Back to Journals » Cancer Management and Research » Volume 11
Safety and efficacy of amrubicin monotherapy in patients with platinum-refractory metastatic neuroendocrine carcinoma of the gastrointestinal tract: a single cancer center retrospective study
Authors Kitagawa Y, Osumi H , Shinozaki E , Ota Y, Nakayama I, Suzuki T , Wakatsuki T, Ichimura T , Ogura M , Ooki A , Takahari D , Suenaga M , Chin K , Yamaguchi K
Received 10 January 2019
Accepted for publication 29 May 2019
Published 25 June 2019 Volume 2019:11 Pages 5757—5764
DOI https://doi.org/10.2147/CMAR.S201048
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Rituraj Purohit
Yusuke Kitagawa, Hiroki Osumi, Eiji Shinozaki, Yumiko Ota, Izuma Nakayama, Takeshi Suzuki, Takeru Wakatsuki, Takashi Ichimura, Mariko Ogura, Akira Ooki, Daisuke Takahari, Mitsukuni Suenaga, Keisho Chin, Kensei Yamaguchi
Department of Gastroenterology, Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan
Purpose: Patients with gastrointestinal neuroendocrine carcinoma (GI-NEC) have poor prognoses. Although platinum-based combination chemotherapy is commonly used as first-line treatment, the benefit of amrubicin (AMR) and salvage chemotherapy in those who develop platinum-refractory GI-NEC remains unknown. This study aimed to evaluate the efficacy and safety of AMR monotherapy in patients with platinum-refractory GI-NEC.
Patients and methods: Platinum-refractory GI-NEC patients who received AMR monotherapy between April 2012 and September 2017 were retrospectively analyzed. The overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and adverse events were evaluated. PFS and OS were estimated using Kaplan-Meier methods and compared using log-rank tests.
Results: In total, 16 patients were enrolled. Of them, 13 (81.3%), 1 (6.2%), and 2 (12.5%) received cisplatin plus irinotecan, cisplatin plus etoposide, and fluoropyrimidine plus platinum, respectively, before AMR monotherapy. The primary sites of NEC included the esophagus (N=3, 18.8%), stomach (N=10, 62.5%), duodenum (N=1, 6.2%) and colorectum (N=2, 12.5%). Patients were administered a median of 3 (range, 1–15) cycles of AMR. The ORR was 6.3%, and the median PFS and OS were 2.9 months (95% CI: 1.7–7.4) and 13.8 months (95% CI: 7.9–23.5), respectively. Neutropenia was the most serious adverse event. Grade 3 or higher neutropenia and febrile neutropenia occurred in 50.0% and 6.2% of patients, respectively. Other nonhematological toxicities were not severe, and no treatment-related deaths occurred. The 10 patients who received subsequent chemotherapy after AMR had significantly longer OS than those who did not (17.3 months vs 8.9 months; p=0.018). The median PFS of those who received organ-specific subsequent chemotherapy after AMR was 3.8 months, which was longer than that of those who received prior AMR.
Conclusion: AMR is feasible with minimal side effects for platinum-refractory GI-NEC. Organ-specific subsequent chemotherapy after AMR may improve patient survival.
