Back to Journals » Cancer Management and Research » Volume 12
Safety and Efficacy of PD-1 Inhibitors Plus Chemotherapy in Advanced Soft Tissue Sarcomas: A Retrospective Study
Authors Tian Z, Yang Y, Yang J, Zhang P, Zhang F, Du X, Li C, Wang J
Received 4 November 2019
Accepted for publication 14 February 2020
Published 24 February 2020 Volume 2020:12 Pages 1339—1346
DOI https://doi.org/10.2147/CMAR.S237300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Beicheng Sun
Zhichao Tian,1 Yonghao Yang,2 Jinpo Yang,3 Peng Zhang,1 Fan Zhang,1 Xinhui Du,1 Chao Li,1 Jiaqiang Wang1
1Department of Orthopedics, The Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, Henan Province 450008, People’s Republic of China; 2Department of Immunotherapy, The Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, Henan Province 450008, People’s Republic of China; 3Department of Medical Oncology, The Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, Henan Province 450008, People’s Republic of China
Correspondence: Jiaqiang Wang
Department of Orthopaedics, The Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, Henan Province 450008, People’s Republic of China
Tel +86-187 3718 7831
Fax +86-371-6596 1505
Email [email protected]
Purpose: Programmed cell death 1 (PD-1) inhibitors are ineffective as monotherapy for the treatment of soft tissue sarcomas (STS). However, increasing evidence shows that the combination of PD-1 inhibitors and chemotherapy is efficacious and safe for many types of malignancies, including STS. This study aimed to assess the safety and efficacy of doxorubicin chemotherapy plus PD-1 inhibitor in the treatment of metastatic STS.
Patients and Methods: We retrospectively reviewed 21 patients with metastatic STS who received doxorubicin chemotherapy plus a PD-1 inhibitor between November 2017 and October 2018.
Results: The objective response rate was 47.6%, the disease control rate was 71.40%, and the median progression-free survival was 6 months (95% CI, 2– 8 months). The average change in target lesion diameter from baseline was − 25.15 ± 41.61. Majority of the patients experienced grade 1/2 adverse events (AEs), the grade 3/4 AEs were few. The most common grade 3/4 AEs were as follows: leukopenia (23.8%) and anemia (19.0%). Immune-related AEs were common and included hypothyroidism (14.3%) and pneumonitiss (9.5%). No drug related deaths occurred.
Conclusion: This study provides preliminary evidence that the combination of doxorubicin chemotherapy and PD-1 inhibitor for advanced STS is safe and effective. We plan to conduct randomized clinical trials to confirm and characterize the activity of the chemotherapy-immunotherapy combinations in the treatment of sarcomas.
Keywords: pembrolizumab, camrelizumab, doxorubicin, immunotherapy
Introduction
As a malignant tumor of mesenchymal origin, soft tissue sarcomas (STS) have a low incidence.1,2 However, approximately 40,000 incident cases of more than 70 STS subtypes are still reported in China annually.3,4 A significant proportion of STS will eventually metastasize,2,4 which primarily occurs through the blood and with the lung and liver being the most common sites for primary metastases.2,5 The first line of the treatment of advanced STS is doxorubicin-based chemotherapy.4,6,7 Multi-target receptor tyrosine kinase inhibitors (TKIs) have been shown to be effective against STS.8,9 However, most patients who receive chemotherapy or TKI-targeted therapy relapse after a certain period of time. The mean overall survival (OS) of metastatic STS after comprehensive treatment is 12 to 18 months.10 Therefore, more effective alternatives need to be developed.
