Back to Journals » Clinical Ophthalmology » Volume 9
Safety and efficacy of switching from dorzolamide 1.0%/timolol maleate 0.5% eye drops to brinzolamide 1.0%/timolol maleate 0.5% eye drops
Authors Inoue K, Shiokawa M, Ishida K, Tomita G
Received 24 December 2014
Accepted for publication 18 February 2015
Published 9 April 2015 Volume 2015:9 Pages 619—623
DOI https://doi.org/10.2147/OPTH.S79843
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Kenji Inoue,1 Minako Shiokawa,1 Kyoko Ishida,2 Goji Tomita2
1Inouye Eye Hospital, Tokyo, Japan; 2Department of Ophthalmology, Toho University Ohashi Medical Center, Tokyo, Japan
Purpose: To evaluate the safety and efficacy of switching from dorzolamide 1.0%/timolol maleate 0.5% fixed-combination (DTFC) eye drops to brinzolamide 1.0%/timolol maleate 0.5% fixed-combination (BTFC) eye drops in patients with primary open-angle glaucoma or ocular hypertension.
Methods: A total of 35 eyes of 35 patients with primary open-angle glaucoma or ocular hypertension using DTFC eye drops were included. Participants discontinued DTFC drops and immediately began using BTFC drops. All other eye drops currently being used were continued. Intraocular pressure (IOP) 1 and 3 months after switching medications was compared with baseline IOP. One month after switching medications, participant preference and adherence were evaluated. Adverse reactions were monitored at each study visit.
Results: The IOP was 17.9±2.6 mmHg at baseline and 18.3±4.1 mmHg and 17.8±3.4 mmHg 1 month and 3 months after switching medications, respectively (P=0.633). The frequency of missing a dose was not different before (6.1%) and after (6.1%) switching to BTFC. Significantly fewer participants reported stinging after switching to BTFC (15.2%) than while using DTFC (51.5%, P<0.01). Blurred vision was reported with the same frequency before (39.4%) and after (42.4%) switching to BTFC. A total of 33.3% and 27.3% of patients preferred DTFC and BTFC, respectively. Several patients withdrew from the study because of discomfort (n=2, 5.7%), discharge (n=1, 2.9%), dizziness (n=1, 2.9%), or increased IOP (n=2, 5.7%).
Conclusion: Switching from DTFC to BTFC was well tolerated and did not result in IOP changes or a decreased patient adherence. When glaucoma patients complain of stinging with DTFC administration, switching to BTFC is an acceptable treatment option.
Keywords: brinzolamide/timolol fixed-combination, dorzolamide/timolol fixed-combination, intraocular pressure, switch, safety
Introduction
The ultimate goal of treating glaucoma is to reduce intraocular pressure (IOP) and to preserve the visual field. Initial glaucoma therapies generally consist of prostaglandin analog monotherapy.1,2 However, when inadequate IOP control with a single analog occurs, a different monotherapy agent or an additional medication must be used.3 Multiple medications increase the number of eye drops instilled, which can lead to poor patient adherence. Therefore, a fixed-combination eye drop that contains two different medications is recommended. Several fixed-combination eye drops have been approved for use in Japan and have been available since 2011. These include fixed combinations of a prostaglandin analog and timolol (latanoprost/timolol [Xalacom®, Pfizer, Inc., New York, NY, USA], travoprost/timolol [DuoTrav®, Alcon Laboratories Inc., Fort Worth, TX, USA]) and a fixed combination of a carbonic anhydrase inhibitor and timolol (dorzolamide/timolol [Cosopt®, Merck & Co., Inc., Whitehouse Station, NJ, USA]). In November 2013, a fixed-combination eye drop containing brinzolamide and timolol (Azarga, Alcon Laboratories, Inc.) was approved for use in Japan. This brinzolamide/timolol fixed-combination (BTFC) eye drop was shown to be extremely effective in reducing IOP in Japanese glaucoma patients.4–6 However, dorzolamide/timolol fixed-combination (DTFC) eye drops have been shown to be better for reducing IOP reduction than both latanoprost7 and brimonidine/timolol fixed-combination drops.8 It has been shown that switching patients from a DTFC eye drop to a BTFC eye drop is both safe and effective in reducing IOP,9–11 but this has not been specifically examined in a Japanese population.
