the ATP-binding site of ABC transporter, and thereby, inhibits drug efflux and maintains the intracellular chemotherapeutic drug concentration. Apatinib was shown to reverse ATP-binding cassette subfamily B member 1(ABCB1)- and ATP binding cassette subfamily G member 2(ABCG2)-mediated MDR and improve the efficacy of doxorubicin and paclitaxel in drug-resistant cell transplantation in nude mice.15
In the present study, apatinib was administered at a dose of 125 mg/d, which is less than the standard doses of 850 mg/d reported in gastric cancer and 500 mg/d in other tumors.5 However, the lower dose of apatinib was found to be effective. The common adverse reactions to apatinib in the clinic are reductions of leukopenia, neutrophils or platelets as well as non-hematological toxicities, including fatigue, hand-foot syndrome, hypertension, proteinuria, oral ulcers, etc. These toxic side effects are often severe, leading to reduced quality of life and tolerance among patients. The most common adverse reaction reported during the administration of apatinib is hypertension, with an incidence rate of 69.5%,16 and common hypertension drugs were not found to be effective. The mechanism may be decreased endothelial cell/platelet secretion of nitric oxide (NO)/prostacyclin (PGI2), abnormal blood vessel density, blood vessel stiffening, and endothelin function disorders. In the present study, the ORR was 34.1% and the DCR was 80.4%. We also found trends of decreasing ORR and shortening time to benefit with an increase in the line of chemotherapy, taking into account factors such as MDR, decreased human immune function, and nutritional status. The median PFS was 9.7 months, the median OS was 22.1 months, and the 2-year survival rate was 41.5%. Compared with previous studies,2,17 low-dose apatinib plus S-1 yielded an excellent survival benefit, and the toxicities were tolerable and reversible. Grade 3 adverse reactions were rare. Only 7 patients required dose reduction of apatinib to 125 mg every other day. It was particularly important to pay attention to the occurrence of dyslipidemia (34.1%). One case had cerebral infarction, and thus, required drug therapy to control and lower blood lipids.
In summary, low-dose apatinib combined with S1 was feasible and resulted in a certain treatment efficacy for metastatic NPC. Moreover, the side effects were generally tolerated. This treatment offered good clinical benefits for metastatic NPC patients after multi-line treatments. In the future, more trials are needed to further explore the mechanism of the drugs in the treatment of NPC, and to expand the sample size for clinical trials to determine the best drug dosage and method.
Acknowledgments
This study was supported by the Foundation of Hainan Provincial Health Committee, China (No. 19A200032). The authors thank all the patients and the research staff for their contributions to this project. Liya Zhou and Jie Lin are co-first authors for this work.
Disclosure
We declare that we have no conflicts of interest in this work.
References
1. Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403. doi:10.1016/j.radonc.2013.10.020
2. Zhang S, Chen J, Yang S, Lin S. An open-label, single-arm phase II clinical study of docetaxel plus lobaplatin for Chinese patients with pulmonary and hepatic metastasis of nasopharyngeal carcinoma. Anticancer Drugs. 2016;27(7):
3. Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 2017;35(36):4050–4056. doi:10.1200/JCO.2017.73.3675
4. Wang Y-J, Zhang Y-K, Rishil J, et al. Repositioning of tyrosine kinase inhibitors as antagonists of atp-binding cassette transporters in anticancer drug resistance. Cancers. 2014;6(4):1925–1952. doi:10.3390/cancers6041925
5. Zhao D, Hou H, Zhang X. Progress in the treatment of solid tumors with apatinib: a systematic review. Onco Targets Ther. 2018;11:4137–4147. doi:10.2147/OTT
6. Wen L, You C, Lu X, et al. Phase II trial of concurrent chemoradiotherapy with S-1 versus weekly cisplatin for locoregionally advanced nasopharyngeal carcinoma. Mol Clin Oncol. 2015;3(3):687–691. doi:10.3892/mco.2015.529
7. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2018;68(6):394–424. doi:10.3322/caac.21492
8. Yeh S, Tang Y, Lui C, et al. Treatment outcomes and late complications of 849 patients with nasopharyngeal carcinoma treated with radiotherapy alone[J]. Int J Radiat Oncol. 2005;62(3):672–679. doi:10.1016/j.ijrobp.2004.11.002
9. Tuan JKL, Ha TC, Ong WS, et al. Late toxicities after conventional radiation therapy alone for nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):305–311. doi:10.1016/j.radonc.2011.12.028
10. Lee AWM, Ng WT, Chan LLK, et al. Evolution of treatment for nasopharyngeal cancer – success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110(3):377–384. doi:10.1016/j.radonc.2014.02.003
11. Wang W, Feng M, Fan Z, et al. Clinical outcomes and prognostic factors of 695 nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Biomed Res Int. 2014;2014:814910–814948.
12. Sun Y, W F L, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–1520. doi:10.1016/S1470-2045(16)30410-7
13. Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388(10054):1883–1892. doi:10.1016/S0140-6736(16)31388-5
14. Shuai Z, Liya Z, Xiaopeng H, et al. A retrospective study of concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy on curative effect for treatment of patients with N3 stage nasopharyngeal carcinoma. Cancer Manag Res. 2018;10:1705–1711. doi:10.2147/CMAR.S165804
15. Yan-jun M, Liang Y-J, Huang H-B, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70(20):7981–7991. doi:10.1158/0008-5472.CAN-10-0111
16. Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN965DI in patients with advanced malignancies. BMC Cancer. 2010;10(1):529. doi:10.1186/1471-2407-10-529
17. Long GX, Lin JW, Liu DB, et al. Single-arm, multicentre phase II study of lobaplatin combined with docetaxel for recurrent and metastatic nasopharyngeal carcinoma patients. Oral Oncol. 2014;50(8):717–720. doi:10.1016/j.oraloncology.2014.04.007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
