Back to Journals » Clinical Ophthalmology » Volume 16
Safety and Efficacy of a New Water Gradient Biomimetic Monthly Replacement Spherical Contact Lens Material (Lehfilcon A)
Authors Wesley G, Giedd B, Hines B, Bickle K, Pearson C, Lorentz H
Received 14 June 2022
Accepted for publication 15 August 2022
Published 30 August 2022 Volume 2022:16 Pages 2873—2884
DOI https://doi.org/10.2147/OPTH.S362926
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Gina Wesley,1 Brad Giedd,2 Bradley Hines,3 Katherine Bickle,4 Christopher Pearson,5 Holly Lorentz6
1Complete Eye Care of Medina, Hamel, MN, USA; 2Kindred Optics at Maitland Vision, Maitland, FL, USA; 3Optometry Group, Memphis, TN, USA; 4ProCare Vision Center, Granville, OH, USA; 5OMEGA Vision Center PA, Longwood, FL, USA; 6Alcon Research, LLC, Johns Creek, GA, USA
Correspondence: Holly Lorentz, Alcon Research, LLC, 11460 Johns Creek Pkwy, Johns Creek, GA, 30097, USA, Tel +1 678 415 5272, Email [email protected]
Purpose: The objective of this study was to evaluate the safety and performance of the investigational lens, lehfilcon A, when worn in a daily wear modality and replaced monthly as compared to the commercially available comfilcon A contact lens.
Methods: This was a multicenter, prospective, controlled, double-masked, randomized, parallel-group clinical study with bilateral lens wear for 3 months. In all, 115 subjects completed the study (77 with test lehfilcon A and 38 with control comfilcon A contact lenses). Distance visual acuity (VA) was assessed using Snellen VA. Lens performance was assessed by examining lens fit/movement, centration, front surface wettability and front/back surface deposits using slit-lamp biomicroscopy.
Results: At the 3-month follow-up visit, all eyes had a distance VA of 20/20 or better. Further, lens fit/movement was assessed as optimal in 92.9% of the eyes with lehfilcon A and 89.2% with comfilcon A. There were no ratings of unacceptably tight or loose fits for either contact lens material. Lens centration was assessed as optimal in 98.7% of eyes with lehfilcon A and 94.6% with comfilcon A. For front and back surface deposits, both materials showed minimal lens surface deposits. Front surface wettability was assessed as grade 0 or 1 for most of the study lenses in both lens groups across all attended study visits. There were no ocular adverse events related to the study lenses.
Conclusion: Overall, lehfilcon A showed excellent VA, optimal lens fitting characteristics, a clean surface, high wettability, and low risk for adverse events after 3 months of lens wear.
Keywords: biomimetic, comfilcon A, lehfilcon A, visual acuity, water gradient, contact lens
Introduction
Contact lens wearers and their prescribing eye care practitioner today have many different contact lens materials, wear schedules, and replacement plans to choose from; however, no single lens, wear modality, or replacement schedule is suitable for all individuals. Survey results from the United States have shown that 39% to 50% of the wearers use daily disposable lenses, whereas 32% to 36% wear monthly reusable lenses.1 Monthly replacement lenses may be preferred by some people due to cost considerations and concerns about the potential ecological consequences of daily lens disposal.2 It has been shown that people fitted with monthly reusable lenses are more compliant with replacement schedules than those fitted with 2-week reusable lenses.3–5
Recent advances in the technology for reusable daily wear contact lenses generally have been limited compared with those for daily disposable contact lenses.6 The most recent technological development in daily disposable silicone hydrogel contact lenses has been the development of “water gradient” delefilcon A lenses. These lenses have low water content in the core and then transition to higher water content (approaching 100%) at the surface, creating a hydrophilic layer aimed at producing superior on-eye performance in terms of comfort.7–10 Delefilcon A lenses have been shown to be comfortable and convenient to use.11,12 Recently, 2-methacryloyloxyethyl phosphorylcholine (MPC)—a substance designed using a biomimetic approach to match the structure of phospholipids on cellular membrane surface and which has many properties that make it highly suitable for incorporation into a monthly replacement contact lens13,14—has been included in the development of a new lehfilcon A monthly replacement daily wear water gradient contact lens. This substance was designed to have multiple properties with the potential to enhance the on-eye performance of contact lenses. These properties emerge from extreme hydrophilicity, electrical neutrality and a unique structure with hydrophilic phosphorylcholine groups in the side chain which decrease the surface tension of water.13–17 Further, MPC mimics the surface of the cornea with polymer nanofibers, similar to the gel-like layer of protection provided by the corneal glycocalyx and microvilli. Addition of MPC results in a lens surface that selectively attracts smaller water molecules while deflecting larger bacteria and lipids.14 Thus, inclusion of MPC in lehfilcon A contact lenses helps to provide a lubricious surface that has fewer deposits, helps resist bacterial adhesion, and is durable to last 30 days.14,15,18–24
The purpose of the present study was to describe the clinical performance of lehfilcon A contact lenses with a daily wear monthly replacement schedule. This study was a registration study and thus the study design, endpoints, sample size, and descriptive analysis in this study adhered to ISO 11980:2012 and the US FDA 510(k) guidance document for daily wear contact lenses.