Here, we prospectively evaluate IOP reduction, patient comfort, and treatment safety when switching from a DTFC eye drop to a BTFC eyed drop in patients with primary open-angle glaucoma and ocular hypertension. Patients were followed for 3 months following the medication switch.
Materials and methods
A total of 35 eyes of 35 patients (12 men, 23 women) with primary open-angle glaucoma or ocular hypertension who were using DTFC eye drops at Inouye Eye Hospital were enrolled in this study between November 2013 and August 2014. The protocol was approved by the hospital’s ethical committee, and all participants provided written informed consent before any study procedure or examination was performed.
Participants discontinued their twice-daily use of DTFC eye drops (Cosopt®, Merck & Co., Inc.) and immediately switched to a twice-daily use (morning and evening instillation) of BTFC eye drops (Azarga, Alcon Laboratories, Inc.) (twice a day, morning and evening instillation). All other topical medication used remained the same. When participant were using DTFC eye drops in both eyes, the eye with the higher IOP was selected as the study eye. If both eyes had the same IOP, the right eye was selected as the study eye.
The IOP was measured at approximately the same time each visit using a Goldmann tonometer. The magnitude of IOP reduction was defined as the IOP 3 months after switching medication minus the IOP at baseline. One month after switching medications, participant preference and adherence to BTFC therapy use were evaluated (Table 1). Participants were also asked about experiencing specific adverse reactions at each study visit. Reasons for participant dropout were also recorded.
  | Table 1 Questionnaire regarding brinzolamide/timolol fixed-combination eyed drop use |
Data analyses
All data are presented as mean ± standard deviation, where applicable. Differences in mean IOP at baseline and at 1 and 3 months were analyzed using paired t-tests. Commercially available statistical software (SPSS, version 22; SPSS, Inc., Chicago, IL, USA) was used to perform all statistical analyses. Statistical significance was defined as P<0.05.
Results
Subject characteristics
Mean subject age at baseline was 67.5±9.0 years (range: 46–84 years). A total of 33 eyes had primary open-angle glaucoma and 2 eyes had ocular hypertension. The number of drops used before switching medications was 3.5±0.6 drops per day, including 19 (54.3%) patients who used 3 drops per day, 14 (40.0%) patients who used 4 drops per day, and 2 (5.7%) patients who used 5 drops per day. The DTFC eye drop counted as 2 drops. Baseline IOP was 17.9±2.6 mmHg (range: 14.0–23.5 mmHg) and Humphrey visual field (30-2 SITA Standard) mean deviation was –8.5±6.5 dB (range: –19.1–1.6 dB).
IOP after switching medications
The IOP 1 and 3 months after switching medications was 18.3±4.1 mmHg (n=33 eyes) and 17.8±3.4 mmHg (n=29 eyes), respectively. These slight differences from baseline (17.9±2.6 mmHg, n=35 eyes) were not statistically significant (P=0.633, Figure 1). Three months after switching medication, 10 (34.5%) eyes had a decrease in IOP ≥2 mmHg, 13 (44.8%) eyes had no change in IOP (within 2 mmHg of baseline), and 6 (20.7%) eyes had an increase in IOP ≥2 mmHg.
  | Figure 1 Intraocular pressure values before and after switching medications. |
Subject compliance and comfort
Subject comfort and compliance were evaluated 1 month after switching medications. Two (6.1%) subjects reported missing BTFC drop doses, which was not significantly different than the 2 (6.1%) subjects who missed DTFC drop doses (P=0.999, Table 2).
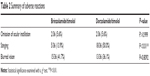  | Table 2 Summary of adverse reactions |
Five (15.2%) subjects reported stinging with BTFC drop use, which was significantly less than the 17 (51.5%) subjects who reported stinging with DTFC drop use (P<0.01). Thirteen (39.4%) subjects reported blurred vision with DTFC drop use, which was not significantly different than the 14 (42.4%) subjects who reported blurred vision with BTFC drop use (P=0.999). Figure 2 summarizes the stinging and blurred vision that subjects experienced during BTFC drop use.
  | Figure 2 Stinging and blurred vision in subjects using brinzolamide/timolol fixed-combination eye drops. |
A total of 9 (27.3%) participants preferred to use BTFC drops, 11 (33.3%) participants preferred to use DTFC drops, and 13 (39.4%) participants expressed no preference (Figure 3). Subjects preferred BTFC drops because they had less stinging (n=5 subjects) and better IOP reduction (n=5 subjects). Subjects preferred DTFC drops because they had less blurred vision (n=7 subjects), less stinging (n=2 subjects), and less discomfort (n=2 subjects).
  | Figure 3 Patient preferences for each medication examined. |
Six (17.1%) participants dropped out of the study before the end of the 3-month study period. Participants withdrew from the study because of increased IOP (n=2 [5.7%] subjects, 1 and 2 months after switching), ocular discomfort (n=2 [5.7%] subjects, ≤1 month after switching), ocular discharge (n=1 [2.9%] subject, 1 month after switching), and dizziness (n=1 [2.9%] subject, 3 days after switching).
