Back to Journals » Vascular Health and Risk Management » Volume 11
Safety and effectiveness of a fixed-dose combination of olmesartan, amlodipine, and hydrochlorothiazide in clinical practice
Authors Bramlage P, Fronk E, Wolf W, Smolnik R, Sutton G, Schmieder R
Received 3 October 2014
Accepted for publication 4 November 2014
Published 17 December 2014 Volume 2015:11 Pages 1—8
DOI https://doi.org/10.2147/VHRM.S75380
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Daniel Duprez
Peter Bramlage,1 Eva-Maria Fronk,2 Wolf-Peter Wolf,3 Rüdiger Smolnik,3 Gemma Sutton,1 Roland E Schmieder4
1Institut für Pharmakologie und präventive Medizin, Mahlow, Germany; 2Daiichi Sankyo Europe GmbH, Munich, Germany; 3Daiichi Sankyo Deutschland GmbH, Munich, Germany; 4Abteilung für Nephrologie und Hypertensiologie, Universitätsklinikum Erlangen, Erlangen, Germany
Background: Clinical trials indicate that the use of fixed-dose combinations (FDCs) is associated with a higher level of treatment adherence and prolonged blood pressure (BP) control. The aim of this study was to document the safety and effectiveness of the FDC olmesartan/amlodipine/hydrochlorothiazide in patients with essential hypertension in clinical practice.
Methods: This multicenter, prospective, 24-week, noninterventional study enrolled 5,831 patients from primary care offices in Germany and Austria. Inclusion criteria were a diagnosis of essential hypertension and newly initiated treatment with the FDC.
Results: The mean age of patients was 63.5 years, almost 50% of patients had a time since diagnosis of essential hypertension of over 5 years, and approximately 70% of patients had at least one cardiovascular risk factor, including 29.4% of patients with diabetes mellitus. Following approximately 24 weeks of treatment, the mean reduction in systolic/diastolic BP was 29.0/14.0 mmHg, a BP response was observed by 94.2% of patients, and a target BP of <140/90 mmHg was attained in 67.5% of patients. At least one adverse drug reaction (ADR) was experienced by 1.2% of patients, with the most common being peripheral edema. Subanalyses demonstrated that the following factors did not have a significant influence on the ADR rate: age (<65 years versus ≥65 years), diabetes mellitus (no/yes), cardiovascular risk (low/high), and concomitant medication (no/yes).
Conclusion: This study demonstrates that in clinical practice, treatment with the three-drug combination as an FDC tablet resulted in a very high proportion of patients with a BP response and control, accompanied by a very low rate of ADRs.
Keywords: hypertension, clinical practice, fixed-dose combination, blood pressure, adverse drug reactions
Introduction
Hypertension is an independent cardiovascular risk factor, and each reduction of either 20 mmHg in systolic (S) blood pressure (BP) or 10 mmHg in diastolic (D)BP correlates with a twofold decrease in the likelihood of a fatal coronary event.1 To achieve the currently recommended BP goal of <140/90 mmHg (lower for specific patient populations), it is estimated that at least 25% of patients will require triple-agent therapy.2–4 The combined use of three different classes of antihypertensive drugs enables targeting of distinct biological pathways, thus enhancing efficacy compared with mono- or dual therapy. In addition, side effects caused by one drug class may be negated by the actions of another drug class that elicits opposing physiological compensatory mechanisms, resulting in a more favorable tolerability profile.5–7
Among the five main classes of antihypertensive drugs, the combination of either an angiotensin converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB), which both inhibit the renin–angiotensin system, a calcium channel blocker, and a diuretic is the most commonly used triple-drug regimen.5,8–10 Compared with separate tablets for each drug, fixed-dose combination (FDC) tablets are associated with a higher rate of adherence to treatment, and, as a consequence, a greater proportion of patients attaining their BP goal over time.6,11,12 At the time of publication, only three fixed-dose triple-antihypertensive drug combination tablets were available, all containing the dihydropyridine calcium channel blocker amlodipine besylate and the thiazide diuretic hydrochlorothiazide (HCT). These two drugs are combined with the renin inhibitor aliskiren hemifumarate, the ARB valsartan, or the more recently developed ARB olmesartan medoxomil.13
A randomized 12-week clinical trial (TRINITY) of separate-tablet triple-drug therapy comprising olmesartan, amlodipine, and HCT demonstrated that the combination was well tolerated and efficacious in patients with moderate-to-severe hypertension, including those with cardiovascular disease, chronic kidney disease, and diabetes mellitus.14,15 In a 4-week, single-center, open-label study that evaluated the olmesartan/amlodipine/HCT FDC tablet in patients with inadequate BP control on single-, dual- or triple-agent therapy, all patients attained SBP goals with no reports of hypotension.16 Thus, clinical trials indicate that the three-drug combination of olmesartan, amlodipine, and HCT displays efficacy, tolerability, and safety when administered as separate tablets for each drug or, alternatively, as an FDC tablet.
