Back to Journals » Journal of Pain Research » Volume 14
Safety and Efficacy of Ultrasound-Guided Retrolaminar Block of Multiple Injections in Retroperitoneal Laparoscopic Nephrectomy: A Prospective Randomized Controlled Study
Authors Liu D, Xu X, Zhu Y, Liu X , Zhao F, Liang G, Zhu Z
Received 22 September 2020
Accepted for publication 12 January 2021
Published 5 February 2021 Volume 2021:14 Pages 333—342
DOI https://doi.org/10.2147/JPR.S282500
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Dexing Liu,1,2 Xinpeng Xu,2 Yuhang Zhu,2 Xingxing Liu,1,2 Faliang Zhao,1,3 Guobiao Liang,1,3 Zhaoqiong Zhu1,2
1Soochow University Medical College, Suzhou, 215000, People’s Republic of China; 2Department of Anesthesiology, Affiliated Hospital of Zunyi Medical University, ZunYi, 563000, People’s Republic of China; 3Department of Urology, Affiliated Hospital of Zunyi Medical University, ZunYi, 563000, People’s Republic of China
Correspondence: Zhaoqiong Zhu Email [email protected]
Purpose: Ultrasound-guided retrolaminar block (RLB) has the potential to provide postoperative analgesia in retroperitoneal laparoscopic surgery. This study was conducted to evaluate the effects of RLB when compared with local infiltration analgesia (LIA) in retroperitoneal laparoscopic nephrectomy.
Patients and Methods: One hundred and fifteen patients scheduled for laparoscopic nephrectomy were divided into two groups: the RLB group (n = 57) received an ultrasound-guided RLB, while the LIA group (n = 58) received LIA. At 2, 4, 6, 24, and 48 hours after operation, the maximal visual analog score (VAS), sufentanil and rescue analgesia consumption, and the utilization of patient-controlled intravenous analgesia (PCIA) were assessed. The incidence rates of postoperative nausea and vomiting (PONV); time of leaving bed (at the first instance); and the levels of plasma β-Endorphin (β-EP), Interleukin-1β (IL-1β), and prostaglandin E2 (PEG2) 30 min after extubation were noted.
Results: Patients in the RLB group had significantly lower VAS scores; lower sufentanil cumulative consumption; lower manual addition frequency of PCIA; lower proportion of using rescue analgesia within 48 hours after operation; lower incidence rate of PONV; shorter resuscitation times; earlier time of leaving the bed; and lower β-EP, IL-1 β, and PEG2 levels.
Conclusion: Ultrasound-guided RLB of multiple injections is both safe and controllable for postoperative analgesia after retroperitoneal laparoscopic nephrectomy. When compared with LIA, RLB has better and longer-lasting analgesic effect, lower incidence rates of PONV, and the potential to reduce the level of postoperative inflammatory factors.
Trial Registration: China Clinical Trials Registration Center (http://www.chictr.org.cn, No. ChiCTR1800017526, Date of registration: 2018– 08-02).
Keywords: ultrasound guidance, retrolaminar block, retroperitoneal laparoscopic nephrectomy, local infiltration anesthesia
Introduction
Laparoscopic nephrectomy is a common surgical method for the treatment of renal parenchyma diseases and was first reported by Clayman et al.1 When compared with traditional open operations, laparoscopy has the advantages of reduced intraoperative bleeding, earlier time of leaving bed, and faster recovery of gastrointestinal function.2 Although the operation can also be completed through transperitoneal or retroperitoneal approaches,3 the retroperitoneal approach is capable of achieving a better perioperative outcome,4 and therefore, this method is currently favored by urologists.