Programmed cell death-1 (PD-1) targeted immunotherapy has been consistently shown to be effective in the treatment of advanced STS.11–13 However, PD-1 inhibitors are ineffective as monotherapy for STS. In a multicenter, open-label, Phase II trial of the PD-1 inhibitor pembrolizumab for the treatment of patients with advanced STS and bone sarcomas, the objective response rate (ORR) of patients with STS was only 18% (7 out of 40 patients).11 In another non-comparative multicenter randomized Phase 2 trial of nivolumab (a PD-1 inhibitor) +/− ipilimumab for the treatment of patients with advanced sarcoma, the confirmed ORR was only 5% among 38 patients that received nivolumab monotherapy.12 The combination therapy of chemotherapy with PD-1 inhibitor has been proposed to improve the efficacy of PD-1 inhibitor against malignancies. The combination of PD-1 inhibitor and chemotherapy has been shown to be effective for the treatment of non-small cell lung cancer (NSCLC),14,15 breast cancer and nasopharyngeal cancer, with fewer adverse events (AEs).16,17 It has also been reported that doxorubicin can enhance the efficacy of PD-1 inhibitors.18 Accordingly, we combined PD-1 inhibitors with doxorubicin chemotherapy and started treating a cohort of patients with advanced STS since 2017. In this study, we aimed to assess the safety and efficacy of doxorubicin chemotherapy plus PD-1 inhibitor in the treatment of metastatic STS.
Materials and Methods
Patients
This was a single center retrospective study of 21 patients who received doxorubicin chemotherapy plus PD-1 inhibitor as treatment for STS between November 2017 and October 2018 at The Affiliated Cancer Hospital of Zhengzhou University. The inclusion criteria were as follows: (1) age 16 to 65 years; (2) histologically diagnosed nonspecific subtype STS; (3) acceptable blood, liver and kidney functions; (4) locally unresectable or multiple metastases; (5) interval of more than 3 months between the end of previous chemotherapy and enrollment; (6) The Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1; (7) The target lesions were measurable according to the response evaluation criteria in solid tumors (RECIST), version 1.1.
This study was approved by the Ethics Committee for Clinical Investigation of The Affiliated Cancer Hospital of Zhengzhou University and was conducted according to the tenets of the Declaration of Helsinki. All patients provided written informed consent for data collection and research purposes.
Treatment Protocol
The initial patients received 200 mg pembrolizumab (Merck Sharp & Dohme Corp, USA), Patients later in the study received 200 mg camrelizumab (Hengrui Medicine, China) via a 30-min intravenous infusion on day 1 every 21 days until disease progression (PD) or the occurrence of unacceptable AEs. In parallel, patients were administered chemotherapy (37.5 mg/m2 doxorubicin per day via intravenous bolus) on days 2 and 3 every 21 days for a maximum of six cycles unless PD or AEs occurred. AEs were evaluated according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0. The treatment was delayed until recovery when the patients developed grade 3 or 4 AEs. The treatment was terminated if the delay was more than 2 weeks.
Evaluation of Safety and Efficacy
Efficacy was evaluated according to the RECIST 1.1 criteria every 30 or 60 days via magnetic resonance imaging or computed tomography. Anti-tumor efficacy was categorized as complete response (CR), partial response (PR), stable disease (SD) or PD, according to RECIST 1.1 criteria. The median progression-free survival (m-PFS), ORR, disease control rate (DCR) and AEs were also evaluated. PFS was defined as the time from the first day of treatment to the occurrence of PD or death, whichever occurred first.
Statistical Analysis
All the data were analysed using SPSS 21.0 software. Quantitative variables are presented as numbers (percentage) or medians (range). PFS was calculated using the Kaplan-Meier method, with 95% confidence interval (CI). the corresponding figures were drawn by using GraphPad Prism 5.0. The cutoff date for data collection was October 2019, and the present study is a descriptive analysis.
Results
Patient Characteristics
The cohort included 9 (42.86%) men and 12 (57.14%) women. The average patient age was 45.81±18.25 years. The basic patient characteristics are as shown in Table 1. All patients had stage IV disease. The primary tumor site varied greatly and was distributed throughout the body, but it was mainly in the extremities. All the target lesions were metastatic lesions. The histological subtypes were also markedly different, with the most common being synovial sarcoma (n=4) and undifferentiated pleomorphic sarcoma (UPS) (n=4) followed by angiosarcoma (n=2), clear cell sarcoma (n=2), malignant peripheral nerve sheath tumor (MPNST) (n=2), myxofibrosarcoma(n=2), and myxoid liposarcoma (n=2), and the least common being dedifferentiated liposarcoma (n=1), epithelioid sarcoma (n=1), and leiomyosarcoma (n=1). 66.67% (14/21) patients underwent excision of the primary lesion, and 28.58% (6/21) of patients were previously treated with doxorubicin. Sixteen patients received pembrolizumab, and five patients received camrelizumab.