Materials and Methods
This was a prospective, randomized, controlled, double-masked, parallel-group, daily wear clinical trial (ClinicalTrials.gov number: NCT04178720; the trial started on January 28, 2020, and was completed on June 10, 2020). The study design, endpoints, and descriptive analysis in this registration study adhered to ISO 11980:2012 and the US FDA 510(k) guidance document for daily wear contact lenses. Eligible subjects requiring vision correction were randomized in a 2:1 ratio to wear in both eyes either lehfilcon A/test or comfilcon A/control contact lenses. Sample size is based upon ISO clinical standard and FDA 510(k) requirements with the recommendations of at least 50 subjects in the test group, in a 2:1 test-to-control ratio. Subjects and study personnel conducting evaluations were masked to treatment. There were 6 scheduled study visits: screening/baseline/dispense day 1, week 1 follow-up, week 2 follow-up, month 1 follow-up, month 2 follow-up, and month 3 follow-up/exit.
Ethical Considerations
The study was conducted in accordance with the ethical principles contained within the Declaration of Helsinki, and in compliance with the ICH E6 Good Clinical Practice Guideline, ISO 14155:2011, and US Food & Drug Administration Code of Federal Regulations Title 21. Before initiation of study-related procedures, the protocol and all amendments, the informed consent form, and any other written information given to subjects were approved by an institutional review board/independent ethics committee. The ethics committee included Sterling IRB, an independent institutional review board. Voluntary informed consent was obtained from every subject prior to the initiation of any study-specific procedures.
Contact Lenses
The contact lenses evaluated were lehfilcon A, an investigational new water gradient monthly replacement silicone hydrogel lens, and commercially available comfilcon A (Table 1).
 |
Table 1 Characteristics of Contact Lenses |
Subjects
Potential subjects were aged ≥18 years, with non-diseased eyes and manifest cylinder ≤0.75 D in each eye, were frequent replacement daily wear soft contact lens wearers, had ≥3 months of soft contact lens wearing experience, wore their habitual lenses ≥5 days/week and ≥8 hours/day, and required contact lens spherical correction from −1.00 D to −6.00 D, with a best corrected visual acuity (VA) of 20/25 or better in each eye. Potential subjects were excluded if they were wearing habitual contact lenses in an extended wear modality (routinely sleeping in lenses for ≥1 night/week) over the last 3 months prior to enrollment, monovision contact lens wearers, or habitually wearing comfilcon A contact lenses.
Lens Wear
Subjects were instructed to wear the study lenses during waking hours for ≥8 hours/day and ≥5 days/week. The total duration of study lens exposure was 3 months. Lenses were to be removed at the end of each day for cleaning, disinfection, and storage with CLEAR CARE® Cleaning & Disinfecting Solution (Alcon Vision LLC, Fort Worth, TX) prior to reinsertion the next day. Subjects were to replace the assigned study lenses with a fresh pair each month. Subjects were instructed to wear their study lenses for at least 4 hours prior to each follow-up visit.
Endpoints
The primary effectiveness endpoint was distance VA with study lenses collected by the Snellen method for each eye. Additional exploratory endpoints included lens fit/movement characteristics, lens surface characteristics, and lens wear time. A slit-lamp examination was performed at all study visits for lens fit/centration, lens surface evaluation, and safety. The contact lens characteristics were assessed with the lens on-eye and utilizing the biomicroscope with low magnification and white light with the scales provided per ISO 11980:2012. Safety endpoints included biomicroscopy and adverse events (AEs).
