Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Safety and efficacy of the perioperative administration of recombinant human brain natriuretic peptide (rhBNP): a systematic review and meta-analysis
Authors Hua P , Liu JY, Tao J, Lin XF, Zou RJ, Zhang DW, Yang SR
Received 5 June 2017
Accepted for publication 1 December 2017
Published 20 February 2018 Volume 2018:14 Pages 313—321
DOI https://doi.org/10.2147/TCRM.S143247
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Ping Hua,1 Jianyang Liu,2 Jun Tao,1 Xifeng Lin,1 Rongjun Zou,1 Dingwen Zhang,1 Songran Yang3,4
1Department of Cardiovascular Surgery, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 2Department of Vascular Surgery, Henan Provincial People’s Hospital, Zhengzhou, 3The Biobank of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 4Guangdong Province Key Laboratory of Brain Function and Disease, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
Objective: Retrospective studies and a meta-analysis were performed to evaluate the safety and effectiveness of the perioperative administration of recombinant human brain natriuretic peptide (rhBNP) during cardiac surgery under extracorporeal circulation.
Methods: Computerized literature searches were performed in Medline, Embase, The Cochrane Library, CNKI, CBM, and WANFANG to find randomized controlled trials (RCTs) related to the perioperative administration of rhBNP during cardiac surgery starting from the database inception until December 2016. Two researchers independently performed study screening, information extraction, and quality evaluation according to the inclusion/exclusion criteria, and a meta-analysis was performed using RevMan 5.2 software.
Results: A total of 12 studies were analyzed, including 12 RCTs and 727 patients. The meta-analysis results indicated that the perioperative administration of rhBNP could reduce the occurrence rate of postoperative complications, length of intensive care unit (ICU) stay, length of hospital stay, and serum creatinine (Scr) levels, and increase the 24-hour urine volume; however, it did not affect the postoperative mortality rate.
Conclusion: The perioperative administration of rhBNP during cardiac surgery was safe and effective, and could improve the prognosis of the patients.
Keywords: recombinant human brain natriuretic peptide, perioperative administration, meta-analysis
Introduction
Technology in cardiac surgery, anesthesia, and perioperative care continues to improve; however, risks of severe postoperative complications or death are still high among the patients who undergo cardiac surgical procedures.1,2 Studies showed that the postoperative death rate within 30 days was between 2.8% and 4.8%, which might be related to the multi-organ ischemia-reperfusion injuries and systemic inflammation resulting from the extracorporeal circulation (ECC) procedures used during cardiac surgery.3–5 Therefore, treatment strategies accounting for perioperative interventions, reduction of ECC injuries, and improvement of patient prognosis are particularly important.
Brain natriuretic peptide (BNP) is a circulating hormone secreted by the heart ventricle muscles and plays an important role in the regulation of blood pressure, osmotic equilibrium, and cardiovascular functions.6 Recently, BNP has become a major marker in the diagnosis of cardiovascular diseases and a reliable indicator for prognosis estimation.7 Meanwhile, it is also being used to treat heart failure due to its potent effects on natriuresis, diuresis, vasodilation, blood pressure reduction, and renin-angiotensin-aldosterone system inhibition.8 Both recombinant human BNP (rhBNP) and endogenous BNP have similar structures and biological activities. Recent studies have shown that rhBNP administration during the perioperative period could improve the prognosis of patients undergoing cardiac surgery, shorten their hospital stays, lower the postoperative mortality rate, and reduce myocardial injuries, pulmonary edema, kidney injury, and systemic inflammation.9,10
The present study investigated the data of randomized controlled trials (RCTs) conducted during the past 10 years that were relevant to the effects of rhBNP application on the prognosis of patients who underwent cardiac surgery and applied meta-analysis from the perspective of evidence-based medicine, to obtain better clinical evidence.11
Materials and methods
Literature retrieval
Manual/computerized literature retrieval was carried out to collect the results from RCTs evaluating the perioperative administration of rhBNP in patients who underwent extracorporeal circulation procedures. The English databases included Medline, Embase, and The Cochrane Library. The Chinese databases included CNKI, CBB, CQVIP, and WANFANG. The relevant registered clinical data from Google Scholar and the clinical trials website were also included. The search included publications from the beginning of the database inception until December 2016. Medical subject headings and text-word searches were performed. English medical subject headings included “natriuretic peptide, brain,” “Nesiritide,” “cardiopulmonary bypass,” “cardiac surgical procedures,” “thoracic surgery,” and “cardiac surgery.” The Chinese search words included “BNP,” “Nesiritide,” “Xinhuosu,” “cardiac surgery,” “ECC,” “heart valve replacement surgery,” and “coronary artery bypass.” A retrospective literature review was performed, and the authors were contacted so that the materials could be as detailed as possible.