 |
Table 1 Patient Demographics and Characteristics |
Efficacy of Therapy
Of the 21 patients, 2 patients (one with UPS and one with angiosarcoma) achieved CR; 8 patients had PR, 5 patients had SD and 6 patients had PD (Tables 1 and 2). The ORR was 47.6%, the DCR was 71.4%, and the m-PFS was 6 months (95% CI, 2–8 months) (Table 3). The Average change in target lesion diameter from baseline was −25.15 ± 41.61 (Figure 1). The Kaplan-Meier estimate of PFS is shown in Figure 2.
 |
Table 2 Responses of Various Histological Subtypes to Treatment |
 |
Table 3 Clinical Efficacy |
 |
Figure 2 Kaplan–Meier estimates of progression-free survival. |
Toxicity and Safety
In general, the combination therapy of PD-1 inhibitor immunotherapy and doxorubicin chemotherapy was relatively well tolerated. The majority of the AEs were grade 1 or 2, and grade 3 or 4 AEs were rare (Table 4). No treatment-related deaths occurred. The most common grade 1/2 AEs were alopecia (90.5%), leukopenia (85.7%), anemia (76.2%), fatigue (76.2%), nausea (66.7%), and vomiting (47.6%). The most common grade 3/4 AEs were leukopenia (23.8%) and anemia (19.0%). The immune-related AEs included hypothyroidism (n=3, 14.3%, all three patients received camrelizumab), and pneumonitis (n=2, 9.5%, one received camrelizumab and another received pembrolizumab). Immune-related AEs were treated with prednisone, and all patients recovered accordingly. there were no treatment delays due to the AEs.
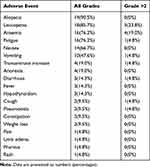 |
Table 4 Adverse Events |
Discussion
This retrospective study evaluated the safety and efficacy of combination treatment of chemotherapy and PD-1 inhibitor (pembrolizumab or camrelizumab) for STS in a cohort of 21 patients with advanced-stage disease. The ORR was 47.6%, DCR was 71.4%, and m-PFS was 6 months (95% CI, 2–8 months). The average change of target lesion diameter from baseline was −25.15% ± 41.61%.
Doxorubicin-based chemotherapy remains the first line treatment for metastatic or locally unresectable STS, as recommended by the National Comprehensive Cancer Network guidelines.7 However, the efficacy of doxorubicin chemotherapy for STS is low.6,19 AEs and drug resistance have also limited the continued use of doxorubicin.20 Therefore, there is an urgent need for new systemic therapies for advanced STS. The emergence of broad-spectrum TKIs has made a significant progress in the treatment of those patients. However, broad-spectrum TKIs for advanced STS yields an ORR of only approximately 14% and an m-PFS of approximately 4.6 months.8,9 Fortunately, a 2017 phase II trial of pembrolizumab in advanced STS showed promising results.11 Since then, many patients with advanced STS in China started to use PD-1 inhibitors off-label, particularly due to the introduction of local and cheaper PD-1 inhibitors into the market.21,22 However, as shown in the phase 2 trial of pembrolizumab in STS, PD-1 inhibitor monotherapy has limited efficacy in most patients with advanced STS.
Increasing evidence shows that the anti-tumor effects of chemotherapy are mediated not only through cytotoxic effects, but also through stimulating and modulating tumor immunity by enhancing tumor antigen presentation, immunogenic cell death, and inhibiting regulatory T cells.18,23–26 These results lend credence to the argument that the combination of chemotherapy and immunotherapy is the next step for cancer treatment. Based on these evidences and on the results of previous studies in other malignant tumors,27,28 we started using PD-1 inhibitors and doxorubicin in advanced STS.