The objective of the present noninterventional study was to determine whether findings from clinical trials can be applied to an unselected patient population in real-life clinical practice. This report covers the safety and effectiveness of the FDC tablet following 24 weeks of treatment, with an emphasis on the safety of the FDC according to age, cardiovascular risk profile, including diabetes mellitus, and concomitant medications.
Methods
Study design
Between November 2012 and December 2013, this binational, multicenter, noninterventional, open-label, prospective, noncontrolled observational study recruited 5,831 patients from primary care centers in Austria and Germany. The protocol was approved by the relevant ethics committees in Austria and Germany, and the study was performed according to the ethical standards of the Declaration of Helsinki. Signed informed consent was obtained from all patients prior to enrollment. It was registered with the “Verband Forschender Arzneimittelhersteller” (VFA).
Patient population and schedule
Adult (≥18 years old) patients with essential hypertension (ie, with no known cause) were eligible for inclusion, providing that the olmesartan/amlodipine/HCT FDC tablet was indicated according to the summary of product characteristics, and treatment with the FDC had been initiated less than 2 weeks before the baseline visit. Exclusion criteria included contraindications to the FDC (eg, known hypersensitivity to any of the active substances of the FDC, to dihydropyridine derivatives, to other sulphonamide-derived substances or to any excipients of the compound), impaired renal function, treatment-resistant hypokalemia, hypercalcemia, hyponatremia, or symptomatic hyperuricemia, severely impaired liver function, cholelithiasis or biliary tract obstruction, as well as severe hypotension, (cardiogenic) shock, left ventricular obstruction, hemodynamically unstable heart failure after acute myocardial infarction, planned or existing treatment with the direct renin inhibitor aliskiren, and planned or current pregnancy.
The following five dose levels of the olmesartan/amlodipine/HCT FDC tablet were used: 20/5/12.5 mg, 40/5/12.5 mg, 40/5/25 mg, 40/10/12.5 mg, and 40/10/25 mg. Up- or down-titration of the FDC tablet dose level was permitted at the discretion of the treating physician. The study had a planned follow-up period of 24±2 weeks, with optional interim visits at 8±2 weeks and 16±2 weeks. At baseline, details of patient demographics and other characteristics were obtained, including time since diagnosis, relevant prior and concomitant diseases/risk factors, and concomitant antihypertensive and non-antihypertensive pharmacotherapies.
Objectives
The primary objective was to gain further insight into the safety profile of the olmesartan/amlodipine/HCT FDC tablet in clinical practice by documenting adverse drug reactions (ADRs) and their possible associations with concomitant pharmacotherapy. Secondary objectives were to evaluate the BP-lowering effectiveness of the FDC in clinical practice.
ADRs
All ADRs that occurred during the observation period and had a suspected causal relationship to the FDC tablet were recorded. Each ADR was coded using the Medical Dictionary for Regulatory Activities (latest version available at database lock). Subsequently, specific ADRs were translated into more general terms categorized by organ system. A serious ADR was recorded if any of the following events occurred: death, a life-threatening reaction, inpatient hospitalization or prolongation of existing hospitalization, persistent or significant disability, a medically significant reaction, or a congenital disease/birth defect.
BP measurement
Office sitting BP was measured at each visit using a calibrated standard sphygmomanometer and a cuff size appropriate for each patient. We recommended that measurements be performed after the patient had been seated for at least 5 minutes with the arm rested at the level of the heart. Physicians were advised to submit the mean BP value obtained from three separate readings.