Discussion
Several reports on the efficacy of BTFC eye drops in Japanese patients have been published.4–6 Yoshikawa et al4 treated 301 patients with primary open-angle glaucoma or ocular hypertension with timolol for 4 weeks. Patients were then divided into two groups, with one group switched to BTFC drops (150 cases) and the other group continuing on timolol drops (151 cases). Treatment was continued for an additional 8 weeks in both groups. Eight weeks after group differentiation, IOP in the BTFC group was 3.2 mmHg lower (measured 2 hours after drop instillation) than before the switch. Adverse reactions experienced were stinging (5%), blurred vision (1%), and hyperemia (1%). Nagayama et al5 administered timolol therapy to 319 patients with primary open-angle glaucoma or ocular hypertension for 4 weeks. Patients were then divided into two groups, with one group switching to BTFC therapy (158 cases) and the other group switching to brinzolamide and timolol therapy (161 cases). Treatment was continued for an additional 8 weeks in both groups. After 8 weeks, the decrease in IOP was not significantly different between the BTFC group (range: 2.5–3.4 mmHg) and the brinzolamide-added group (range: 2.7–3.3 mmHg). Adverse reactions observed in the BTFC group were punctate keratitis (1%), blurred vision (1%), ocular pruritus (1%), and dysgeusia (1%). Nakajima et al6 administered BTFC eye drops to 126 patients with primary open-angle glaucoma or ocular hypertension for 52 weeks. After the 52-week treatment period, IOP had been reduced between 4.1 mmHg and 5.7 mmHg in each patient. Adverse reactions included, but were not limited to, punctate keratitis (10%), ocular irritation (6%), keratitis (3%), and dysgeusia (3%).
Several studies reporting what happens after switching from DTFC to BTFC therapy have been published.9–11 Lanzl and Raber9 reported what happened after switching from various therapies to BTFC drops. In the 2,937 patients who switched from DTFC drops to BTFC drops, IOP significantly decreased from 18.5±4.1 mmHg to 16.5±3.2 mmHg. Moreover, IOP was significantly lower in 823 patients who switched from a prostaglandin analog plus DTFC drops (18.3±5.0 mmHg) to a prostaglandin analog plus BTFC drops (16.4±3.9 mmHg). Auger et al10 evaluated stinging, blurred vision, eye redness, and abnormal taste in 31 patients who switched from DTFC to BTFC. Patients using DTFC drops experienced more stinging and patients using BTFC drops experienced more blurred vision. There were no differences in eye redness and abnormal taste. Rossi et al11 investigated tear film breakup time, corneal stinging, and glaucoma symptom scale score12 in 72 patients switching from DTFC. A significant improvement was reported for all evaluations. Adverse reactions included blurred vision (4.2%) and dysgeusia (2.7%). IOP was not affected by switching medications.
In our study, no patients used DTFC drops concomitantly with an analog. We observed no changes in IOP that resulted from switching to a prostaglandin analog plus BTFC drops (n=18 cases). These results differed from those reported by Lanzl and Raber.9 However, IOP decreased or increased by ≥2 mmHg in 55.2% of subjects. Therefore, we conclude that changes in IOP vary between patients.