Assessments
Visual Acuity
Snellen distance VA assessments were performed using conventional methods.
Lens Fit/Movement
Lens fit/movement was evaluated immediately after blinking with the eye in the primary position and following digitally applied push-up of the lower lid margin. Lens fit/movement was assessed as follows: −2, unacceptably tight (reduced movement, unacceptable fit); −1, acceptably tight (reduced movement, acceptable fit); 0, optimal fit/movement; +1, acceptably loose (excessive movement, acceptable fit); or +2, unacceptably loose (excessive movement, unacceptable fit).
Lens Position/Centration
Lens position and centration were evaluated with the eye in the primary position (relaxed, looking straight ahead) and assessed as follows: 0, optimal lens centration; 1, acceptable decentration; or 2, unacceptable decentration.
Contact Lens Surface Characteristics
Front surface wettability was recorded according to the following scale: 0, smooth uniformly reflecting surface; 1, coarse hazy surface that resolved momentarily with each blink and became exacerbated with staring; 2, one stable dry (nonwetting) area of some magnitude; 3, >1 stable dry area of some magnitude; and 4, nonwettable lens surface. Front surface deposits were assessed according to the following scale: 0, absent clean surface; 1, very slight, only visible after tear film drying; 2, slight visible deposits easily removable; 3, moderate deposits adherent and not removable; and 4, severe nonremovable deposits and comfort affected. Back surface deposits were recorded according to the following scale: 0, absent (clean surface); 1, very slight (≤3 spots of moving particles); 2, slight (≤10 spots of moving particles); 3, moderate (≤3 nonmoving deposits adherent to lens); or 4, severe (≥4 deposits adherent to the lens or corneal indentation).
Study Lens Wear Time
At each post-baseline visit, subjects provided the approximate times of lens insertion and removal for each day. Subjects completed a daily diary indicating the total number of days the lenses were worn each month.
Safety
Safety was evaluated by recording ocular and nonocular AEs and biomicroscopic findings, all of which were collected at each study visit. Biomicroscopy findings included the following assessments: limbal hyperemia, bulbar hyperemia, corneal staining, conjunctival staining, palpebral conjunctival observations, corneal epithelial edema, corneal stromal edema, corneal vascularization, conjunctival compression/indention, chemosis, corneal infiltrates, and other findings. The following scale was used to grade severity in accordance with the ISO11980:2012 scales: 0 = none, 1 = trace, 2 = mild, 3 = moderate, 4 = severe.
Data Analysis
Descriptive summary statistics were provided at each visit when applicable, using number and percentage for categorical variables; number of observations, mean, standard deviation, median; and range for continuous variables.
Results
Subjects
In all, 119 subjects (238 eyes) were enrolled (signed consent) in the study and 1 subject failed screening due to the exclusion criteria. A total of 118 subjects (236 eyes) were randomized and dispensed study lenses; 115 subjects completed the study and 3 discontinued early; 2 subjects from the comfilcon A group withdrew and 1 from the lehfilcon A group as a result of COVID-19-related circumstances. This study was conducted during the national health emergency response to the COVID-19 outbreak in March 2020. Study enrollment was completed at the time of the national emergency declaration and although most sites continued to see study subjects in-person in the study clinic, the COVID-19 emergency did impact the conduct of the study depending on the sites’ unique features and local governances. Provisional contingency measures were implemented to ensure the safety of trial subjects and the integrity of the study conduct. Month 2 visits were the most impacted by COVID-19 and the implemented contingency measures, which can be seen in the data where there is a temporary decrease in available subject data for both lehfilcon A and comfilcon A. Subjects had an overall mean age of 33.9 years, with 74.6% women and 25.4% men. Subjects were generally white and not of Hispanic or Latino ethnicity (Table 2).
 |
Table 2 Baseline Subject Characteristics |
Effectiveness
Visual Acuity
All eyes had distance Snellen VA with study lenses of 20/30 or better at every visit, except 1 eye from the lehfilcon A group (Figure 1). Results at 3 months also showed that 99.4% of the eyes in the lehfilcon A and 100% in the comfilcon A group had VA 20/20 or better. For 1 eye in the lehfilcon A group, distance VA was worse than 20/30 (20/200) at month 3. It was discovered that the subject was wearing two lenses in the same eye. Once the contact lenses were removed and best corrected VA with manifest refraction was assessed, a distance VA of 20/20 was achieved. At each study visit, ≥98.6% of the eyes wearing comfilcon A and ≥98.4% wearing lehfilcon A lenses had distance VA with the study lenses of 20/20 or better.
