Back to Journals » Neuropsychiatric Disease and Treatment » Volume 12
Safety and efficacy of paliperidone extended-release in Chinese patients with schizophrenia: a 24-week, open-label extension of a randomized, double-blind, placebo-controlled study
Authors Hongyan Z, Li H , Liu Y, Wu C, Wu Q, Nuamah I, Jianguo S, Shiping X, Gang W, Gopal S
Received 19 May 2015
Accepted for publication 30 September 2015
Published 8 January 2016 Volume 2016:12 Pages 69—77
DOI https://doi.org/10.2147/NDT.S88875
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Hongyan Zhang,1 Huafang Li,2 Yanning Liu,3 Cathy Wu,3 Qingqi Wu,3 Isaac Nuamah,4 Jianguo Shi,5 Shiping Xie,6 Gang Wang,7 Srihari Gopal4
1Peking University 6th Hospital, Peking University Institute of Mental Health, Key Laboratory of Mental Health, Ministry of Health (Peking University), Beijing, 2Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, 3Janssen Research & Development, Shanghai, People’s Republic of China; 4Janssen Research & Development, LLC, Titusville, NJ, USA; 5Xian Mental Health Center, Xian, 6Department of Psychiatry, Nanjing Brain Hospital, Nanjing, 7Mood Disorders Center, Beijing Anding Hospital, Beijing, People’s Republic of China
Objectives: The long-term safety, tolerability, and efficacy of paliperidone extended-release (ER) were evaluated in Chinese patients with schizophrenia.
Methods: Patients (aged ≥18 years) with schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria) who had completed run-in (8-week), stabilization (6-week), and double-blind (DB) phases (variable) of a phase-3, placebo-controlled study entered this 24-week, open-label extension (OLE) study. These patients, who had either experienced a relapse or remained relapse-free through DB phase of the study, were treated with flexible-dose paliperidone-ER (3–12 mg/day) during the OLE phase. Major safety evaluations included treatment-emergent adverse events (TEAEs) and extrapyramidal symptoms. Efficacy endpoints included changes in Positive and Negative Syndrome Scale total score, Clinical Global Impression-Severity scale, and Personal and Social Performance scale from OLE baseline to OLE endpoint.
Results: Out of 106 patients who entered the OLE phase (placebo: 59, paliperidone-ER: 47), a total of 85 (80%) completed it. Thirty-five (33%) patients experienced at least one TEAE; most common were akathisia, somnolence, nasopharyngitis, and constipation (3.8% each). Serious TEAEs were noted in two patients (completed suicide; schizophrenia worsening). No TEAEs with an onset during the OLE phase led to discontinuation. Extrapyramidal symptoms related-TEAEs were reported in eight (7.5%) patients. Mean (standard deviation) changes in Positive and Negative Syndrome Scale total scores (–10.4 [13.2]), Clinical Global Impression-Severity scores (–0.6 [0.96]) and Personal and Social Performance scores (7.4 [13.2]) from OLE baseline to OLE endpoint showed patients who had been treated with placebo during the DB phase experienced more pronounced improvements.
Conclusion: In this OLE study, flexibly dosed paliperidone-ER (3–12 mg/day) was tolerable and efficacious in Chinese patients with schizophrenia.
Keywords: CGI-S score, paliperidone, PANSS score, PSP score
Introduction
Schizophrenia is a major psychiatric disability, accounting for more than 50% of all psychotic disorders in the adult Chinese population.1 As a result of improved treatment effectiveness and tolerability profiles, use of atypical antipsychotic medications in Chinese patients has increased significantly over the past decade, although clinical trial data in this population remains very limited.2
Paliperidone extended-release (ER) is a once-daily atypical antipsychotic, approved in many countries, including People’s Republic of China, for the treatment of schizophrenia in adults. Several clinical studies were conducted to demonstrate both the short-term and long-term efficacy and safety of paliperidone-ER in patients with schizophrenia.3 The majority of these studies were conducted in Western populations. However, physiological differences between populations can impact drug metabolism and thus its efficacy and safety. Symptom presentation, including suicide prevalence, as well as treatment response to antipsychotic therapy may relate to race and ethnicity differences among patients.4–6
To date, only three studies of paliperidone-ER have been conducted by the sponsor in Chinese patients: an 8-week, open-label, flexible-dose study of paliperidone-ER in Chinese patients with first-episode psychosis;7 a 12-week, non-randomized, single-arm, multicenter study in Chinese patients with non-acute schizophrenia,8 and a randomized, double-blind (DB), placebo-controlled relapse prevention study.9 Patients enrolled in these studies typically were exposed to paliperidone-ER 2 to 3 months and published data on the long-term safety and efficacy of paliperidone-ER in Chinese patients are lacking.