Although laparoscopic nephrectomy narrows down the surgical incision point, it still fails to reduce patients’ postoperative pain and requires the same level of postoperative analgesia that open surgeries do.5 Multimodal analgesia, combined with regional block anesthesia, is an important component of contemporary clinical anesthesia management.6 However, the incision of retroperitoneal laparoscopic surgery is located on the lateral side of the abdomen, which poses a challenge for anesthesiologists to perform a nerve block. Retrolaminar block (RLB) was first reported by Pfeiffer in 2006,7 and an ultrasound-guided operation was completed in 2013.8 The efficacy of RLB has been confirmed by comparison of postoperative analgesia in breast surgery; however, it is characteristic of weak diffusion at the craniocaudal extension, and therefore, it limits the clinical application of this technique.9 In this randomized controlled study, it was hypothesized that through the improvement of three injection points, ultrasound-guided RLB could potentially provide better management of postoperative analgesia in posterior laparoscopic nephrectomy when compared with that provided by local infiltration analgesia (LIA).
Patients and Methods
Study Design and Participants
We performed a prospective, randomized, double-blind controlled trial registered with the Chinese Clinical Trial Register (ChiCTR1800017526). This study was approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical College (ethical review of the Affiliated Hospital of Zunyi Medical College in 2018, No. 56), and written informed consent was obtained from all subjects or their relative. This trial was conducted in accordance with the Declaration of Helsinki. All patients were informed of the purpose of the study and signed an informed consent. Because some patients were illiterate and could not write their own names, a consent form was signed by their surrogates during hospitalization. A total of 120 patients, who underwent elective operations of retroperitoneal laparoscopic nephrectomy at the Affiliated Hospital of Zunyi Medical University from September 2018 to August 2019, were recruited for the study. Inclusion criteria were as follows: age 18–65 years; BMI 18–28kg/m2; American Society of Anesthesiologists (ASA) grade I–II; elective operation of laparoscopic nephrectomy. Exclusion criteria were as follows: severe heart, liver, and lung dysfunction; abnormal blood coagulation; history of allergy to anesthetic drugs; history or family history of malignant hyperthermia; history of long-term drinking, chronic pain, long-term use of psychotropic drugs or history of taking or injecting analgesics; scar, infection, and tumor of puncture site; pregnancy and lactation; patients with diabetes; patients with severe visual or auditory disorders; patients with endocrine system diseases; patients transferred to the intensive care unit after operation; loss of core data during follow-up; withdrawal from the study for any reason.
Anesthesia Implementation
After entering the operating room, patients were given peripheral venous passage routinely and continuous mask oxygen inhalation, and their electrocardiogram (ECG), blood pressure, and peripheral oxygen saturation (SpO2) were monitored using a cardiogram monitor (T8, Mindray Ltd, Shenzhen, China). All patients were treated with general anesthesia under tracheal intubation. Midazolam of 0.05–0.1mg/kg, etomidate of 0.2–0.3mg/kg, sufentanil of 0.3–0.5μg/kg, and rocuronium of 0.5mg/kg were used for inducing anesthesia. Mechanical ventilation was employed with tidal volume 6–8mL/kg, breath rate 12–16bpm, and partial pressure of end-tidal carbon dioxide (PETCO2) of 35–45mmHg. After induction, radial artery puncture and catheterization were performed to monitor arterial blood pressure. According to routine usage in the anesthesia department of our institute, propofol of 4–10mg·kg−1·h−1 and remifentanil of 6–10μg·kg−1·h−1 were injected intravenously. The final dose of propofol and remifentanil to maintain anesthesia was calculated on the basis of the patient’s weight. Sevoflurane (only an appropriate supplement) was inhaled intermittently, and rocuronium was injected intravenously to maintain anesthesia according to the need of operation. All general anesthetic drugs were stopped immediately after operation, and sufentanil of 5μg and flurbiprofen axetil of 50mg were given as loading infusion. Remifentanil was discontinued using the Gradual Withdrawal method at the end of the operation.10 The patients were sent back to the post-anesthesia care unit (PACU) after the operation without extubation. The resuscitation of the two groups of patients was completed by PACU’s full-time doctors and nurses. Recovery from anesthesia was assessed using a modified Steward score. If a patient’s Steward score was more than 4, he/she was sent back to the general ward. Immediately before leaving the PACU, patients were treated with patient-controlled intravenous analgesia (PCIA) according to the hospital standardized analgesia scheme: sufentanil of 2μg/kg and normal saline of 100mL were injected at the speed of 2mL/h, with single compression of 0.5mL and locking time of 15min. If patients were given insufficient analgesia after the operation, doctors were informed and a follow-up with a bedside evaluation was performed at the time. Incidence of postoperative nausea and vomiting (PONV) and postoperative pain level were assessed by Visual Analog Scale (VAS). PONV was defined as VAS ≥1. If the VAS score was >4, a single intramuscular injection of parecoxib sodium of 20mg was the first choice for analgesia, with an interval of 6 hours. If there was still a need for analgesia during this interval, a single intravenous injection of sufentanil of 5μg was added as a secondary remedy.
