Back to Journals » Journal of Pain Research » Volume 11
Safety and efficacy of an intravenous nanocrystal formulation of meloxicam in the management of moderate to severe pain following laparoscopic abdominal surgery
Authors Singla N , McCallum SW , Mack RJ, Freyer A , Hobson S , Du W
Received 25 January 2018
Accepted for publication 10 July 2018
Published 18 September 2018 Volume 2018:11 Pages 1901—1903
DOI https://doi.org/10.2147/JPR.S163736
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Neil Singla,1 Stewart W McCallum,2 Randall J Mack,2 Alex Freyer,2 Sue Hobson,2 Wei Du3
1Lotus Clinical Research, Pasadena, CA, USA; 2Research and Development, Recro Pharma, Inc., Malvern, PA, USA; 3Clinical Statistics Consulting, Blue Bell, PA, USA
Postoperative pain after laparoscopic surgery is one of the major concerns of patients. Improper pain management can be associated with varied respiratory, cardiovascular, gastrointestinal, and psychological complications. Pain after laparoscopic surgery may be transient or persistent2 and opioids have traditionally been the mainstay treatment for postoperative pain.3 Increasing evidence exists to support a multimodal treatment approach for postoperative pain that reduces opioid side effects and decreases pain intensity (PI) scores.3 A proprietary nanocrystal intravenous (NIV) formulation of the non-steroidal anti-inflammatory meloxicam, a long-acting preferential cyclooxygenase-2 inhibitor, is being evaluated with the aim of providing rapid and sustained analgesia.
Introduction
Postoperative pain after laparoscopic surgery is one of the major concerns of patients.1 Improper pain management can be associated with varied respiratory, cardiovascular, gastrointestinal, and psychological complications.1 Pain after laparoscopic surgery may be transient or persistent2 and opioids have traditionally been the mainstay treatment for postoperative pain.3 Increasing evidence exists to support a multimodal treatment approach for postoperative pain that reduces opioid side effects and decreases pain intensity (PI) scores.3 A proprietary nanocrystal intravenous (NIV) formulation of the non-steroidal anti-inflammatory meloxicam, a long-acting preferential cyclo-oxygenase-2 inhibitor, is being evaluated with the aim of providing rapid and sustained analgesia.
Material and methods
This was a phase II, randomized, double-blind, placebo and active controlled study in subjects undergoing laparoscopic abdominal surgery. Subjects were randomized 1:1:1:1:1 to intravenous (IV) doses of placebo, ketorolac every 6 hours (Q6H), meloxicamNIV 7.5 mg Q12H, meloxicamNIV15 mg Q12H, or meloxicamNIV30 mg once daily (QD) for up to 48 hours. Each subject was dosed Q6H (with placebo as applicable) to maintain blinding. Subjects were discharged from the study unit 48 hours after the initial dose of study drug and followed as outpatients through Day 28.
Efficacy was assessed by subject reports of PI on a 100 mm Visual Analog Scale anchored at 0 for no pain and 100 for worst possible pain. Rescue medication (parenteral morphine 2–6 mg) was available any time after the initial dose of study drug. Missing PI scores were imputed for the efficacy analysis using a 2-hour windowed last observation carried forward (W2LOCF) approach.
Safety was monitored through the reporting of adverse events, clinical laboratory testing, vital sign assessments, 12-lead electrocardiograms (ECGs), and wound site assessments.
The study was expected to enroll 200 subjects; however, the study was terminated for business reasons after 50 subjects enrolled. As a result, the original analyses were limited to disposition, demographics, and safety. Efficacy data were subsequently analyzed post hoc. No formal statistical analysis was performed.
The Aspire Institutional Review Board approved the protocol and the study was conducted according to Good Clinical Practices. All subjects provided written informed consent. Trial registration: ClinicalTrials.gov (Identifier: NCT01436032).
Results
Fifty subjects received at least one dose of study drug and 47 completed the study. The mean age overall was 41.8 years and most subjects were white and male.
Safety
Headache, dry mouth, dysuria, and nausea were the most common treatment emergent adverse events (TEAEs) reported across the meloxicam groups. Reported TEAE rates were similar to placebo (Table 1). Most TEAEs were mild in intensity and unrelated to treatment.
No deaths were reported. One ketorolac-treated subject who underwent hernia repair had a serious adverse event of post procedural hemorrhage. One meloxicamNIV 7.5 mg Q12H treated subject had a TEAE of mild truncal maculopapular rash that led to discontinuation. This subject had no signs of anaphylaxis.
The mean changes from baseline in laboratory parameters were small and similar across treatment groups. Leukocytosis in the meloxicamNIV 7.5 mg Q12H group and anemia secondary to post procedural hemorrhage in the ketorolac group were TEAEs. Mean changes from baseline in vital signs and ECG parameters were small and similar across treatment groups. Wound examinations showed normal healing in the meloxicamNIV and ketorolac groups.
Efficacy
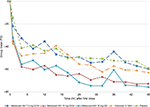
Mean PI differences over the 48-hour assessment period were similar between the meloxicamNIV15 mg Q12H and 30 mg QD groups and similar to ketorolac IV Q6H (Figure 1).
Conclusions
In this small study, meloxicam NIV was generally well tolerated with a safety profile similar to placebo. PI differences over time suggest meloxicamNIV15 mg Q12H, meloxicamNIV30 mg QD, and ketorolac IV Q6H may provide pain relief after laparoscopic abdominal surgery.
Acknowledgment
Funding for this research was provided by Recro Pharma, Inc., Malvern, PA 19355, USA.
Author contributions
NS contributed to the design of the study and its implementation. NS managed the subjects and contributed to acquisition of the data. WD provided statistical guidance for data analysis and interpretation. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
NS is an employee of Lotus Research, which conducted this trial. WD received consultancy fees from Recro Pharma, Inc., Malvern, PA, USA. RJM, SWM, AF, and SH are employees and security holders of Recro Pharma, Inc., Malvern, PA, USA. The authors report no other conflicts of interest in this work.
References
Golzari SE, Nader ND, Mahmoodpoor A. Underlying mechanisms of postoperative pain after laparoscopic surgery. JAMA Surg. 2016;151(3):295–296. | ||
Alexander JI. Pain after laparoscopy. Br J Anaesth. 1997;79(3):369–378. | ||
Veerabhadram G, Cellini C. Postoperative pain control. Clinic Colon Rectal Surg. 2013;26(3):191–196. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.