Keywords: amrubicin monotherapy, platinum refractory, gastrointestinal neuroendocrine carcinoma
Introduction
Neuroendocrine tumors (NETs) are diagnosed using their characteristic histologic features and immunoprofiles. In the recent World Health Organization (WHO) classification for gastroenterohepatic NETs, a grading system based on mitotic count and the Ki-67 proliferation index is recommended for the classification of NETs. Using this system, gastrointestinal NETs are classified into one of these three categories: NET grade G1, NET grade G2, or neuroendocrine carcinoma (NEC).1 Among these three categories, NEC is a poorly differentiated, high-grade malignant tumor. It was previously termed poorly differentiated neuroendocrine carcinoma and includes small-cell carcinoma and large-cell NEC. There are various primary sites of NEC, with gastrointestinal NEC (GI-NEC) being the most common, accounting for 20–68% of cases of extra-pulmonary NEC.2
GI-NEC has an aggressive natural history that is characterized by early and widespread metastasis; at least 50.0% of patients have distant metastases at diagnosis.3 Platinum-containing chemotherapy is commonly used for the treatment of advanced GI-NEC. In Japan, this includes etoposide plus cisplatin (EP) or irinotecan plus cisplatin (IP), which are also used for the treatment of small-cell lung carcinoma (SCLC).2,4 For esophageal lesions, Chin et al reported an 83% response rate to IP.5 In addition, Okita et al reported a 75% response rate in patients administered IP for gastric NEC.6 The first randomized phase III trial evaluating the differences between EP and IP regimens in GI-NEC patients is currently being conducted by the Japan Clinical Oncology Group.7
Amrubicin (AMR), a topoisomerase II inhibitor, showed an activity and tolerability when used as a second-line treatment in patients with refractory-relapsed SCLC after first-line platinum-based chemotherapy.8 However, although AMR is similarly used as a second-line treatment for GI-NEC patients for whom first-line platinum-based chemotherapy has failed,4,9–11 its efficacy in this context remains unclear. Regarding its safety, in a phase III trial in which AMR was administered to non-small-cell lung cancer patients, grade 3 or higher neutropenia occurred in 82.7% of all patients.12 Although similar adverse events are predicted in GI-NEC, its safety in GI-NEC patients remains unclear. Therefore, the present study aimed to evaluate the efficacy and safety of AMR monotherapy as a second-line treatment in patients with recurrent GI-NEC after first-line platinum-based chemotherapy.
Materials and methods
Patients
This retrospective study included patients diagnosed with GI-NEC according to the WHO 2010 NET Grading system and received AMR monotherapy after failure of a platinum-based chemotherapy regimen between April 2012 and September 2017 at the Cancer Institute Hospital of Japanese Foundation for Cancer Research. All diagnoses were performed, based on immunohistochemistry (IHC) in addition to hematoxylin-eosin staining of the biopsy or surgical specimen. Chromogranin and synaptophysin were used for the IHC staining. The present study was performed in accordance with the Declaration of Helsinki and was approved by the institutional review board (Registry no: 2018–1006). The protocol was described in the Web site of the hospital, and the subjects were provided with the opportunity to opt out, and therefore, no new consent was required from the patients.
Data collection
The medical records and imaging results were reviewed. Data on the patient’s age, sex, Eastern Cooperative Oncology Group Performance Status, primary lesion of GI-NEC, histological type of the primary site, metastatic sites, history of prior chemotherapy, starting dose of AMR, number of cycles, tumor response, toxicity, date of disease progression, and date of the last follow-up were obtained.
Treatment and evaluation
AMR was administered at a dose of 30–45 mg/m2 on days 1–3 every 3–4 weeks. Chest and abdominal tumors were assessed at screening and after every 2–3 months using computed tomography or magnetic resonance imaging. The overall tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors v1.1, and toxicity was graded according to the Common Terminology Criteria for Adverse Events v4.0. Progression-free survival (PFS) was measured from the date of initiation of AMR therapy to the date on which disease progression was confirmed or the final day of follow-up without disease progression. Overall survival (OS) was measured from the date of initiation of AMR therapy until the final date of follow-up.
Statistical analysis
PFS and OS rates were estimated using the Kaplan-Meier method and compared using the log-rank test. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). All P-values are two-sided, and P<0.05 was considered significant.
Results
Patient characteristics
The baseline clinical characteristics of the 16 patients are summarized in Table 1. The median follow-up period at the time of the analysis was 9.0 months (range, 1.1–25.8 months). The primary site of the lesion was the esophagus, stomach, duodenum, and colorectum in 3, 10, 1, and 2 patients, respectively. All patients were diagnosed with NEC based on a biopsy specimen. Chromogranin and synaptophysin were used for all patients (100%) as the IHC staining and CD56 was used for 9 patients (56.3%). For first-line chemotherapy, 15 patients received a cisplatin-containing regimen: 13 patients were treated with cisplatin plus irinotecan, 1 patient with cisplatin plus etoposide, and 1 patient with cisplatin plus capecitabine. The remaining patient who was not administered a cisplatin-containing regimen was treated with oxaliplatin plus S-1.