The present study was conducted to support the long-term safety, tolerability, and efficacy of paliperidone-ER (3–12 mg/day) in Chinese patients with schizophrenia and is the 6-month, open-label extension (OLE) of the relapse prevention study.9
Methods
Study participants
This study is registered at ClinicalTrials.gov: NCT01662310. Patients of either sex, aged ≥18 years, diagnosed with schizophrenia, based on Diagnostic and Statistical Manual of Mental Disorders, 4th version, for at least 1 year before screening, and a Positive and Negative Syndrome Scale (PANSS) total score between 70 and 120 (inclusive), at screening and baseline were eligible for enrollment in the study.
Major exclusion criteria for the study included: drug dependence (excluding nicotine and caffeine dependence) within 6 months before screening according to Diagnostic and Statistical Manual of Mental Disorders, 4th edition, history of cardiovascular, respiratory, neurologic, renal, hepatic, endocrine, or immunologic diseases, presence of circumstances that may increase the risk of the occurrence of torsade de pointes or sudden death, heart rate <50 beats per minute, presence of congenital prolongation of the QT interval or demonstration of repeated prolonged QTc Fridericia interval >450 ms in >1 electrocardiogram (ECG), neuroleptic malignant syndrome and hypersensitivity to risperidone, paliperidone, or their excipients. Patients treated with clozapine for treatment-refractory or treatment-resistant schizophrenia, monoamine oxidase inhibitor antidepressants within 4 weeks before screening, depot antipsychotic drugs within 120 days, paliperidone palmitate within 10 months or electroconvulsive therapy within 60 days before screening, and pregnant and lactating women, were all excluded from the study.
Only patients who experienced a relapse or remained relapse-free during the DB phase, or who were still enrolled in the DB or run-in/stabilization phase of the study at the time of study termination were eligible to enter the OLE.
The Independent Ethics Committee or Institutional Review Board at each study site approved the protocol and the study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Clinical Practices and applicable regulatory requirements. All patients provided written informed consent before enrollment.
Study design
This was an OLE (24-week) phase of a randomized, DB, placebo-controlled, parallel group study conducted at 18 sites within the People’s Republic of China from June 2011 to April 2013. Only the data collected from patients who participated in the OLE phase of the study are included in this paper. However, a brief description of the prior phases is provided in Figure 1.
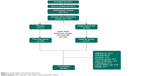  | Figure 1 Study design and patient disposition. |
Patients were treated with flexibly dosed paliperidone-ER (3 to 12 mg/day) during the OLE phase. All patients who entered the OLE were provided a starting dose of 6 mg which was given at termination of DB phase of the study. Investigators could adjust the dose with increments or decrements of 3 mg at their discretion. At trial visits, patients were provided medication in a blister pack. Inventory of each blister pack, and the amount of drug used was noted at each study visit. A patient was considered to have completed the OLE phase of the study on completion of all 24 weeks of the OLE phase and having assessments at week 24.
Concomitant medication
Oral acetaminophen, non-steroidal anti-inflammatory drugs, antihypertensives, beta-adrenergic blockers, oral benzodiazepine, and non-benzodiazepine hypnotic agents were allowed during the OLE phase. Monoamine oxidase inhibitors and antipsychotics (oral or injectable) other than paliperidone-ER were not allowed during the OLE phase.