We could not calculate the exact overall survival in this study because majority of the patients received other treatments after PD. Hence, PFS, ORR, and DCR were selected as endpoints. The ORR of PD-1 inhibitor plus doxorubicin treatment was higher than that of doxorubicin alone (14%) or PD-1 inhibitor alone (18%) reported in other studies.6,11 This suggests that doxorubicin may have a synergistic effect with PD-1 inhibitor. Our results are similar to those of the TONIC trial in breast cancer, which indicated that short-term doxorubicin may induce a more favorable tumor microenvironment and increased the likelihood of response to PD-1 inhibitor.18 The PFS in this study was also higher compared to that of monotherapy regimens reported in another study.6 As a cytotoxic chemotherapy drug, doxorubicin works rapidly, while PD-1 inhibitors have a later onset but a longer duration of action.11,15,28 When doxorubicin is combined with PD-1 inhibitor, the cytotoxic effect is achieved immediately from doxorubicin and is then maintained for long periods from the PD-1 inhibitor. From this perspective, these two drugs complement each other with respect to both effectiveness and duration.
The results of this study showed that the therapeutic effect of PD-1 inhibitor combined with doxorubicin differs according to the sarcoma subtype. The subtypes that responded well (including UPS, synovial sarcoma, and angiosarcoma) are all sensitive to at least one of the two drugs. Meanwhile, the subtypes that showed poor therapeutic response, such as MPNST, myxofibrosarcoma, and myxoid liposarcoma, appeared insensitive either to doxorubicin or PD-1 inhibitor. Studies have shown that in NSCLC treatment, the addition of standard chemotherapy to PD-1 inhibitor treatment resulted in a significant longer PFS and OS irrespective of PD-L1 expression.27,29 In other words, adding chemotherapy can increase the sensitivity of malignant tumors to PD-1 inhibitors. From this perspective, for sarcoma subtypes that showed poor sensitivity to either doxorubicin or PD-1 in this study, it may be beneficial to switch to chemotherapy drugs to which they may be sensitive to.
Compared with monotherapy, combination therapy was associated with more types of AEs in this study, but the severity did not increase significantly, consistent with other studies.6,11 The AEs observed were common grade 1–2 complications associated with chemotherapy or immunotherapy. There was no indication for increased frequency or severity of immune-related or chemotherapy-related AEs. However, immune-related thyroid disorders appear to be more common in patients treated with camrelizumab than in those treated with pembrolizumab, in line with other studies using camrelizumab.16,30 Because the number of patients in this study is too small, we cannot determine whether there is a significant difference in AEs between the two PD-1 inhibitors, and further studies are needed.
The combination of chemotherapy, targeted therapy, and immunotherapy in the treatment of advanced STS is logical. This study preliminarily demonstrated the safety and efficacy of doxorubicin combined with the PD-1 inhibitor. However, this study has some limitations, including its retrospective design, small sample size, the absence of a control group, and the inclusion of uncommon sarcoma subtypes. The optimal dose of chemotherapy drugs also needs to be further evaluated. To further investigate the efficacy of PD-1 inhibitor combined with doxorubicin in the treatment of sarcoma, we are soon conducting a randomised clinical trial (Chinese Clinical Trial Registry NO. ChiCTR1900027009).