Nerve Block and Local Anesthesia
After the induction of general anesthesia, the patients in the RLB group were placed in a lateral position, T7 was located at the subscapular angle of the patient’s back, and T8, T9, and T10 were then marked in turn. After preparing the skin and completing draping, portable color Doppler ultrasonography (M9, Mindray Ltd, Shenzhen, China) was used to identify the laminae corresponding to T8, T9, and T10 via a convex array probe of 1–5Hz, in the transverse plane of centrum, and through the short axis of the spine (Figure 1A, Supplementary Table S1). The puncture needle was then inserted, and it made contact with the lamina under the guidance of ultrasonography. Afterwards, the needle tip was withdrawn approximately 1mm to the lamina and then further withdrawn to ensure that was no blood or cerebrospinal fluid. Through real-time imaging, 10mL of 0.4% ropivacaine was injected into the laminae of T8, T9, and T10, respectively, for a total of 30mL (Figure 1B and C).
Referring to the published literature11,12 and the surgeon’s opinion, patients in the LIA group were given 30mL of 0.4% ropivacaine to selectively perform local infiltration of the subcutaneous and muscular layer of incision in accordance with incision conditions.
All patients were covered with the same dressing at the puncture point and incision, and dressings were intact until the end of the last follow-up at 48 hours to ensure appropriate blinding of the study methodology.
Follow-Up
Drug dosage during operation along with heart rate (HR), mean blood pressure (MAP), and SpO2 at 10 min after RLB, 10 min after local anesthesia infiltration, at the end of operation, immediately after extubation, 10 min after extubation, 20 min after extubation, and 30 min after extubation were recorded. The main outcomes recorded were as follows: maximal VAS score and the cumulative consumption of sufentanil at 2, 4, 6, 24, and 48 hours after operation, the cumulative times of effective PCIA compression, and the proportion of remedial analgesia at 24 and 48 hours after operation. The secondary outcomes included the incidence of PONV, the period from back to ward to leaving bed (at the first instance) for activity, and the levels of plasma β-EP, IL-1β, and PEG2 30 min after extubation.
Study Design and Sample Estimation
When all of the patients had entered the operating room and completed recording of baseline data, opaque random envelopes were opened, and researchers from the two groups with different responsibilities completed the study. Group I included two anesthesiologists who were trained in the same period and had valuable experience in clinical regional blocks. After opening the envelope, they checked the sequence to determine random grouping. Researchers in Group II included two other anesthesiologists who were subspecialized in urology, and they were responsible for anesthesia management, postoperative follow-up, and data recording. The induction of general anesthesia was completed by researchers in Group II, who then left the operating room. Patients from the RLB group were treated with RLB by researchers in Group I. Patients in the LIA group received anesthetic management only while in the room. Researchers in Group I left the operating room before operation, and then, researchers in Group II entered the operation room to continue anesthetic management. When the operation was completed, researchers in Group II left the operating room again. If patients were in the LIA group, surgeons were instructed by researchers in Group I to perform LIA, and patients in the RLB group had anesthetic management done while indoors only. After skin suturing was completed, researchers in Group I left the operating room again, and researchers in Group II immediately entered the operation room to stop general anesthesia and sent patients to PACU for resuscitation. To make the time for patients to receive local infiltration anesthesia consistent, the patients in Group I were designed to complete LIA before the incision was sutured after surgery. Researchers from the two groups completed the transfer of patient anesthesia management twice to ensure the safety of patients without disturbing their clinical decisions. The flow chart of the study design is shown in Supplementary Figure S1.