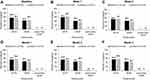 |
Figure 1 Snellen visual acuity at baseline (A) and at week 1 (B), week 2 (C), month 1 (D), month 2 (E), and month 3 (F). |
Lens Fit/Movement and Position
At month 3, 89.2% of the eyes with comfilcon A and 92.9% with lehfilcon A lenses were assessed as having optimal fit/movement (Table 3). There were no reports of unacceptably tight or unacceptably loose-fitting lenses at any visit. Lens position at month 3 was assessed as having optimal centration for 94.6% of the eyes with comfilcon A and 98.7% with lehfilcon A lenses. There were no reports of unacceptable decentration at any visit. Despite the two study lens brands having different base curves and diameters, both study lenses fit well in the diverse range of subject corneal curvatures as randomized.
 |
Table 3 Fit and Movement Assessments |
Lens Deposits/Wettability
For most worn lenses, front and back surface deposits were assessed as absent (grade 0) or very slight (grade 1) in both groups and across all study visits. At month 3, the respective percentages of lenses assessed as either grade 1 or 0 for front and back surface deposits were 95.9% and 100% with comfilcon A and 98.7% and 100% with lehfilcon A (Table 4). Front surface wettability was assessed as grade 0 or 1 for most of the study lenses in both lens groups across all attended study visits (Table 4). At month 3, 86.5% of comfilcon A and 82.5% of lehfilcon A lenses were assessed as grade 0, and each lens group had 1 lens assessed as grade 2.
 |
Table 4 Lens Surface Assessments |
Lens Wear Time
For subjects who completed the 3-month trial, mean daily wear time was 14.3 hours for the comfilcon A and 14.6 hours for the lehfilcon A group. Mean number of days the lenses were worn ranged from 28.0 to 32.9 for the comfilcon A and 28.8 to 33.8 for the lehfilcon A group.
Safety
There were no deaths or nonfatal serious AEs, adverse device effects, or discontinuations due to AEs. There were 8 nonserious ocular AEs reported by 6 subjects, all in the lehfilcon A group (hordeolum, OD; eye irritation, OU; eye irritation OD; conjunctival hyperemia, OU; conjunctival edema, OS; ocular discomfort, OD). In 6 out of the 8 nonserious ocular AEs, no action was taken, and no treatment was required. For conjunctival edema, the use of SYSTANE® eye drops prior to lens insertion was recommended. For ocular discomfort, the study device was temporarily discontinued. All of these AEs were mild in severity, resolved, and judged as unrelated to the study lens by the investigator. There were 4 nonocular AEs, all in 3 subjects in the comfilcon A group (bronchitis, influenza, and nasopharyngitis). All of these AEs were mild or moderate in severity, resolved, and judged as unrelated to the study lens.
No biomicroscopic findings of grade >2 (mild) were reported for subjects in either lens group. There were no reports of corneal edema or infiltrates in the study. Overall, there were no clinically relevant differences in biomicroscopic findings between the lens groups.
Discussion
The results of this registration clinical study indicate that the new lehfilcon A contact lens provided good VA over 3 months of follow-up, and that it also performed well with respect to fit/movement, position, and surface characteristics. There were no safety concerns for the lehfilcon A lens based on assessment of AEs, adverse device reactions, or biomicroscopy. No formal hypotheses were formulated for the primary effectiveness endpoint of VA; hence, no inferential testing was performed. Descriptive statistics were provided on the Snellen categories. Similarly, all supportive endpoints were summarized descriptively according to their measurement scale. There were no pre-specified safety hypotheses in this study. The focus of the safety analysis was a comprehensive descriptive assessment of occurrence of AEs and biomicroscopy findings. Although there are multiple in vitro publications highlighting the features and structure of the lehfilcon A contact lens material, this is the first clinical trial manuscript, as this is a brand-new contact lens material. Despite limitations of no inferential testing and limited subject numbers, this pivotal study did provide evidence that the lehfilcon A contact lens demonstrates good safety and efficacy.