Conclusion
Our study has preliminarily demonstrated that the combination therapy of PD-1 inhibitor and doxorubicin is safe and effective for advanced STS, providing a basis for further studies investigating doxorubicin and PD-1 inhibitor combination regimens.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. Lindner LH, Litière S, Sleijfer S, et al. Prognostic factors for soft tissue sarcoma patients with lung metastases only who are receiving first-line chemotherapy: an exploratory, retrospective analysis of the European Organization for Research and treatment of cancer-soft tissue and bone sarcoma. Int J Cancer. 2018;142(12):2610–2620. doi:10.1002/ijc.31286
3. Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization classification of tumors of soft tissue and bone. Cancer. 2014;120(12):1763–1774. doi:10.1002/cncr.28657
4. Sarcoma Committee of Chinese Anti-Cancer Association; Chinese Society of Clinical Oncology. Chinese expert consensus on diagnosis and treatment of soft tissue sarcomas (version 2015). Zhonghua Zhong Liu Za Zhi. 2016;38(4):310–320. doi:10.3760/cma.j.issn.0253-3766.2016.04.013
5. Trans-Atlantic Retroperitoneal Sarcoma Working Group. Management of metastatic retroperitoneal sarcoma: a consensus approach from the Trans-Atlantic Retroperitoneal Sarcoma Working Group (TARPSWG). Ann Oncol. 2018;29(4):857–871. doi:10.1093/annonc/mdy052
6. Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled Phase 3 trial. Lancet Oncol. 2014;15(4):415–423. doi:10.1016/s1470-2045(14)70063-4
7. von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(5):536–563. doi:10.6004/jnccn.2018.0025
8. van der Graaf WTA, Blay J-Y, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi:10.1016/s0140-6736(12)60651-5
9. Tian Z, Wang X, Liu Z, et al. Safety and efficacy of combination therapy with apatinib and doxorubicin in metastatic soft tissue sarcomas: an observational study from multiple institutions. Cancer Manag Res. 2019;11:5293–5300. doi:10.2147/cmar.S207150
10. In GK, Hu JS, Tseng WW. Treatment of advanced, metastatic soft tissue sarcoma: latest evidence and clinical considerations. Ther Adv Med Oncol. 2017;9(8):533–550. doi:10.1177/1758834017712963
11. Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493–1501. doi:10.1016/s1470-2045(17)30624-1
12. D’Angelo SP, Mahoney MR, Van Tine BA, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19(3):416–426. doi:10.1016/s1470-2045(18)30006-8
13. Zuo W, Zhao L. Recent advances and application of PD-1 blockade in sarcoma. Onco Targets Ther. 2019;12:6887–6896. doi:10.2147/ott.S220045
14. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
15. Wang C, Kulkarni P, Salgia R. Combined checkpoint inhibition and chemotherapy: new era of 1st-line treatment for non-small-cell lung cancer. Mol Ther Oncolytics. 2019;13:1–6. doi:10.1016/j.omto.2019.02.001
16. Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, Phase 1 trials. Lancet Oncol. 2018;19(10):1338–1350. doi:10.1016/s1470-2045(18)30495-9
17. Cyprian FS, Akhtar S, Gatalica Z, Vranic S. Targeted immunotherapy with a checkpoint inhibitor in combination with chemotherapy: a new clinical paradigm in the treatment of triple-negative breast cancer. Bosn J Basic Med Sci. 2019;19(3):227–233. doi:10.17305/bjbms.2019.4204
18. Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920–928. doi:10.1038/s41591-019-0432-4
19. Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–497. doi:10.1016/s0140-6736(16)30587-6
20. Li J, Zhang B, Yue C, et al. Strategies to release doxorubicin from doxorubicin delivery vehicles. J Drug Target. 2017;26(1):9–26. doi:10.1080/1061186x.2017.1363209
21. Keam SJ. Toripalimab: first global approval. Drugs. 2019;79(5):573–578. doi:10.1007/s40265-019-01076-2
22. Markham A, Keam SJ. Camrelizumab: first global approval. Drugs. 2019;79(12):1355–1361. doi:10.1007/s40265-019-01167-0
23. Kepp O, Galluzzi L, Martins I, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30(1):61–69. doi:10.1007/s10555-011-9273-4
24. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714. doi:10.1016/j.ccell.2015.10.012
25. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi:10.1016/j.immuni.2013.06.014
26. Mathew M, Enzler T, Shu CA, Rizvi NA. Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther. 2018;186:130–137. doi:10.1016/j.pharmthera.2018.01.003
27. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi:10.1016/s1470-2045(16)30498-3
28. Lazzari C, Karachaliou N, Bulotta A, et al. Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: is this the beginning of the end for cancer? Ther Adv Med Oncol. 2018;10:1758835918762094. doi:10.1177/1758835918762094
29. Borghaei H, Langer CJ, Gadgeel S, et al. 24-month overall survival from KEYNOTE-021 cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2019;14(1):124–129. doi:10.1016/j.jtho.2018.08.004
30. Huang J, Mo H, Zhang W, et al. Promising efficacy of SHR-1210, a novel anti–programmed cell death 1 antibody, in patients with advanced gastric and gastroesophageal junction cancer in China. Cancer. 2018;125(5):742–749. doi:10.1002/cncr.31855
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