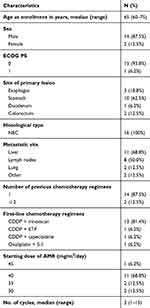 | Table 1 Baseline patient characteristics (N=16) |
Treatment outcomes and adverse events
The initial dose of AMR was determined by the treating physician with consideration of the patient’s characteristics. The dose varied between patients, but the majority (N=11) received a dose of 40 mg/m2/day. All patients received AMR monotherapy every 3 or 4 weeks. The median number of cycles per patient was three (range, 1–15) (Table 1). The overall response rate (ORR) was 6.2%, and the disease control rate was 43.8% (Table 2). The median PFS and OS were 2.9 months (95% confidence interval [CI], 1.7–7.4) and 13.8 months (95% CI, 7.9–23.5), respectively (Figure 1). Median PFS in esophageal, gastric, duodenum and colorectal were 2.1 month, 3.7 months, 1.1 months and 5.4 months, respectively (p=0.09). Ten patients (62.5%) received subsequent chemotherapy after AMR administration (Table 3). Although the median PFS of the patients who received AMR was not significantly different between those who did and did not receive subsequent chemotherapy (2.1 months vs 3.7 months, p=0.89), the median OS for those who received supportive care only after AMR was significantly lower than those who were administered subsequent chemotherapy (8.9 months (95% CI, 1.1-not applicable (NA)) vs 17.3 months (95% CI, 5.9–NA); p=0.018; Figure 2). The median PFS of those who received organ-specific subsequent chemotherapy after AMR was 3.7 months, which was longer than that of those who received prior AMR. The most common adverse event was hematological toxicity, with grade 3 or higher neutropenia observed in 8 (50.0%) patients (Table 4). Febrile neutropenia was observed in one patient (6.2%). Other nonhematological toxicities were less frequent; any grade 3 or higher adverse events were not observed. No treatment-related deaths occurred.
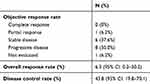 | Table 2 Efficacy of AMR monotherapy |
 | Figure 1 (A) Progression-free survival and (B) overall survival in all patients after first-line chemotherapy. Abbreviations: PFS, progression free survival; OS, overall survival. |
 | Table 3 Subsequent chemotherapy after AMR monotherapy |
 | Figure 2 Overall survival of patients who received subsequent chemotherapy after amrubicin or supportive care only. Abbreviations: OS, overall survival; NA, not applicable. |
 | Table 4 Grade 3 or higher treatment-related toxicities |
Discussion
This retrospective study evaluated the efficacy and safety of AMR monotherapy as a second-line treatment for GI-NEC. The results indicate that AMR monotherapy is a feasible treatment and that organ-specific subsequent chemotherapy after AMR may result in improved OS.
When the efficacy of AMR monotherapy as a second-line treatment for GI-NEC in this study and in previous reports are compared, the following is observed. In this study, the disease control rate was 43.8%, and the median PFS and OS were 2.9 and 13.8 months, respectively. Meanwhile, in the first report of AMR monotherapy for 5 case series of platinum-refractory GI-NEC, Asayama et al4 reported a 60% (3/5 patients) partial response rate. Moreover, the OS in the four previous reports of AMR was shorter than that of the present study, ranging from 5 months to 7.7 months (Table 5).4,9–11
 | Table 5 Patient characteristics and adverse events with AMR monotherapy in previous reports |
For subsequent chemotherapy, the chemotherapeutic regimen was selected according to the primary organ based on previous studies reporting the possibility of differences in treatment efficacy depending on the primary site of the tumor.13,14 Dasari et al reported that survival differed depending on the primary site of the tumor even in patients with GI-NEC.15
In our study, we diagnosed NEC using biopsy specimen only and gastric NEC was most frequent in this analysis (62.5%). Regarding gastric cancer, several studies have demonstrated its intratumor heterogeneity at molecular, histological, and phenotypic levels.16 Thus, we thought several cases in gastric NEC contained adenocarcinoma component, and in those cases, organ-specific chemotherapy might have efficacy. Furthermore, 2nd line chemotherapy of gastric cancer contained ramucirumab, might be effective for NEC. Mishima et al reported that ramucirumab-containing chemotherapy has greater efficacy for gastric NEC than that in gastric adenocarcinoma. They showed that the median degree of vascular VEGFR2 expression was higher in gastric NEC, and this might be because ramucirumab-containing chemotherapy showed a promising activity.17 These results indicate that chemotherapy after AMR, selected according to the primary site of the lesion, has the potential to improve patient survival.