The sample size of this study was based on the results of pre-research (n = 15 in the RLB group, n = 15 in the LIA group). Superiority test was conducted to compare the maximum VAS in 24 hours after surgery (2.86±1.46 VS 4.52±0.99), SM = 1, α = 0.05, β = 0.2, and the sample proportion was 1:1, with each group requiring 45 patients. Because the VAS score pre-research was non-normal distribution, we refer to the calculation method of non-normal distribution in the study by Valerie J Page.13 Finally, after increasing the number of recruits by1.053 times and allowing for 20% loss to follow-up, the target sample size was 60 per group (120 total).
Statistical Analysis
SPSS 17.0 software was used for analysis. Measurement data from the normal distribution were represented by mean ± standard deviation (SD), and independent sample t-tests were used for comparison between groups. Measurement data of non-normal distribution were represented by median (interquartile range) and analyzed by a nonparametric rank sum test. Numerical data were represented by case numbers and percentages, and the χ2 test was used for comparison between groups. A p-value of less than 0.05 (P < 0.05) represented a statistically significant difference.
Results
Of the 155 cases recruited for this study, 120 were included in the study, and 115 completed follow-up and data analysis (Figure 2). There was no significant difference in demographic information, anesthesia time, surgical incision size, operation site, or surgical classification between the two groups (Table 1). None of the subjects involved in this study showed clear opioid-induced hyperalgesia during postoperative follow-up.
 |
Figure 2 A flow chart illustrating patient inclusion. |
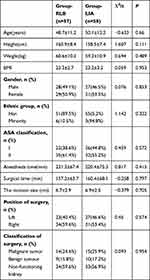 | 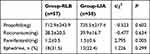 |
Table 1 Patient Characteristics and Clinical Data in the Study |
There was no significant difference in the utility of propofol and rocuronium between the two groups. The dosage of remifentanil in the RLB group (1.2±0.5 mg) was lower than that in the LIA group (1.5±0.6mg) (P = 0.005). Although 31.5% (n = 18) of the RLB patients used ephedrine during operation, there was no significant difference when compared with its usage in the LIA group (22.4%, 13) (Table 2).
After 10 min of finishing RLB, HR (64.6±9.2bpm) and mean arterial pressure (MAP) (70.3±10.7mmHg) in patients of the RLB group were lower than those in patients of the LIA group (67.9±7.2bpm, 76.6±10.3 mmHg) (PHR =0.036, PMAP= 0.002); however, in terms of Pulse Oxygen Saturation (SpO2), there was no significant difference. After 10 min of completing local anesthesia, there was no significant difference in HR, MAP, or SpO2 in patients between the two groups (Figure 3). The HRs of patients in the RLB group were lower than those of patients in the LIA group after extubation, 20 min after extubation, and 30 min after extubation (90.5±11.3bpm, 80.7±10.2bpm, 76.4±9.5bpm vs 96.2±13.1bpm, 86.3±11.9bpm, 85.1±10.3bpm) (P = 0.013, P = 0.008 and P < 0.001). Besides, MAP of patients in the RLB group was lower than that of patients in the LIA group after extubation, 10 min after extubation, 20 min after extubation, and 30 min after extubation (91.7±11.8mmHg, 86.9±12.4mmHg, 82.8±11.8mmHg, 81.1±8.3mmHg vs 109.2±16.9mmHg, 97.6±14.5mmHg, 94.7±12.7mmHg, 93.8±10.7mmHg) (P < 0.001). There was no significant difference in SpO2 between the two groups after extubation (Figure 3).