Statistical analyses
Data were documented using a paper case report form and were entered into an electronic data capture system/project database. Exploratory descriptive statistical analysis was performed using Statistical Analysis System (SAS Institute, Cary, NC, USA). The safety set included all patients who received at least one dose of the FDC. The full analysis set, which was used to analyze efficacy, comprised all patients in the safety set who also had valid and available data on SBP and DBP at baseline as well as at least one postbaseline visit. Continous parameters are described by means of absolute numbers ± standard deviation (SD; including the number of nonmissing and missing observations), while for discrete parameters, frequencies (%) are presented.
Results
A total of 5,831 patients were enrolled, of whom 451 and 5,380 were cared for by physicians in Austria and Germany, respectively. The safety set comprised 5,831 patients, and the full analysis set included 5,818 patients.
Patient characteristics
The mean age of patients was 63.5 (±11.79) years, including 50.5% aged between 40 and 65 years, 29.2% between 65 and 75 years, and 18.1% aged ≥75 years. There were fewer females (47.0%) than males (53.0%) in the study population. The mean body mass index was 29.4 (±4.89) kg/m2, and 36.9% of patients were obese (≥30 kg/m2). Essential hypertension was the indication for 97.9% of patients, and the time since diagnosis was more than 5 years for 47.5% of patients, 1–5 years for 30.1% of patients, less than 1 year for 11.1% of patients, and unknown for 6.5% of patients (4.8% missing). Risk factors were present in approximately 70% of the study population, with 54.7% having one or two risk factors, and 10.2% having three risk factors or more. The most common risk factors were diabetes mellitus (29.4%), metabolic syndrome (21.1%), smoking (17.8%), left ventricular hypertrophy (9.9%), and cardiac failure (7.4%; Table 1). In 24.3% of the patients “other risk factors” were reported.
  | Table 1 Patient demographics at baseline (n=5,831, safety set) |
Treatment patterns
Prior to the study, ARBs, calcium channel blockers, and diuretics were being administered to 25.8%, 33.2%, and 44.9% of the study population, while ACE inhibitors, beta-blockers, renin inhibitors, and other antihypertensive drug classes were being taken by 52.4%, 39.4%, 0.9.%, and 6.8% of patients respectively. With regard to the number of antihypertensive drugs and the use of separate tablets or an FDC, the majority of patients were receiving antihypertensive monotherapy (25.1%), separate dual-agent therapy (23.9%), or separate triple-agent therapy (20.3%). The most common reason for switching to the FDC was a perceived lack of efficacy (87.7%; Table 2).
During the follow-up period (24±2 weeks), mean exposure to the FDC tablet was 180.7 (±41.16) days. The mean daily dose was 32.8 (±9.48) mg for olmesartan, 6.2 (±2.16) mg for amlodipine, and 15.0 (±5.07) mg for HCT. After baseline and at the final assessment, the most frequently prescribed dose regimen was 20/5/12.5 mg, which was administered to 37.4% and 32.0% of patients at the two respective time points, followed by 40/5/12.5 mg (29.3% and 29.1% of patients, respectively), 40/10/25 mg (13.4% and 15.9% of patients, respectively), 40/10/12.5 mg (10.4% and 13.0% of patients, respectively), and 40/5/25 mg (9.6% and 9.9% of patients, respectively; Table 3).
Concomitant antihypertensive treatments were being administered to 39.2% and 35.2% of patients at baseline and at follow-up (V4), respectively, with beta-blockers being prescribed to 30.5% and 29.2% of patients at the two respective time points, and all other classes of antihypertensive agent being prescribed to <10% of the study population. Concomitant non-antihypertensive medications were being taken by 66.1% and 66.1% of patients at baseline and follow-up, respectively. These agents included lipid-lowering drugs (32.3% and 32.6%, at the two respective time points), oral antidiabetic agents (22.0% and 22.7%, respectively), and acetylsalicylic acid (19.1% and 19.5%, respectively), as well as psychotropic agents (7.2% and 7.5%, respectively), nonsteroidal anti-inflammatory drugs (NSAIDs)/cyclo-oxygenase (COX) II antagonists (6.4% and 6.6%, respectively), insulin (6.2% and 6.2%, respectively), anticoagulants (4.5% and 4.6%, respectively), and other types of non-antihypertensive treatment (24.4% and 23.7%, respectively; Table 3).