Contact lens fit is an important consideration for both ocular health and avoiding wearer dropout.25,26 Poor fitting soft lenses have negative effects on ocular physiology that may be reflected by increased fluorescein staining, and higher levels of bulbar and limbal hyperemia.25,27 Results from two surveys indicated that poor fit is a frequent reason for discontinuation of contact lens wear.28,29
Contact lenses and other biomaterials incorporating MPC have been evaluated in multiple nonclinical studies that demonstrated high resistance to protein absorption and bacterial adhesion, a low coefficient of friction and high wettability, and high oxygen permeability.14,21,30,31 Additional in vitro studies focusing on lehfilcon A contact lenses demonstrated properties that contribute to enhanced performance and compatibility with a monthly replacement schedule, including high hydrophilicity and lubricity, and a lower coefficient of friction compared with senofilcon C and comfilcon A lenses.22,23 The aqueous gel layers of lehfilcon A contact lenses are protective against friction,22 which can result in an increase in inflammatory biomarkers and lead to cell damage and death.32–34
In vitro data also demonstrate that lehfilcon A contact lenses have similar wettability after 30 days of daily wear compared with lenses evaluated immediately after removal from packaging.22 Nanoindentation testing of lehfilcon A indicated high degrees of softness and durability after 30 days of daily wear. Comparison with five other commercial lenses indicated that lehfilcon A was ~5 times softer than all other lenses tested.35 Class 1 ultraviolet blocking with lehfilcon A contact lenses is comparable to that achieved with existing class 1 ultraviolet-blocking contact lenses (ie, senofilcon A and C). In addition, lehfilcon A lenses provide a level of high-energy visible light filtration similar to that for spectacle lenses marketed as providing this attribute.36 The water gradient lehfilcon A contact lens has high in vitro oxygen transmissibility37; it should be noted that addition of MPC does not compromise this property, which is important for corneal health.38 MPC shows high oxygen permeability due to high water content, similar to the case of corneal surface.39
Protein adsorption is also an important consideration for a contact lens with a monthly replacement schedule, and the MPC polymer can effectively prevent protein adsorption at the surface.21 In vitro adhesion of microorganisms Escherichia coli to lehfilcon A lenses is lower than for samfilcon A and comfilcon A lenses, and this is also the case for adherence of Pseudomonas aeruginosa to lehfilcon A vs samfilcon A, senofilcon C, and comfilcon A lenses.14 These differences are potentially important for maintenance of ocular health, particularly for contact lenses with a monthly replacement schedule.
Use of contact lenses with a monthly replacement schedule may be limited by discomfort and discontinuation in a high percentage of patients.40 Results from several studies have indicated that use of daily disposable contact lenses provides greater comfort, decreased signs of eye irritation, and fewer complaints for tired eyes, irritated eyes, blurred vision, redness, discomfort, deposits, and dryness vs use of lenses with longer replacement schedules.41–43 Results from one comparison, however, indicated no significant differences in signs and patient complaints between monthly and daily disposable lenses.44 Results from a 2-year study of 242 subjects (naïve contact lens wearers) wearing lotrafilcon B silicone hydrogel contact lenses with a monthly replacement schedule indicated a dropout rate of 29.2%, with 10.8% of subjects discontinuing in the first month,45 and it has been repeatedly shown that the most common reason for contact lens discontinuation is discomfort.29,40,44,45,47 Other reasons cited for contact lens discontinuation include poor vision and problems with insertion or handling.28,46,47 All of these results indicate there is a significant unmet need for advancement in the technology of contact lens materials with a monthly replacement schedule. The on-eye and in vitro characteristics of the lehfilcon A lens, including excellent fit/movement and vision, high lubricity, oxygen transmissibility, low risk for adherence of proteins and ocular microorganisms, and protection against transmission of high-energy light, suggest that it may fulfill these needs.
Conclusions
Overall, lehfilcon A, a new biomimetic water gradient monthly replacement daily wear silicone hydrogel contact lens, showed excellent VA, optimal lens fitting/movement characteristics, a clean surface, high wettability, and seemingly low risk for AEs after 3 months of daily wear.