Patients in neither group developed adverse events, such as death, allergy, or poisoning concerning local anesthesia, pneumothorax, and so on. The incidence rate of PONV in patients of the RLB group (15.7%, 9) was lower than that in patients of the LIA group (34.5%, 20)(P = 0.031). There were two cases that showed excessive sedation in the RLB group and four cases in the LIA group during the perioperative period; however, the difference between them was not statistically significant. In the RLB group, patients’ retention time in PACU (54.9±16.2 min) and first time of leaving bed (44.8±7.9 hours) were lower than those of patients in the LIA group (67.1±20.3 min, 53.7±11.7 hours) (P = 0.001, P < 0.001). There was no significant difference in total hospital stay time after operation between the two groups (Table 3).
 |
Table 3 Complications Within 48 Hours After Surgery |
Patients in neither group used remedial drugs in PACU or remedial sufentanil during the postoperative follow-up period. There was no significant difference in patients’ VAS score between the two groups at 2 and 4 hours after operation. The VAS score of patients in the two groups showed no difference at 2 or 4 hours after operation ((2 [2, 3], 3 [2, 4] and 3 [3, 4]), while VAS scores of patients in the RLB group at 6, 24, and 48 hours after operation were lower than those of patients in the LIA group (3 [2, 4.25], 5 [4, 6], and 5 [4,5.25]) (P = 0.022, P < 0.001, and P < 0.001) (Figure 4A). There was no significant difference in the cumulative consumption of sufentanil for patients between the two groups at 2 and 4 hours after operation, while the cumulative consumption of sufentanil for patients in the RLB group at 6, 24, and 48 hours after operation was lower than that for patients in the LIA group (P = 0.042, P = 0.001, and P = 0.005) (Figure 4B). Manual additional times of PCIA per capita in the RLB group at the first 24 hours, the second 24 hours, and the cumulative 48 hours were lower than those in the LIA group (P < 0.001) (Figure 4C). There was no significant difference in the proportion of patients using rescue analgesia between the RLB group and the LIA group in the second 24 hours. The proportion of patients using rescue analgesia in the RLB group (8.7%, 5) was lower than that in the LIA group (29.3%, 17) within the first 24 hours. The proportion of patients using rescue analgesia in the RLB group (14%, 10) was also lower than that in the LIA group (32.3%, 23) for the cumulative 48 hours (P = 0.009) (Figure 4D).
In patients in the RLB group, the levels of plasma inflammatory factors β-EP, IL-1 β, and PEG2 were lower in comparison with those in the LIA group (P β-EP=0.007, PIL-1β<0.001, and PPEG2<0.001) (Figure 5).
 |
Figure 5 The levels of plasma β-EP (A), IL-1β (B), and PEG2 (C) 30 min after extubation. Data are expressed as means ± SD. t-test was used for comparison between groups. |
Discussion
Peripheral nerve block can not only reduce acute pain but also reduce mortality, pulmonary complications, and hospital stay time during the perioperative period.14,15 With regard to the incision in the lateral abdominal area, it poses a new challenge for anesthesiologists when choosing a suitable approach for postoperative analgesia. Comparison between the quadratus lumborum block and the traditional intrathecal block and between the quadratus lumborum block and the paravertebral block (PVB) in laparoscopic renal surgery had been reported,16,17 which showed that the trunk nerve block can be involved in postoperative analgesia management after laparoscopic nephrectomy with the introduction of ultrasound. However, performing PVB is relatively complex, and there has been no breakthrough for traditional epidural analgesia yet.18 With ongoing development in nerve block technology, newer PVB approaches have been the focus of many studies in recent years, such as RLB. It was speculated that RLB would be more inclined to work through the PVB pathway, that is, through “deep” penetration.19 The clinical effect of RLB has been reported in ipsilateral thoracic surgery.8,20 In this study, the effect of RLB when compared with that of LIA in retroperitoneal laparoscopic nephrectomy was assessed, which resulted in a better and longer-lasting analgesic effect, lower incidence rates of PONV, and lower level of postoperative inflammatory factors.
RLB has the disadvantage of weak craniocaudal diffusion ability,21 which limits its clinical application. Inspired by analgesia in breast surgery completed by a two-point block,9 a three-point block at lateral abdominal region of T8-10 was designed for this study. Considering that there was still capacity dependence in deep infiltration,22 a total local anesthetic capacity of 30mL was designed, and anesthesia at three injection sites was carried out respectively. Weak craniocaudal diffusion can be observed in RLB of the traditional sagittal plane of the spinal long axis section; however, deep and shallow drug diffusion can be observed in the spinal short-axis section of the transverse plane, that is, whether drugs diffuse to the spinous process or to the transverse process. Because the superior costotransverse ligament may play a role in deep penetration of the drug into paraspinal and even epidural space after RLB,23 we hold the view that the technique of in-plane insertion with the transverse plane can better exert the effect of RLB.