Adverse drug reactions, concomitant treatment, and risk factors
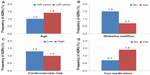
Among the 5,831 patients in the safety data set, 1.23% (n=75) had at least one ADR, including 0.09% (n=5) with at least one serious ADR, and 0.82% (n=48) with at least one ADR that led to study discontinuation (Table 4). The most frequent types of ADR by primary organ system were general disorders and administration site conditions (0.6%; predominantly peripheral edema, 0.3%), skin and subcutaneous tissue disorders (0.19%), nervous system disorders (0.17%; primarily dizziness, 0.12%), vascular disorders (0.15%; the majority of which were hypotension, 0.14%), and gastrointestinal disorders (0.10%). Further analyses to determine the influence of age (<65 years versus ≥65 years; Figure 1A), diabetes mellitus (no/yes; Figure 1B), and cardiovascular risk (low/high; Figure 1C) demonstrated that the rate of ADRs was lower among patients aged <65 years (1.0% versus 1.4%), those with diabetes mellitus (0.7% versus 1.5%), and those with low cardiovascular risk (1.3% versus 1.0%). For patients who received any type of concomitant medication (antihypertensive and/or non-antihypertensive), the rate of ADRs was higher (1.4%) than for those who received only the FDC tablet (0.7%; Figure 1D).
BP-lowering effectiveness
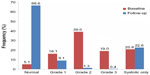
Using data from the 5,818 patients in the full analysis set, the mean SBP and DBP changed from 162.1 (±17.01) mmHg and 93.6 (±10.56) mmHg, respectively, at baseline, to 133.1 (±10.38) mmHg and 79.7 (±7.02) mmHg, respectively, at the 24 weeks follow-up, corresponding to a mean reduction in SBP/DBP of 28.8/13.9 mmHg (Figure 2). The frequency of patients with BP <140/90 mmHg was 5.1% at baseline, rising to 67.5% at follow-up. A BP response, defined as SBP <140 mmHg and DBP <90 mmHg or a change from baseline of ≥20 mmHg in SBP and/or ≥10 mmHg in DBP, was observed in 94.2% of patients at follow-up. With regard to the severity of hypertension, 16.1%, 39.0%, 19.0%, and 20.8% of patients were considered to have grade 1, grade 2, grade 3, and isolated systolic hypertension at baseline, respectively, compared with 9.1%, 1.3%, 0.4%, and 22.6% of patients with each respective class of hypertension at the follow-up visit (Figure 3).
Discussion
The results of the present study are in accordance with those from randomized controlled clinical trials that demonstrate that the FDC olmesartan/amlodipine/HCT tablet is associated with very few ADRs, even among patients who are elderly, have diabetes mellitus or other cardiovascular risk factors, and those receiving concomitant medications. Furthermore, after 24±2 weeks of treatment with the FDC tablet, 68% of patients uncontrolled at baseline had BP <140/90 mmHg, and 94% of patients had a favorable BP response. Thus, this study indicates that data from clinical trials provide an accurate translation of the safety and efficacy of the FDC olmesartan/amlodipine/HCT tablet in a real-life environment.