Abbreviations
AE, adverse event; MPC, 2-methacryloyloxyethyl phosphorylcholine; VA, visual acuity.
Data Sharing Statement
The data used to support the primary findings of this study are available at ClinicalTrials.gov (NCT04178720).
Ethics Approval and Informed Consent
This study was conducted in accordance with the principles of Declaration of Helsinki and in compliance with the ICH E6 GCP Consolidated Guideline, ISO 14155:2011, and the applicable US FDA 21 CFR Regulations. Before clinical study initiation, the protocol and all amendments, the informed consent form, any other written information given to subjects, and any advertisements planned for subject recruitment was approved by an IRB/IEC.
Acknowledgments
The authors wish to thank the other study investigators, including Drs. Wilson Movic, Mark Perry, and David Evans. Editorial assistance was provided by BioScience Communications, New York, NY and funded by Alcon Research, LLC.
Funding
This study was funded by Alcon Research, LLC., which participated in the design of the study; collection, analysis, and interpretation of data; writing of the report; and decision to submit the report for publication.
Disclosure
Gina Wesley is a consultant for Bausch + Lomb Incorporated, Orasis and Lumenis and runs clinical trials for Alcon, Bausch + Lomb, CooperVision and Johnson & Johnson Vision Care. Brad Giedd conducts clinical trials for Alcon, Johnson & Johnson and Oculos Development Services, LLC. Bradley Hines is a study investigator for Alcon and Johnson & Johnson Vision Care. Katherine Bickle is a study investigator for Alcon, Johnson & Johnson, and CooperVision. Christopher Pearson is a study investigator for Alcon, Bausch + Lomb, CooperVision, Johnson & Johnson, and Ocuphire Pharma, Inc. Holly Lorentz is an employee of Alcon.
References
1. Nichols JJ, Starcher L. Contact lenses 2019. Contact Lens Spectrum. 2020. Available from: https://www.clspectrum.com/issues/2020/january-2020/contact-lenses-2019.
2. Smith SL, Orsborn GN, Sulley A, Chatterjee NB, Morgan PB. An investigation into disposal and recycling options for daily disposable and monthly replacement soft contact lens modalities. Cont Lens Anterior Eye. 2021;45. doi:10.1016/j.clae.2021.03.002
3. Dumbleton K, Richter D, Bergenske P, Jones LW. Compliance with lens replacement and the interval between eye examinations. Optom Vis Sci. 2013;90(4):351–358. doi:10.1097/OPX.0b013e318288afcb
4. Dumbleton K, Woods CA, Jones LW, Fonn D. The relationship between compliance with lens replacement and contact lens-related problems in silicone hydrogel wearers. Cont Lens Anterior Eye. 2011;34(5):216–222. doi:10.1016/j.clae.2011.03.001
5. Rueff EM, Wolfe J, Bailey MD. A study of contact lens compliance in a non-clinical setting. Cont Lens Anterior Eye. 2019;42(5):557–561. doi:10.1016/j.clae.2019.03.001
6. Pawson A, Bailey GM. Total 30 contact lens coming in early 2022. Optometry Times. 2021. Available from: https://www.optometrytimes.com/view/total-30-contact-lens-coming-in-early-2022.
7. Krysztofiak K, Ciężar K, Kościński M. Raman imaging of layered soft contact lenses. J Appl Biomater Funct Mater. 2017;15(2):e149–152. doi:10.5301/jabfm.5000329
8. Pruitt J, Bauman E. The development of Dailies Total1 water gradient contact lenses. Contact Lens Spectrum. 2013:40–44. Available from: https://www.clspectrum.com/content/alcon/10/cls_13_final_edit_pgs.pdf.
9. Pruitt J, Qiu Y, Thekveli S, Hart R. Surface characterisation of a water gradient silicone hydrogel contact lens (delefilcon A). Invest Ophthalmol Vis Sci. 2012;53:E–abstract6107.