Our observed efficacy in reducing IOP was not significantly different from a previous report comparing dorzolamide 2.0%/timolol fixed-combination drops with BTFC drops.13 However, this previous study reported that participants withdrew from the study because of adverse reactions, including discomfort, discharge, and dizziness. In our study, 14.3% and 40% of participants reported stinging and blurred vision, respectively. This was not significantly different than that reported in a previous study (stinging in 42%, blurred vision in 55%).10 Moreover, we observed no severe adverse reactions, which is in agreement with past reports.4–6,9–11,13,14 Also in agreement with past reports,10,13,14 less stinging was reported in the BTFC group than in the DTFC group. We believe that less stinging occurred because of pH differences between medications (dorzolamide/timolol pH =5.6, brinzolamide/timolol pH =7.2). The frequency of blurred vision in the DTFC group was not significantly different from that observed in the BTFC group. This is in agreement with one previous study,13 but two other studies10,14 found that BTFC drops caused blurred vision more often than DTFC drops. BTFC drops are a liquid suspension, which is known to cause blurred vision. In a previous study, 79.2% of patients preferred BTFC drops over DTFC drops.14 Likewise, Lanzl and Raber9 reported that patients preferred BTFC drop (82.0%) significantly more often than they preferred DTFC drops (8.8%). We found that 33.3% of our participants preferred using DTFC drops and that 27.3% of our participants preferred using BTFC drops. The reason cited for preferring DTFC was less vision blurring. Participants preferred BTFC drops because they caused less stinging. Consequently, we considered the importance of sensation in determining patient preference.
In conclusion, we found no significant difference in IOP, subject compliance, or blurred vision in participants who switched from DTFC drops to BTFC drops. However, stinging was reported more frequently during treatment with DTFC drops. Therefore, in patients who experience stinging with the administration of DTFC drops, switching to BTFC drops is an acceptable glaucoma treatment option.
Disclosure
No author has any conflicts of interest to declare that is relevant to the subject of this article.
References
Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126(4):498–505. | ||
The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. | ||
Djafari F, Lesk MR, Harasymowycz PJ, Desjardins D, Lachaine J. Determinants of adherence to glaucoma medical therapy in a long-term patient population. J Glaucoma. 2009;18(3):238–243. | ||
Yoshikawa K, Kozaki J, Maeda H. Efficacy and safety of brinzolamide/timolol fixed combination compared with timolol in Japanese patients with open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2014;8:389–399. | ||
Nagayama M, Nakajima T, Ono J. Safety and efficacy of a fixed versus unfixed brinzolamide/timolol combination in Japanese patients with open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2014;8:219–228. | ||
Nakajima M, Iwasaki N, Adachi M. Phase III safety and efficacy study of long-term brinzolamide/timolol fixed combination in Japanese patients with open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2014;8:149–156. | ||
Quaranta L, Miglior S, Floriani I, Pizzolante T, Konstas AG. Effects of the timolol-dorzolamide fixed combination and latanoprost on circadian diastolic ocular perfusion pressure in glaucoma. Invest Ophthalmol Vis Sci. 2008;49(10):4226–4231. | ||
Konstas AG, Quaranta L, Yan DB, et al. Twenty-four hour efficacy with the dorzolamide/timolol-fixed combination compared with the brimonidine/timolol-fixed combination in primary open-angle glaucoma. Eye. 2012;26(1):80–87. | ||
Lanzl I, Raber T. Efficacy and tolerability of the fixed combination of brinzolamide 1% and timolol 0.5% in daily practice. Clin Ophthalmol. 2011;5:291–298. | ||
Auger GA, Raynor M, Longstaff S. Patient perspectives when switching from Cosopt® (dorzolamide-timolol) to Azarga™ (brinzolamide-timolol) for glaucoma requiring multiple drug therapy. Clin Ophthalmol. 2012;6:2059–2062. | ||
Rossi GC, Pasinetti GM, Sandolo F, Bordin M, Bianchi PE. From dorzolamide 2%/timolol 0.5% to brinzolamide 1%/timolol 0.5% fixed combination: a 6-month, multicenter, open-label tolerability switch study. Expert Opin Pharmacother. 2011;12(16):2425–2431. | ||
Lee BL, Gutierrez P, Gordon M, et al. The glaucoma symptom scale. A brief index of glaucoma-specific symptoms. Arch Ophthalmol. 1998;116(7):861–866. | ||
Manni G, Denis P, Chew P, et al. The safety and efficacy of brinzolamide 1%/timolol 0.5% fixed combination versus dorzolamide 2%/timolol 0.5% in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2009;18(4):293–300. | ||
Mundorf TK, Rauchman SH, Williams RD, Notivol R; Brinzolamide/Timolol Preference Study Group. A patient preference comparison of Azarga™ (brinzolamide/timolol fixed combination) vs Cosopt® (dorzolamide/timolol fixed combination) in patients with open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2008;2(3):623–628. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