PVB has been considered associated with risk concerning serious complications, such as pneumothorax, hypotension, or nerve injury.19 In this study, no serious adverse events, such as allergic reactions, poisoning of anesthetics, and pneumothorax, or uncontrollable persistent hypotension were observed, suggesting that ultrasound-guided RLB of multiple injections is safe for postoperative analgesia after retroperitoneal laparoscopic nephrectomy. It has been demonstrated that PVB provides better hemodynamic stability when compared with epidural analgesia.24 In this study, HR and MAP in the RLB group were lower than those in the LIA group after extubation, implying RLB could provide more stable hemodynamics than LIA in retroperitoneal laparoscopic nephrectomy.
In addition, the maximal VAS score from the 6th hour through to the end of the follow-up period and the proportion of PONV in patients in the RLB group were both lower than those in the LIA group, which may be the reason for the shorter time of leaving bed in RLB group during the early stages after operation. It was reported that the intraoperative use of remifentanil in breast surgery was higher in patients receiving retrolaminar injection with saline than that in patients receiving RLB with local anesthetic mixture.25 In our study, we also found that RLB clearly reduced the perioperative consumption of remifentanil, which led to shorter retention time in the recovery room of patients in the RLB group. However, the difference in the cumulative amount of sufentanil, cumulative PAIC bolus at 24 and 48 hours, and the frequency of using remedial drugs might be the reason for which the proportion of PONV in patients in the RLB group was lower than that in patients in the LIA group. These findings proved that the management of postoperative analgesia in RLB was effective in retroperitoneal laparoscopic nephrectomy and significantly better than that of LIA. Although there was a statistical difference in postoperative sufentanil consumption within 48 hours between the RLB group and the LIA group, the difference in medians between the two groups was small (90μg vs 99μg). The results were limited by the current standards of the hospital PCIA regimen, and both groups used background dose; hence, the ability of RLB to reduce the use of postoperative opioids was underestimated in this study.
The puncture site of RLB is relatively superficial and is located away from the operation area. It was previously shown that anesthetics could effectively diffuse to paraspinal and even epidural spaces.20 A human imaging study uncovered direct evidence of drugs involved with RLB diffused to the epidural space.22 It was suggested that RLB could not only have the effect of incision analgesia but also produce visceral analgesia and reduce neuroinflammation. As we all know, surgical injury can activate the immune system and cause peripheral inflammation.26 Effective analgesia has been considered to reduce stress levels, thereby reducing systemic inflammation responses.27 In this study, we revealed that the levels of plasma inflammatory factors β-EP, IL-1β, and PEG2 in the RLB group were significantly lower than those in the LIA group, suggesting that RLB has the distinct advantage of inhibiting inflammatory outbreaks. These results may be attributed to RLB’s effectiveness in penetrating to the paravertebral cavity, blocking the transmission of noxious stimuli in the abdominal cavity, reducing stress levels, and thereby reducing perioperative inflammation.20,22 Because the present study did not include a comparison of β-EP, IL-1β, and PEG2 levels before surgery or during follow-up, further evidence to confirm these results is still needed.
There are some limitations to this study. First, because of the limitation of the standardized analgesia scheme, we did not implement opioid-free analgesia management, which limited the significance of the results to a certain extent; hence, it is necessary to conduct further clinical studies on opioid-free postoperative pain. Second, paracetamol was not used in the multimodal analgesia of this study because of its unavailability in clinical practice in our hospital. Third, the study only held preliminary discussions about the relationship between RLB and postoperative inflammation, and thus, the study lacked stronger evidence to verify its benefits to the overall rehabilitation of patients. However, it does point to the imperative that further study is warranted in this area. Fourth, the depth of anesthesia cannot be monitored using bispectral index (BIS) monitoring because of the impact of local medical insurance policies and for the fact that the anesthesiology department lacks consumables for BIS monitoring all year round. The monitoring of the depth of anesthesia in the maintenance phase of anesthesia in this study can only rely on the anesthesiologist to judge on the basis of the changes in blood pressure and heart rate. Fifth, the use of antiemetic drugs was not incorporated into the study design, which led to differences in the evaluation of VAS and the use of HTs receptor inhibitors by different surgeons on duty in the formal study. In addition, because the study only included random daily elective operations, patients who came to the operating room between 8am and 4pm were admitted, and as a result, most patients were in the state of late-night sleep at the 12th and 36th hours and failed to carry out more intensive follow-ups. To avoid jeopardizing blindness, patients from neither group carried out an assessment on the range of abnormal skin sensation at each follow-up time.