10. Thekveli S, Qiu Y, Kapoor Y, Kumi A, Liang W, Pruitt J. Structure–property relationship of delefilcon A water gradient contact lenses. Contact Lens Anterior Eye. 2018;35(Suppl 1):e14. doi:10.1016/j.clae.2012.08.044
11. Marx S, Lauenborg B, Kern JR. Performance evaluation of delefilcon a water gradient daily disposable contact lenses in first-time contact lens wearers. Cont Lens Anterior Eye. 2018;41(4):335–341. doi:10.1016/j.clae.2017.12.019
12. Michaud L, Forcier P. Comparing two different daily disposable lenses for improving discomfort related to contact lens wear. Cont Lens Anterior Eye. 2016;39(3):203–209. doi:10.1016/j.clae.2015.11.002
13. Goda T, Ishihara K. Soft contact lens biomaterials from bioinspired phospholipid polymers. Expert Rev Med Devices. 2006;3(2):167–174. doi:10.1586/17434440.3.2.167
14. Ishihara K, Fukazawa K, Sharma V, et al. Antifouling silicone hydrogel contact lenses with a bioinspired 2-methacryloyloxyethyl phosphorylcholine polymer surface. ACS Omega. 2021;6(10):7058–7067. doi:10.1021/acsomega.0c06327
15. Ishihara K. Revolutionary advances in 2-methacryloyloxyethyl phosphorylcholine polymers as biomaterials. J Biomed Mater Res A. 2019;107(5):933–943. doi:10.1002/jbm.a.36635
16. Ishihara K, Mu M, Konno T, Inoue Y, Fukazawa K. The unique hydration state of poly(2-methacryloyloxyethyl phosphorylcholine). J Biomater Sci Polym Ed. 2017;28(10–12):884–899. doi:10.1080/09205063.2017.1298278
17. Iwasaki Y, Ishihara K. Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces. Sci Technol Adv Mater. 2012;13(6):064101. doi:10.1088/1468-6996/13/6/064101
18. Chen M, Briscoe WH, Armes SP, Cohen H, Klein J. Polyzwitterionic brushes: extreme lubrication by design. European Polymer J. 2011;47(4):511–523. doi:10.1016/j.eurpolymj.2010.10.007
19. Gipson IK. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2007;48(10):4390–4398. doi:10.1167/iovs.07-0770
20. Kurpakus Wheater M, Kernacki KA, Hazlett LD. Corneal cell proteins and ocular surface pathology. Biotech Histochem. 1999;74(3):146–159. doi:10.3109/10520299909047967
21. Shi X, Cantu-Crouch D, Sharma V, et al. Surface characterization of a silicone hydrogel contact lens having bioinspired 2-methacryloyloxyethyl phosphorylcholine polymer layer in hydrated state. Colloids Surf B Biointerfaces. 2021;199:111539. doi:10.1016/j.colsurfb.2020.111539
22. Varde PS, Tucker RC. Water gradient surface coating durability and wettability of worn new monthly contact lens material. Presented at British Contact Lens Association; 2021. Available from: https://clinicalconference2021.bclaevents.com/login?user[email]=anonymous.
23. Wu J, Shi C, Sharma V, Cantu-Crouch D. Surface characterization of a silicone hydrogel contact lens having bioinspired 2-methacryloyloxyethyl phosphorylcholine polymer layer in hydrated state. Presented at British Contact Lens Association; 2021. Available from: https://clinicalconference2021.bclaevents.com/login?user[email]=anonymous.
24. Wu J, Shi C, Sharma V, et al. Antifouling studies of lehfilcon A (TOTAL30®) contact lens. Presented at American Academy of Optometry Annual Meeting; 2021.
25. Cui L, Li M, Shen M, Lu F, Wang J. Characterization of soft contact lens fitting using ultra-long scan depth optical coherence tomography. J Ophthalmol. 2017;2017:5763172. doi:10.1155/2017/5763172
26. Wolffsohn JS, Hunt OA, Basra AK. Simplified recording of soft contact lens fit. Cont Lens Anterior Eye. 2009;32(1):37–42. doi:10.1016/j.clae.2008.12.004
27. Young G, Coleman S. Poorly fitting soft lenses affect ocular integrity. CLAO J. 2001;27(2):68–74.
28. Pritchard N, Fonn D, Brazeau D. Discontinuation of contact lens wear: a survey. Int Contact Lens Clin. 1999;26(6):157–162.