Safety assessments
Safety evaluations in the OLE phase included treatment-emergent adverse events (TEAEs), Columbia Suicide Severity Rating Scale, serum and urine pregnancy tests for female patients, clinical laboratory parameters, ECGs, weight, vital signs, and physical examinations. Serum concentrations of prolactin were also determined. TEAEs were assessed at each study visit using elicitation by the investigator with an open-ended question. No specific checklists were used. Extrapyramidal symptoms (EPS) were measured using the Abnormal Involuntary Movement Scale for dyskinesia, Barnes Akathisia Rating Scale for akathisia and the Simpson-Angus Scale for Parkinsonism. These three scales for EPS were collected only in the DB portion of the study.
Efficacy assessments
Changes in PANSS total score, PANSS subscale scores (positive subscale, negative subscale, and the general psychopathology subscale), PANSS factor scores (positive symptoms, negative symptoms, disorganized thoughts, uncontrolled hostility/excitement, and anxiety/depression), Clinical Global Impression-Severity (CGI-S) scale, and Personal and Social Performance (PSP) scale from OLE baseline to OLE endpoint were evaluated as efficacy parameters.
Scales used in this study were translated into Mandarin Chinese and administered by qualified raters at each study site. Measures of rater quality compared to a gold standard were performed at the investigator meeting and it was found that the level of agreement was high (kappa >0.60). The validation of these scales was performed elsewhere.10,11
Statistical methods
Intent-to-treat (OLE) analysis set included all patients who received at least one dose of OLE medication and this set was used for efficacy and safety analyses in the OLE phase. Descriptive summaries for the change in efficacy parameters from OLE baseline over time were presented. Safety and tolerability profile during the OLE phase was also summarized using descriptive statistics. The last observation carried forward (LOCF) approach was implemented when measurements at some intermittent visits were not done or when patients withdrew early from the OLE phase.
Results
Patient population
Of the 201 patients enrolled in the run-in phase of the study, 106 entered the OLE phase. These included 59 patients who had received placebo (Pbo/Pali group) and 47 patients who had received paliperidone-ER (Pali/Pali group) in the DB phase of the study. Out of the 106 OLE patients, 85 (80%) completed the 24-week OLE phase of the study (Figure 1). The most frequent reason for discontinuation was withdrawal of consent (8%).
The majority of patients who entered the OLE phase were women (59%), with a mean (standard deviation [SD]) age of 30.7 (10.5) years at run-in enrollment. At OLE baseline, based on the body mass index (BMI), 62% of the patients were classified as having normal body weight. The mean (SD) OLE baseline body weight was 65.8 (15.0) kg and mean (SD) OLE baseline BMI was 24.5 (4.3) (kg/m2) (Table 1). The mean dose of paliperidone-ER used was 8.62 mg/day and mean exposure duration was 147.1 days.
Safety results
A total of 35 (33%) patients experienced at least one TEAE in the OLE phase; the incidence was higher in patients from the Pbo/Pali ER group (37.3%) than the Pali/Pali group (27.7%). The most common TEAEs were akathisia, somnolence, nasopharyngitis, and constipation (3.8% each) (Table 2). One patient died (completed suicide: Pali/Pali group) during the OLE phase. Two patients (4.3%; including the one who completed suicide) experienced serious TEAEs (completed suicide and worsening of schizophrenia: Pali/Pali group). No TEAEs leading to permanent discontinuation of the study drug were noted in this phase.
Eight out of 106 patients, 7.5% experienced EPS-related TEAEs. The incidence was higher in patients from the Pbo/Pali group (11.9%) than in the Pali/Pali group (2.1%). The most common EPS-related TEAEs were hyperkinesia (5.7%), akathisia (3.8%), and restlessness (1.9%). Prolactin increases were more pronounced in women than in men (Pbo/Pali: 83% women versus 76% men; Pali/Pali: 19% women versus 5% men). Increased prolactin levels were not commonly associated with reported TEAEs. One prolactin-related TEAE (galactorrhea) was noted in a patient from the Pali/Pali group.