Inclusion and exclusion criteria
We included studies with the following characteristics: 1) RCTs, blinding or allocation concealment; 2) data related to the application of rhBNP during the perioperative period in patients who underwent cardiac surgery; 3) data from control groups (placebo or other treatment medicine) to be pooled during analysis; and 4) evaluation of length of intensive care unit (ICU) stay, length of hospital stay, mortality rate at postoperative day 30, serum creatinine (Scr) levels, pulmonary artery pressure, time to ventilator discontinuation, and 24-hour urine volume. Studies were excluded if they were without clear intervention strategies, duplicates, without clear outcome measures, or with measures that could not be pooled.
Literature selection and quality evaluation
Two reviewers performed study screening and quality evaluation independently, and extracted the information according to a predesigned form. Disagreements were discussed or presented to a third reviewer until a consensus was reached. In cases of missing data, authors were contacted directly whenever possible to obtain the missing data. The included studies were evaluated using the Jadad scoring system. The correct description of the randomization method was rated as 2; mention of “random,” “random allocation,” or “randomize” was rated as 1; a correct description of the double-blind procedure was rated as 2; mention of using a “double-blind procedure” was rated as 1; description of causes and number of withdrawal/lost cases was rated as 1. A score of 2 out of ≤5 was considered as a low-quality study, and a score of ≥3 were considered as a high-quality study.
Statistical analysis
Review Manager version 5.2 software (RevMan for Windows 2003; the Nordic Cochrane Center, Copenhagen, Denmark) was used to perform the statistical analysis. Heterogeneity among the same category was evaluated using the χ2 test: if P≥0.1 and I2≤50%, the probability of heterogeneity was considered to be low, and the fixed-effects model would be used; if P<0.1 and I2>50%, heterogeneity was considered to be present and the causes would be analyzed. If the heterogeneity was too high, only a descriptive analysis would be performed instead of a meta-analysis. Count data were analyzed using the odds ratio (OR). Continuous data were analyzed based on the weighted mean differences (MDs) if the variables measured were the same; otherwise, they were analyzed based on the standardized mean difference (SMD). The 95% confidence intervals (CIs) were calculated for all the analyses.
Results
Characteristics of the included studies
A total of 370 related studies resulted from the preliminary screening. After removal of the publications that were duplicated or apparently did not meet the inclusion criteria, full text/reference reading, and search among registered clinical trials, data from 12 studies and one clinical trial [NCT01440881] which was completed but not published, were subject to further analysis. One clinical trial used nesiritide or milrinone in the treatment group without detailed grouping information and thus has been excluded in our study. In addition, two publications that were based on the same population, but different conclusion markers, were both included. Therefore, a total of 12 RCTs were analyzed, including 375 patients in the treatment group and 378 patients in the control group. All the patients had been subject to open-chest cardiac surgery under the ECC. The flowchart in Figure 1 shows the publication selection process and its results. The basic information about the studies included and the evaluation of the corresponding methods used are summarized in Table 1.