Conclusion
In terms of laparoscopic nephrectomy, ultrasound-guided RLB with multiple injections might be able to provide safe and effective postoperative analgesia and reduce postoperative resuscitation time, time in bed after operation, PONV, and postoperative inflammatory factors. Multidimensional clinical studies are still needed to confirm these findings. In addition, determining the nerve block approach that is the optimal choice for laparoscopic nephrectomy remains to be done in the follow-up clinical study.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This work was supported by Joint fund of zunyi science and technology bureau [grant number ZUNSHIKEHE.HZ (2019)97] and The Department of Education Fund of Guizhou Province [grant number No.KY 045-2017]. Special thanks go to Dr. Luo for her support and selfless dedication in the management of anesthesia and postoperative follow-up in this study (mentioned in Supplementary Figure S1).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Clayman R, Kavoussi L, Soper N, et al. Laparoscopic nephrectomy. N Engl J Med. 1991;324(4):1370–1371.
2. Wang B, Tian Y, Peng Y, et al. Comparative study of retroperitoneal laparoscopic versus open ipsilateral nephrectomy after percutaneous nephrostomy: a multicenter analysis. J Laparoendosc Adv Surg Tech A. 2020;30(5):520–524. doi:10.1089/lap.2019.0746
3. Benoit T, Peyronnet B, Roumiguie M, et al. Laparoscopic nephrectomy for polycystic kidney: comparison of the transperitoneal and retroperitoneal approaches. World J Urol. 2016;34(7):901–906. doi:10.1007/s00345-015-1739-5
4. Jiang YL, Qian LJ, Li Z, et al. Comparison of the retroperitoneal versus Transperitoneal laparoscopic Adrenalectomy perioperative outcomes and safety for Pheochromocytoma: a meta-analysis. BMC Surg. 2020;20(1):12. doi:10.1186/s12893-020-0676-4
5. Alper I, Yuksel E. Comparison of acute and chronic pain after open nephrectomy versus laparoscopic nephrectomy: a prospective clinical trial. Medicine (Baltimore). 2016;95(16):e3433. doi:10.1097/MD.0000000000003433
6. Chou R, Gordon DB, de Leon-casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17(2):131–157. doi:10.1016/j.jpain.2015.12.008
7. Pfeiffer G, Oppitz N, Schöne S, Richter-Heine I, Höhne M, Koltermann C. Analgesie der Achselhöhle durch Paravertebralkatheter in Laminatechnik. Der Anaesthesist. 2006;55(4):423–427. doi:10.1007/s00101-005-0969-0
8. Voscopoulos C, Palaniappan D, Zeballos J, et al. The ultrasound-guided retrolaminar block. Can J Anaesth. 2013;60(9):888–895. doi:10.1007/s12630-013-9983-x
9. Onishi E, Murakami M, Nishino R, Ohba R, Yamauchi M. Analgesic effect of double-level retrolaminar paravertebral block for breast cancer surgery in the early postoperative period: a placebo-controlled, randomized clinical trial. Tohoku J Exp Med. 2018;245(3):179–185. doi:10.1620/tjem.245.179
10. Comelon M, Raeder J, Stubhaug A, et al. Gradual withdrawal of remifentanil infusion may prevent opioid-induced hyperalgesia. Br J Anaesth. 2016;116(4):524–530. doi:10.1093/bja/aev547
11. Park JS, Choi GS, Kwak KH, et al. Effect of local wound infiltration and transversus abdominis plane block on morphine use after laparoscopic colectomy: a nonrandomized, single-blind prospective study. J Surg Res. 2015;195(1):61–66. doi:10.1016/j.jss.2014.12.034