A majority of patients (99%) had a maximum Columbia Suicide Severity Rating Scale score of 0 (no suicidal ideation) and 1% of patients had a score of 1 (wish to be dead). There were no significant increases from OLE baseline in fasting glucose values or in body weight. Patients from the Pbo/Pali group showed greater change in mean (SD) BMI (0.39 [1.6] kg/m2) from OLE baseline to endpoint as compared with Pali/Pali group (0.23 [1.2] kg/m2). Similar trend was observed for mean (SD) change in triglycerides (mmol/L) in Pbo/Pali group (0.42 [0.8]) versus Pali/Pali group (0.13 [0.7]). Change in mean (SD) cholesterol (mmol/L) was 0.12 (0.8) for Pbo/Pali group and −0.03 (0.7) for Pali/Pali group. No clinically relevant changes were observed in vital signs, ECG recordings or other clinical laboratory parameters.
Efficacy results
A decrease of −10.4 (13.2) in the mean (SD) PANSS total score was observed from OLE baseline to endpoint in the total group, indicating improvement in the severity of schizophrenic symptoms (Table 3). Patients from the Pbo/Pali group showed greater improvement in mean (SD) PANSS total score (−15.4 [12.4]) from OLE baseline to endpoint as compared with Pali/Pali group (−3.9 [11.3]) (Figure 2). Overall, patients (total group) showed improvement in mean (SD) PANSS subscale scores (positive subscale: −3.7 [5.0]; negative subscale: −1.5 [3.7]; psychopathology subscale: −5.2 [6.9]) and PANSS factor scores (positive symptoms: −3.3 [4.8]; negative symptoms: −1.5 [3.96]; disorganized thoughts: −2.0 [3.4]; uncontrolled hostility/excitement: −2.1 [3.7]; anxiety/depression: −1.4 [2.6]) from OLE baseline to endpoint. Similarly, greater improvements were noted from OLE baseline to OLE endpoint in Pbo/Pali group than the Pali/Pali group for the mean (SD) CGI-S scores (Table 4, Figure 3) and PSP scores (Table 4, Figure 4).
Discussion
The safety and tolerability profile of paliperidone-ER over the course of a 24-week period in the OLE phase of this study was generally consistent with the earlier open-label, phase-3 paliperidone-ER study of shorter duration (8-week) in patients with schizophrenia from People’s Republic of China7 as well as with placebo-controlled studies conducted in more global populations.12 Safety results of this current OLE were also consistent with a previous long-term OLE study conducted in a Western population.13
The incidences of EPS-related TEAEs and increased prolactin levels in this OLE were less than those in the DB phase of the same study, as well as compared with previous short-term paliperidone-ER studies conducted in People’s Republic of China,8,13 short-term pivotal global studies,14–16 and a long-term (1-year) OLE global study17 without any known reason. No TEAEs leading to permanent discontinuation of the study drug were noted in this OLE study, indicating that the TEAEs did not have a major impact on patients continuing on long-term paliperidone-ER treatment. Also, the number of patients receiving anti-EPS medication in the OLE phase of the study was numerically lower as compared with the DB phase.
Patients who entered the OLE phase of the study showed clinically meaningful improvements in efficacy parameters. As would be expected, patients previously treated with placebo in the DB phase had greater improvements in total PANSS-, CGI-S-, and PSP scores during the OLE phase, where they received active drug, as compared to those patients who had been previously treated with paliperidone-ER during the DB phase and then continued with active treatment during the OLE.
There was no active or placebo comparator used in this OLE phase, which limits the ability to draw conclusions relative to a control population. In addition, because of the lack of a comparator group, conducting inferential statistical analysis was not possible, so the results are descriptive only. However, the open-label flexible-dose treatment design used in this study more closely approximates clinical treatment practice used in hospital settings.
In this OLE study, 24-week treatment with flexibly dosed paliperidone-ER (3–12 mg/day) was tolerable and efficacious, consistent with findings from previous pivotal and open-label (1-year) global studies. These results support long-term paliperidone-ER treatment in patients from People’s Republic of China with schizophrenia.
These data were presented as a poster at the XVI Word Congress of Psychiatry, Madrid, Spain, September 14–18, 2014.