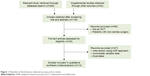  | Figure 1 Flowchart of the literature selection process and its results. |
Meta-analysis results
Adverse events
A total of seven studies reported adverse events during the hospital stay. They included death during hospitalization, acute kidney failure, dialysis, atrial fibrillation, low blood pressure, and dizziness. All data displayed high homogeneity, and the meta-analysis results showed that the incidence of adverse events was significantly lower in the treatment group compared with the control group (Figure 2A) (MD =0.55, 95% CI [0.36, 0.84], P<0.05), and the corresponding symmetric funnel plot indicated the absence of publication bias.
Mortality rates after the surgery
All RCTs reported the mortality during the hospital stay. In the present meta-analysis, the mortality rates among all the hospitalized patients were analyzed (Figure 2B). All data displayed adequate homogeneity (P=0.67, I2=0%). The meta-analysis results indicated that the differences in the mortality rates during the hospital stay were not significant between the treatment group and the control group (OR =0.72, 95% CI [0.29, 1.78] P=0.48), and the symmetric funnel plot indicated the absence of publication bias.
Length of ICU stay and hospital stay
Six studies compared the length of ICU stay (Figure 3A) and four studies compared the length of hospital stay (Figure 3B). The remaining studies displayed some heterogeneity. The meta-analysis results showed that ICU stay was significantly shorter in the treatment group compared with the control group (MD =−12.68, 95% CI [−20.58, −4.79], P=0.002). Similarly, the hospital stay was also significantly shorter in the treatment group when compared with the control group (MD =−1.57, 95% CI [−2.59, −0.55], P=0.003).
24-Hour urine volumes after the surgery
Five studies presented the results of 24-hour urine volumes after surgery (Figure 4A). All the studies display some heterogeneity, I2=28%, and a fixed-effects model was applied. The meta-analysis results showed that 24-hour urine volume after the surgery was significantly higher in the treatment group compared with the control group (MD =397.64, 95% CI [277.28, 518.00], P<0.00001).
Changes in Scr
A total of four studies presented the maximal changes in the Scr levels after the surgery (Figure 4B). All the data displayed fair homogeneity. The meta-analysis results showed that the maximal increase in Scr levels was significantly lower in the treatment group compared with the control group (MD =−19.89, 95% CI [−26.87, −12.91], P<0.00001).
Analysis of inflammatory markers
Three studies compared the changes in postoperative inflammatory markers, which included TNF-α, IL-1β, IL-6, and IL-10. We applied meta-analysis to the peak postoperative values of TNF-α among those three studies (Figure 4C) and found that they were significantly lower in the treatment group compared with the control group (MD =−4.44, 95% CI [−6.27, −2.60], P<0.00001).
Discussion
The BNP was first identified in the porcine brain by Sudoh et al during the 1980s,18 and it has been widely used ever since. It is the only natural antagonist of the renin-angiotensin-aldosterone receptor within the body, and it has extensive biological effects: 1) it inhibits the secretion of renin and aldosterone, increases glomerular filtration rate, and suppresses sodium reabsorption in the inner medullary collecting duct to increase the urine volume; 2) it relaxes vascular smooth muscle, dilates arteries and veins, reduces the cardiac afterload, and decreases the pulmonary wedge pressure; and 3) it inhibits expression of fibrotic and inflammatory genes and myocardial fibrosis, and lessens ventricular remodeling.12–16 The rhBNP has a similar structure and biological activity to the endogenous BNP. In 2001, rhBNP, under the brand name Natrecor® (generic name nesiritide; Johnson Company, NJ, USA), was approved by the US Food and Drug Administration for clinical applications. In China, XinHuoSu® (Nuodikang Company, Chengdu, China), a similar product, has been used in clinical practice since 2005. Since then, large-scale clinical trials have confirmed the safety and effectiveness of rhBNP in the treatment of congestive heart failure, and its use has become widespread.17–19
Animal experiments showed that perfusion with rhBNP during coronary artery bypass surgery could significantly reduce the infarct region, protect vascular endothelial function, and reduce the onset of pulmonary edema.20 Meanwhile, a large number of studies on the safety and effectiveness of the application of rhBNP during the perioperative period in patients who underwent ECC procedures have been carried out in China and across the world.21–24 In the present meta-analysis, we included the majority of the available RCTs that studied the perioperative administration of rhBNP during cardiac surgery. The present results showed that rhBNP could significantly reduce the incidence of adverse effects after cardiac surgery and shorten the ICU stay and hospital stay.25,26 However, it did not lower the postoperative mortality rate in the treated patients.