With regard to adverse events, grade 3 or higher neutropenia was the most common adverse event, occurring in 50.0% of patients. Febrile neutropenia was observed in just one patient (6.3%). These results are similar to those of previous reports, in which the incidence of grade 3 of higher neutropenia ranged between 40% and 80% (Table 5).4,7–9 To manage neutropenia, the initial dose of AMR was adjusted according to patients’ status, such as their performance status or major organ function. To manage hematotoxicity, we found that it was feasible to adjust the dose intensity according to patient status. However, dose adjustment also has the potential to influence treatment efficacy. Additionally, the use of granulocyte-colony stimulating factor (G-CSF) has the potential to shorten the duration and severity of neutropenia. In a previous report on the use of AMR for SCLC, the researchers suggested that routine prophylactic use of G-CSF may be more desirable for the treatment of neutropenia in clinical practice.18 However, as dose intensity reflects treatment outcomes in GI-NEC patients who receive AMR monotherapy remains unclear; therefore, further studies to evaluate the efficacy of AMR monotherapy in combination with PEG G-CSF are needed.
There were several limitations to this study. First, this was a retrospective study with a small sample size. In addition, the adjustment of the initial dose of AMR according to patient status may have influenced the efficacy of AMR monotherapy. Furthermore, as NEC in this study was diagnosed via biopsy specimens, the possibility of a mixture of adenocarcinomas including mixed adenoneuroendocrine carcinoma could not be ruled out. In the viewpoint of efficacy of organ-dependent chemotherapy, the comorbidity of adenocarcinoma should be investigated.
Conclusion
AMR monotherapy was effective and safe when used for the treatment of platinum-refractory GI-NEC. Organ-specific subsequent chemotherapy after AMR may improve patient survival.
Abbreviations list
AMR, amrubicin; EP, etoposide plus cisplatin; G-CSF, granulocyte-colony stimulating factor; GI-NEC, gastrointestinal neuroendocrine carcinoma; IP, irinotecan plus cisplatin; NEC, neuroendocrine carcinoma; NETs, neuroendocrine tumors; ORR, overall response rate; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; SCLC, small-cell lung carcinoma; WHO, World Health Organization; IHC, immunohistochemistry; S-1; tegafur/ gimeracil/ oteracil.
Ethics approval and informed consent
The present study was performed in accordance with the Declaration of Helsinki and was approved by the institutional review board (Registry no: 2018-1006).
Acknowledgments
We thank the data manager Yuki Horiike. An abstract of this paper was presented at the ESMO 20th World Congress on Gastrointestinal Cancer as a poster presentation. The poster’s abstract was published in “Poster Abstracts” in Annals of Oncology, volume 29, Issue supplement 5, 1 June 2018. The authors received no funding for conducting the research.
Disclosure
Dr Kensei Yamaguchi reports grants and personal fees from Daiichi Sankyo, Chugai Pharma, Taiho Pharmaceutical, Eli Lilly, Yakult Honsha, and Ono Pharmaceutical, personal fees from Bristol-Myers Squibb Japan, Merck Serono, Takeda, and Sanofi, grants from MSD Oncology and Dainippon Sumitomo Pharma, and nothing from Gilead Sciences, during the conduct of the study; and no other conflicts of interest in this work. The other authors report no conflicts of interest in this work.