12. Zhu Z, Chen B, Ye W, et al. Clinical significance of wound infiltration with ropivacaine for elderly patients in china underwent total laparoscopic radical gastrectomy: a retrospective cohort study. Medicine (Baltimore). 2019;98(14):e15115. doi:10.1097/MD.0000000000015115
13. Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1(7):515–523. doi:10.1016/S2213-2600(13)70166-8
14. Memtsoudis SG, Sun X, Chiu YL, et al. Perioperative comparative effectiveness of anesthetic technique in orthopedic patients. Anesthesiology. 2013;118(5):1046–1058. doi:10.1097/ALN.0b013e318286061d
15. Perlas A, Chan VW, Beattie S. Anesthesia technique and mortality after total hip or knee arthroplasty: a retrospective, propensity score-matched cohort study. Anesthesiology. 2016;125(4):724–731. doi:10.1097/ALN.0000000000001248
16. Aditianingsih D, Pryambodho AN, Tantri AR, Mochtar CA. A randomized controlled trial on analgesic effect of repeated Quadratus Lumborum block versus continuous epidural analgesia following laparoscopic nephrectomy. BMC Anesthesiol. 2019;19(1):221. doi:10.1186/s12871-019-0891-7
17. Yuan Q, Cui X, Fei Y, Xu Z, Huang Y. Transmuscular quadratus lumborum block versus thoracic paravertebral block for acute pain and quality of recovery after laparoscopic renal surgery: study protocol for a randomized controlled trial. Trials. 2019;20(1):276. doi:10.1186/s13063-019-3359-7
18. El Shora HA, El Beleehy AA, Abdelwahab AA, et al. Bilateral paravertebral block versus thoracic epidural analgesia for pain control post-cardiac surgery: a randomized controlled trial. Thorac Cardiovasc Surg. 2018. doi:10.1055/s-0038-1668496
19. Onishi E, Toda N, Kameyama Y, Yamauchi M. Comparison of clinical efficacy and anatomical investigation between retrolaminar block and erector spinae plane block. Biomed Res Int. 2019;2019:1–8.
20. Sabouri AS, Crawford L, Bick SK, Nozari A, Anderson TA. Is a retrolaminar approach to the thoracic paravertebral space possible? A human cadaveric study. Reg Anesth Pain Med. 2018;43(8):864–868. doi:10.1097/AAP.0000000000000828
21. Yang HM, Choi YJ, Kwon HJ, et al. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia. 2018;73(10):1244–1250. doi:10.1111/anae.14408
22. Damjanovska M, Stopar Pintaric T, Cvetko E, Vlassakov K. The ultrasound-guided retrolaminar block: volume-dependent injectate distribution. J Pain Res. 2018;11:293–299. doi:10.2147/JPR.S153660
23. Yoshida T. Ultrasound-guided proximal obturator block - a reply. Anaesthesia. 2016;71(7):857–858. doi:10.1111/anae.13530
24. Hara K, Sakura S. [Thoracic truncal block: trends and future perspectives]. Masui. 2017;66(3):247–254.
25. Hwang BY, Kim E, Kwon JY, et al. The analgesic efficacy of a single injection of ultrasound-guided retrolaminar paravertebral block for breast surgery: a prospective, randomized, double-blinded study. Korean J Pain. 2020;33(4):378–385. doi:10.3344/kjp.2020.33.4.378
26. Aminsharifi A, Salehipoor M, Arasteh H. Systemic immunologic and inflammatory response after laparoscopic versus open nephrectomy: a prospective cohort trial. J Endourol. 2012;26(9):1231–1236. doi:10.1089/end.2012.0110
27. Jin L, Yao R, Heng L, et al. Ultrasound-guided continuous thoracic paravertebral block alleviates postoperative delirium in elderly patients undergoing esophagectomy: a randomized controlled trial. Medicine. 2020;99(17):e19896. doi:10.1097/MD.0000000000019896
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