Cardiac surgery with ECC can cause multi-organ dysfunction, the most common one being acute kidney disorder (AKD). It was observed that AKD not only prolonged hospital stay but also increased the mortality rate among the patients. Our meta-analysis showed that the administration of rhBNP during the perioperative period could significantly lower the Scr peak levels and increase 24-hour urine volumes. Mentzer et al indicated that patients with weakened kidneys benefitted more significantly from intravenous infusion of rhBNP during the perioperative period than patients with normal kidney function.5 rhBNP inhibits the over-activation of sympathetic nerves, lowers the levels of circulating norepinephrine, suppresses the renin-angiotensin-aldosterone system, and reduces the levels of circulating renin/aldosterone. The protective effects of rhBNP during the perioperative period might be achieved through its functions in improving the heart function and renal perfusion and increasing the glomerular filtration rate.
Additionally, during the ECC, the systemic inflammatory response syndrome (SIRS) can be easily triggered by both the direct contact between the blood and the artificial surface of the ECC system and the surgical trauma itself; thus, the prognosis of the patients can be severely affected. Three studies in the present meta-analysis investigated the effects of rhBNP on SIRS. Our results showed that rhBNP could significantly lower the SIRS marker TNF-α. TNF-α is a potent and key mediator in the development of SIRS. This protection by rhBNP might be related to its capabilities of lowering the pressure and resistance of pulmonary circulation, increasing cardiac output, and improving the systemic perfusion.
In conclusion, the application of rhBNP during the perioperative period may lower the occurrence rate of postoperative complications, length of ICU stay, and length of hospital stay. In addition, rhBNP may mitigate the trend of kidney injury and inflammation response after cardiac surgery. However, rhBNP did not affect the postoperative mortality rate. Collectively, the application of rhBNP during the perioperative period may present a greater clinical benefit in patients with cardiac surgery.
Acknowledgments
Statistical consultation and support were provided by Sun Yat-sen University medical statistics.
This work was supported by the National Natural Science Foundation of China (NSFC No 81771165), the Special Program for Applied Research on Super Computation from the NSFC-Guangdong Joint Fund (the second phase), the Natural Science Foundation Project in Guangdong province in China (Grant No 2016A030313295), the Major Project Development and Emerging, Interdisciplinary Funding Projects of Sun Yat-sen University (Grant No 15ykjc17b), and the Guangzhou science and technology project of Major Special Research Topics on International Collaborative Innovation (Grant No 201704030032).