References
1. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11–16. doi:10.1016/j.anndiagpath.2017.04.005
2. Yamaguchi T, Machida N, Morizane C, et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014;105(9):1176–1181. doi:doi:10.1111/cas.12473
3. Brenner B, Tang LH, Shia J, Klimstra DS, Kelsen DP. Small cell carcinomas of the gastrointestinal tract: clinicopathological features and treatment approach. Semin Oncol. 2007;34(1):43–50. doi:doi:10.1053/j.seminoncol.2006.10.022
4. Asayama M, Fuse N, Yoshino T, et al. Amrubicin for the treatment of neuroendocrine carcinoma of the gastrointestinal tract: a retrospective analysis of five cases. Cancer Chemother Pharmacol. 2011;68(5):1325–1330. doi:10.1007/s00280-011-1619-7
5. Chin K, Baba S, Hosaka H, et al. Irinotecan plus cisplatin for therapy of small-cell carcinoma of the esophagus: report of 12 cases from single institution experience. Jpn J Clin Oncol. 2008;38(6):426–431. doi:10.1093/jjco/hyn041
6. Okita NT, Kato K, Takahari D, et al. Neuroendocrine tumors of the stomach: chemotherapy with cisplatin plus irinotecan is effective for gastric poorly-differentiated neuroendocrine carcinoma. Gastric Cancer. 2011;14(2):161–165. doi:10.1007/s10120-011-0025-5
7. Morizane C, Machida N, Honma Y, et al. Randomized phase III study of etoposide plus cisplatin versus irinotecan plus cisplatin in advanced neuroendocrine carcinoma of the digestive system: a Japan Clinical Oncology Group study (JCOG1213). J Clin Oncol. 2015;33:TPS4143–TPS4143. doi:doi:10.1200/jco.2015.33.15_suppl.tps4143
8. von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol. 2014;32(35):4012–4019. doi:doi:10.1200/jco.2013.54.5392
9. Ando T, Hosokawa A, Yoshita H, et al. Amrubicin monotherapy for patients with platinum-refractory gastroenteropancreatic neuroendocrine carcinoma. Gastroenterol Res Pract. 2015;2015:1–5. doi:doi:10.1155/2015/425876
10. Nio K, Arita S, Isobe T, et al. Amrubicin monotherapy for patients with extrapulmonary neuroendocrine carcinoma after platinum-based chemotherapy. Cancer Chemother Pharmacol. 2015;75(4):829–835. doi:10.1007/s00280-015-2706-y
11. Araki T, Takashima A, Hamaguchi T, et al. Amrubicin in patients with platinum-refractory metastatic neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma of the gastrointestinal tract. Anticancer Drugs. 2016;27(8):794–799. doi:10.1097/cad.0000000000000393
12. Yoshioka H, Katakami N, Okamoto H, et al. A randomized, open-label, phase III trial comparing amrubicin versus docetaxel in patients with previously treated non-small-cell lung cancer. Ann Oncol. 2016;28(2):285–291. doi:10.1093/annonc/mdw621
13. Haider K, Shahid RK, Finch D, et al. Extrapulmonary small cell cancer. Cancer. 2006;107(9):2262–2269. doi:10.1002/cncr.22235
14. Cicin I, Karagol H, Uzunoglu S, et al. Extrapulmonary small-cell carcinoma compared with small-cell lung carcinoma. Cancer. 2007;110(5):1068–1076. doi:10.1002/cncr.22887
15. Dasari A, Mehta K, Byers LA, Sorbye H, Yao JC. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: a SEER database analysis of 162,983 cases. Cancer. 2017;124(4):807–815. doi:10.1002/cncr.31124
16. Fazio N, Milione M. Heterogeneity of grade 3 gastroenteropancreatic neuroendocrine carcinomas: new insights and treatment implications. Cancer Treat Rev. 2016;50:61–67. doi:10.1016/j.ctrv.2016.08.006
17. Mishima S, Kawazoe A, Matsumoto H, et al. Efficacy and safety of ramucirumab-containing chemotherapy in patients with pretreated metastatic gastric neuroendocrine carcinoma. ESMO Open. 2018;3:e000443. doi:10.1136/esmoopen-2018-000443
18. Hata A, Katakami N, Fujita S, et al. Amrubicin at a lower-dose with routine prophylactic use of granulocyte-colony stimulating factor for relapsed small-cell lung cancer. Lung Cancer. 2011;72(2):224–228. doi:10.1016/j.lungcan.2010.08.009
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