Patient demographics
One of the key advantages of an observational study is the inclusion of patients who would be excluded from clinical trials, resulting in a broader patient population that is more representative of that in real-life clinical practice. This is illustrated by considering the differences in baseline demographics between patients included in the present study and those who received the three-drug olmesartan, amlodipine, HCT combination in the TRINITY clinical trial.14 Compared with the three-drug arm of the TRINITY study, patients in the present study were on average older (63.5 years versus 54.7 years), and there was a much higher proportion of patients aged over 65 years (47.3% versus 22.5%). Obesity was less prevalent (36.9% versus 61.7%), but the frequency of diabetes mellitus was almost twofold higher in the present study (29.4% versus 15.3%).14
ADRs and BP-lowering efficacy
The rate of ADRs in the present study was low, with the most frequent ADRs being peripheral edema, dizziness, and hypotension, which is consistent with the safety profile of the three-drug combination in clinical trials.14,16,17 In the TRINITY clinical trial, in which all patients received 12 weeks’ treatment with the highest dose level of each drug (40/10/25 mg), dizziness and peripheral edema were the most common ADRs, followed by headache and fatigue.14 Similarly, in the 4-week study of the FDC tablet, the most prevalent treatment-emergent adverse events were peripheral edema and dizziness.16 With regard to effectiveness, attainment of a BP goal of <140/90 mmHg was experienced by 67% of patients in the present study, 70% in the TRINITY clinical trial,14 and 78% in a 36-week open-label extension study that assessed the three-drug separate-tablet combination of olmesartan, amlodipine, and HCT.17 Of note, subanalyses of the TRINITY trial revealed that the rate of BP goal attainment was lower among patients with diabetes mellitus (41.1%) or chronic cardiovascular disease (38.9%).15 Given that the proportion of patients with diabetes mellitus was approximately twofold higher in the present study, this may have contributed to the slightly lower rate of BP goal attainment.
ADRs and patient subpopulations
Results from this study indicate that the rate of ADRs was only slightly increased among patients who were over 65 years of age, and those with high cardiovascular risk. A study by Weir et al18 of an FDC tablet of olmesartan/amlodipine with separate-pill HCT also indicated that age did not have a relevant influence on the ADR rate. Drug-related hypotension and orthostatic hypotension, however, occurred less frequently in patients aged greater than 65 years (2.2% and 0.0%) compared with under 65 years (2.3% and 0.3%). Although in the present study, subanalyses according to the types of ADRs that were observed in elderly, high-risk, or diabetic patients were not conducted, data from other clinical trials provide some insight into the ADRs that occur more frequently in these patient populations. In the TRINITY clinical trial, subanalyses demonstrated that the most frequent ADRs among patients with diabetes mellitus or chronic cardiovascular disease were peripheral edema and dizziness, which corresponds to findings for the total patient population in both studies.15
Perhaps more surprisingly than the findings in elderly and high-risk patients, the rate of ADRs in the present study was 0.7% lower in patients with diabetes than those without. In the absence of any obvious explanation, it may be hypothesized that patients with diabetes mellitus had greater adherence to treatment because of the need for routine antidiabetic medication. Another possibility is that the combination of oral antidiabetic drugs with antihypertensive medications may have a complementary effect on the tolerability profile of the two types of treatments. Related to this theory, a study by Ogihara et al19 in patients with hypertension and diabetes mellitus demonstrated that the reduction in insulin resistance induced by the thiazolidinedione troglitazone was associated with enhanced BP control.
Concomitant medications
As in other clinical trials of antihypertensive agents, patients in the TRINITY clinical trial were not allowed to receive concomitant medications that may alter BP. In contrast, in the present study, 35%–39% of patients were receiving antihypertensive concomitant medications, and about 66% were receiving concomitant non-antihypertensive medications. These data are relevant because of the potential impact of concomitant medications on the safety, tolerability, and efficacy of the FDC. The rate of ADRs in the present study was only 0.5% higher in patients prescribed any type of concomitant medication compared with those who only received the FDC tablet. In addition, no previously unidentified drug interactions were observed. This suggests that the FDC may be safely administered in combination with a variety of other drugs. Nevertheless, further analyses are warranted to determine whether any individual concomitant medication had a noticeable effect on ADRs. For example, it would be of interest to know if the 0.5% increase in ADRs corresponds to a specific type of ADR or a specific type of concomitant medication.
Strengths and limitations
Because this was an observational study, it allows a more realistic evaluation of the effectiveness of the FDC tablet when applied in a real-life setting.20,21 In addition, this study included elderly, high-risk, and diabetic patients, as well as those receiving concomitant medications. Thus, this report represents an important source of information to inform clinical practice and should help to better define optimal treatment strategies for patient populations that are prevalent in clinical practice, but underrepresented in clinical trials. Limitations of this study include the lack of adjustment for confounding variables and biases in the patient population. For example, one confounding variable is the use of additional medications, both antihypertensive and non-antihypertensive. Also, data from this study are specific to the central European demographic, but it is presently unknown whether these results hold true for different ethnic backgrounds, health systems, and providers. Finally, it is known that the reporting of adverse events is less complete than in clinical trials, potentially leading to an underestimation of the true hazard.