Author contributions
P Hua, SR Yang and JY Liu designed the study, collected the clinical data, performed the statistical analysis, participated in the operation, and drafted the manuscript. J Tao, XF Lin, RJ Zou, and DW Zhang participated in the operation and revised the article. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Beckmann A, Funkat AK, Lewandowski J, et al. Cardiac surgery in Germany during 2012: a report on behalf of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg. 2014;62(1):5–17. | ||
Wei CM, Heublein DM, Perrella MA, et al. Natriuretic peptide system in human heart failure. Circulation. 1993;88(3):1004–1009. | ||
Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343(4):246–253. | ||
Zeng XT, Bao CP, Cao SY, Liy JY. Meta Analysis Series III: Quality control tool for randomized controlled trials. Chinese Journal of Evidence-Based Cardiovascular Medicine. 2012;3:183–185. Chinese. | ||
Mentzer RM Jr, Oz MC, Sladen JM, et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA Trial. J Am Coll Cardiol. 2007;49(6):716–726. | ||
Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC Jr. Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study. Circulation. 2007;116(11 Suppl):I134–I138. | ||
Brackbill ML, Stam MD, Schuller-Williams RV, Dayle AA, et al. Perioperative nesiritide versus milrinone in high-risk coronary artery bypass graft patients. Ann Pharmacother. 2007;41(3):427–432. | ||
Beaver TM, Winterstein A, Hess PJ Jr, et al. Nesiritide following maze and mitral valve surgery. J Card Surg. 2008;23(5):431–436. | ||
Ejaz AA, Martin TD, Johnson RJ, et al. Prophylactic nesiritide does not prevent dialysis or all-cause mortality in patients undergoing high-risk cardiac surgery. J Thorac Cardiovasc Surg. 2009;138(4):959–964. | ||
Liu F, Lin JW. Effects of neogenin on pulmonary arterial hemodynamics in patients with mitral valve replacement. Chinese Journal of Thoracic and Cardiovascular Surgery. 2010;26(2):98–99. Chinese. | ||
Jie XL, Wu PJ, Song L, Yin XQ, Xu CJ. Clinical study of effect of neo-activin on myocardial enzymes and NT-proBNP in patients undergoing valve replacement. Journal of Central South University. 2010;49. Chinese. | ||
Jiang P, Yang YF. Effects of rhBNP on TNF-α and IL-10 in patients with cardiopulmonary bypass. Journal of Central South University. 2015; 46. Chinese. | ||
Wu JB, Wang YJ, Li MQ, Rong XS. The effect of recombinant human brain natriuretic peptide on renal function undergoing cardiac surgery cardiopulmonary bypass. The Journal of Clinical Anesthesiology. 2011;3. Chinese. | ||
Zhao D, Zhu J, Wang CS. Application of recombinant human brain natriuretic peptide in perioperative period of cardiac surgery. Chinese Journal of Clinical Medicine. 2016;19(4):354–355. Chinese. | ||
Lin GQ, Wang QW. Effect of recombinant human brain natriuretic peptide on plasma endotoxin and systemic inflammatory response in patients undergoing cardiopulmonary bypass. Chinese Journal of Thoracic and Cardiovascular Surgery. 2012;28(5):294–297. Chinese. | ||
Zhang KF, Xu D, Shang XB, Liu YH, Li HL. Application of recombinant human brain natriuretic peptide in patients with acute myocardial infarction after coronary artery bypass grafting. Beijing Medical Journal. 2015;7. Chinese. | ||
Gao F, Zhang JB, Jiang JQ, Ding S, Zhou K. Effect of rhBNP on hemodynamics after severe heart valve disease. Medical Journal of National Defending Forces in Southwest China. 2016;8. Chinese. | ||
Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332(6159):78–81. | ||
Wilkins MR, Redondo J, Brown LA. The natriuretic-peptide family. Lancet. 1997;349(9061):1307–1310. | ||
Lazar HL, Bao Y, Siwik D, Frame J, Mateo CS, Colucci WS. Nesiritide enhances myocardial protection during the revascularization of acutely ischemic myocardium. J Card Surg. 2009;24(5):600–605. | ||
Rodrigues AJ, Evora PR, Bassetto S, et al. Risk factors for acute renal failure after heart surgery. Rev Bras Cir Cardiovasc. 2009;24(4):441–446. | ||
Bove T, Calabrò MG, Landoni G, et al. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18(4):442–445. | ||
Ostermann ME, Taube D, Morgan CJ, Evans TW. Acute renal failure following cardiopulmonary bypass: a changing picture. Intensive Care Med. 2000;26(5):565–571. | ||
Abraham WT, Lowes BD, Ferguson DA, et al. Systemic hemodynamic, neurohormonal, and renal effects of a steady-state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure. J Card Fail. 1998;4(1):37–44. | ||
Day JR, Taylor KM. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int J Surg. 2005;3(2):129–140. | ||
Tracey KJ, Beutler B, Lowry SF, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234(4775):470–474. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