29. Young G. Why one million contact lens wearers dropped out. Cont Lens Anterior Eye. 2004;27(2):83–85. doi:10.1016/j.clae.2004.02.006
30. Shimizu T, Goda T, Minoura N, Takai M, Ishihara K. Super-hydrophilic silicone hydrogels with interpenetrating poly(2-methacryloyloxyethyl phosphorylcholine) networks. Biomaterials. 2010;31(12):3274–3280. doi:10.1016/j.biomaterials.2010.01.026
31. Spadafora A, Korogiannaki M, Sheardown H. Antifouling silicone hydrogel contact lenses via densely grafted phosphorylcholine polymers. Biointerphases. 2020;15(4):041013. doi:10.1116/6.0000366
32. Pitenis A, Uruena JM, Hart JM, Levings PP, Sawyer WG. Frictional shear stresses in vitro can trigger apoptotic pathways in human corneal epithelial cells. IOVS. 2019;60(9):E–abstract3890.
33. Sawyer WG. Low friction and low modulus contact lens surfaces improve lubricity and reduce cell damage in corneal epithelial cell models. Optom Vis Sci. 2019;96:E–abstr190051.
34. Sawyer WG, Petinis AA, Uruena JM, Hart S. Friction and pro-inflammatory cytokine production in corneal epithelial cell models. IOVS. 2019;60(9):E–abstact3888.
35. Oyler P. Surface softness comparison of select contact lenses by nanoindentation. Presented at British Contact Lens Association; 2021. Available from: https://clinicalconference2021.bclaevents.com/login?user[email]=anonymous.
36. Loudermilk B, Dalton A. HEVL and UV transmittance properties of a new contact lens material. Presented at British Contact Lens Association; 2021. Available from: https://clinicalconference2021.bclaevents.com/login?user[email]=anonymous.
37. U.S. Food & Drug Administration. Lehfilcon 510k summary. Total30 contact lens. Indications for use; 2021. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf21/K210436.pdf.
38. Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26(4):398–436. doi:10.1016/j.preteyeres.2007.03.003
39. Goda T, Matsuno R, Konno T, Takai M, Ishihara K. Protein adsorption resistance and oxygen permeability of chemically crosslinked phospholipid polymer hydrogel for ophthalmologic biomaterials. J Biomed Mater Res B Appl Biomater. 2009;89(1):184–190. doi:10.1002/jbm.b.31204
40. Pucker AD, Tichenor AA. A review of contact lens dropout. Clin Optom. 2020;12:85–94. doi:10.2147/OPTO.S198637
41. Fahmy M, Long B, Giles T, Wang CH. Comfort-enhanced daily disposable contact lens reduces symptoms among weekly/monthly wear patients. Eye Contact Lens. 2010;36(4):215–219. doi:10.1097/ICL.0b013e3181e5859f
42. Nichols KK, Mitchell GL, Simon KM, Chivers DA, Edrington TB. Corneal staining in hydrogel lens wearers. Optom Vis Sci. 2002;79(1):20–30. doi:10.1097/00006324-200201000-00009
43. Riley C, Young G, Chalmers R. Prevalence of ocular surface symptoms, signs, and uncomfortable hours of wear in contact lens wearers: the effect of refitting with daily-wear silicone hydrogel lenses (senofilcon a). Eye Contact Lens. 2006;32(6):281–286. doi:10.1097/01.icl.0000224522.04723.7a
44. Sapkota K, Franco S, Lira M. Daily versus monthly disposable contact lens: which is better for ocular surface physiology and comfort? Cont Lens Anterior Eye. 2018;41:252–257. doi:10.1016/j.clae.2017.12.005
45. Sankaridurg P, Chen X, Naduvilath T, et al. Adverse events during 2 years of daily wear of silicone hydrogels in children. Optom Vis Sci. 2013;90(9):961–969. doi:10.1097/OPX.0000000000000017
46. Dumbleton K, Woods CA, Jones LW, Fonn D. The impact of contemporary contact lenses on contact lens discontinuation. Eye Contact Lens. 2013;39(1):93–99. doi:10.1097/ICL.0b013e318271caf4
47. Sulley A, Young G, Hunt C. Factors in the success of new contact lens wearers. Cont Lens Anterior Eye. 2017;40(1):15–24. doi:10.1016/j.clae.2016.10.002
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