Acknowledgments
The authors thank Rishabh Pandey (SIRO Clinpharm Pvt. Ltd.) for writing assistance and Dr Wendy P Battisti (Janssen Research & Development, LLC) for additional editorial assistance. The authors also thank the study participants, without whom this study would not have been accomplished, as well as the following investigators for their participation in this study:
Qinggang Chen; Chengge Gao; Tiansheng Guo; Jian Hu; Huafang Li; Keqing Li; Luxian Lv; Yuping Ning; Jianguo Shi; Qingrong Tan; Gang Wang; Xiaoping Wang; Shiping Xie; Xiufeng Xu; Fude Yang; Hongyan Zhang; Kerang Zhang; Jingping Zhao.
Authors’ contributions
Drs Srihari Gopal, Cathy Wu, Qingqi Wu, Isaac Nuamah, and Ms Yanning Liu were involved in study design, study conduct, data interpretation, and drafting the manuscript; Drs Hongyan Zhang, Huafang Li, Jianguo Shi, Shiping Xie and Gang Wang participated as principal investigators and were involved in data collection, data interpretation, and manuscript composition. All authors met ICMJE criteria and all those who fulfilled those criteria are listed as authors. All authors had access to the study data and made the final decision about where to present these data.
Disclosure
This study was funded by Janssen Research and Development, LLC, New Jersey, USA. The sponsor also provided a formal review of this manuscript. Drs Srihari Gopal, Cathy Wu, Qingqi Wu, Isaac Nuamah and Ms Yanning Liu are employed by Janssen Research and Development. The other authors report no conflicts of interest in this work.
References
Li H, Rui Q, Ning X, Xu H, Gu NA. Comparative study of paliperidone palmitate and risperidone long-acting injectable therapy in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):1002–1008. | ||
Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. | ||
Canuso CM, Battisti WP. Paliperidone extended-release: a review of efficacy and tolerability in schizophrenia, schizoaffective disorder and bipolar mania. Expert Opin Pharmacother. 2010;11(15):2557–2567. | ||
Versola-Russo JM. Cultural and demographic factors of schizophrenia. The International Journal of Psychosocial Rehabilitation. 2006;10:89–103. | ||
Williams DR, Earl TR. Commentary: Race and mental health – more questions than answers. Int J Epidemiol. 2007;36(4):758–760. | ||
Zhang XY, Al Jurdi RK, Zoghbi AW, et al. Prevalence, demographic and clinical correlates of suicide attempts in Chinese medicated chronic inpatients with schizophrenia. J Psychiatr Res. 2013;47(10):1370–1375. | ||
Si T, Tan Q, Zhang K, Wang Y, Rui Q. An open-label, flexible-dose study of paliperidone extended-release in Chinese patients with first-onset psychosis. Neuropsychiatr Dis Treat. 2015;11:87–95. | ||
Zhang H, Hao X, Wang X. An open-label study on the safety and efficacy of paliperidone extended-release in non-acute schizophrenic patients. Chinese Journal of Psychiatry. 2012;45(1). | ||
Rui Q, Wang Y, Liang S, et al. Relapse prevention study of paliperidone extended-release tablets in Chinese patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:45–53. | ||
Jiang J, Sim K, Lee J. Validated five-factor model of positive and negative syndrome scale for schizophrenia in Chinese population. Schizophr Res. 2013;143(1):38–43. | ||
Tianmei S, Liang S, Yun’ai S, et al. The Chinese version of the Personal and Social Performance Scale (PSP): validity and reliability. Psychiatry Res. 2011;185(1–2):275–279. | ||
Meltzer HY, Bobo WV, Nuamah IF, et al. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week, placebo-controlled studies. J Clin Psychiatry. 2008;69(5):817–829. | ||
Guoxing C, Guojian X. Observation of efficacy and safety of paliperidone sustained release tablets and domestic olanzapine in treatment of schizophrenia. Chongqing Medicine. 2011;17:13. | ||
Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr Res. 2007;93(1–3):117–130. | ||
Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res. 2007;90(1–3):147–161. | ||
Marder SR, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry. 2007;62(12):1363–1370. | ||
Kramer M, Simpson G, Maciulis V, et al. One-year open-label safety and efficacy study of paliperidone extended-release tablets in patients with schizophrenia. CNS Spectr. 2010;15(8):506–514. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