Conclusion
This study reveals that in real-life clinical practice, the FDC olmesartan/amlodipine/HCT tablet was associated with very few ADRs combined with two-thirds of patients attaining a BP goal of <140/90 mmHg. These data should help to inform clinical practice, and indicate that the higher level of treatment adherence associated with an FDC tablet may translate into superior patient outcomes compared with multiple separate tablets. Furthermore, this study demonstrates that treatment with three different classes of antihypertensive drug, with or without the use of concomitant drugs and using the most appropriate dose regimen for each patient, resulted in 94% of patients attaining a BP response, and a clinically relevant reduction in the risk of cardiovascular events might be expected.
Author contributions
Peter Bramlage, Eva-Maria Fronk, Wolf-Peter Wolf, Rüdiger Smolnik, and Roland E Schmieder designed the study. Eva-Maria Fronk was responsible for the statistical analyses. Peter Bramlage and Gemma Sutton designed the analyses, interpreted the findings and drafted the first version of the manuscript. All authors revised the article for important intellectual content. All authors approved the final version of the manuscript that was submitted. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The study was funded and conducted by Daiichi Sankyo Europe GmbH, Munich, Germany. Peter Bramlage and Roland E Schmieder have received research funds and consultancy honoraria from Daiichi Sankyo. Eva-Maria Fronk, Wolf-Peter Wolf, and Rüdiger Smolnik are employees of Daiichi Sankyo. Gemma Sutton has no conflict of interest related to this manuscript. The authors report no other conflicts of interest in this work.
References
Collins R, MacMahon S. Blood pressure, antihypertensive drug treatment and the risks of stroke and of coronary heart disease. Br Med Bull. 1994;50:272–298. | |
Mancia G, Laurent S, Agabiti-Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. | |
Gradman AH. Rationale for triple-combination therapy for management of high blood pressure. J Clin Hypertens (Greenwich). 2010;12:869–878. | |
Wright JT Jr, Dunn JK, Cutler JA, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293:1595–1608. | |
Salahuddin A, Mushtaq M, Materson BJ. Combination therapy for hypertension 2013: an update. J Am Soc Hypertens. 2013;7:401–407. | |
Kizilirmak P, Berktas M, Uresin Y, Yildiz OB. The efficacy and safety of triple vs dual combination of angiotensin II receptor blocker and calcium channel blocker and diuretic: a systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2013;15:193–200. | |
Neutel JM, Smith DH. Hypertension management: rationale for triple therapy based on mechanisms of action. Cardiovasc Ther. 2013;31:251–258. | |
Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. | |
Kjeldsen SE, Aksnes TA, Ruilope LM. Clinical implications of the 2013 ESH/ESC hypertension guidelines: targets, choice of therapy, and blood pressure monitoring. Drugs R D. 2014;14:31–43. | |
de la Sierra A, Barrios V. Blood pressure control with angiotensin receptor blocker-based three-drug combinations: key trials. Adv Ther. 2012;29:401–415. | |
Panjabi S, Lacey M, Bancroft T, Cao F. Treatment adherence, clinical outcomes, and economics of triple-drug therapy in hypertensive patients. J Am Soc Hypertens. 2013;7:46–60. | |
Bramlage P, Hasford J. Blood pressure reduction, persistence and costs in the evaluation of antihypertensive drug treatment–a review. Cardiovas Diabetol. 2009;8:18. | |
Drugs@FDA [homepage on the Internet] Silver Spring: U.S. Food and Drug Administration; 2014. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed November 21, 2014. | |
Oparil S, Melino M, Lee J, Fernandez V, Heyrman R. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double-blind, 12-week, parallel-group study. Clin Ther. 2010;32:1252–1269. | |
Kereiakes DJ, Chrysant SG, Izzo JL Jr, et al. Olmesartan/amlodipine/hydrochlorothiazide in participants with hypertension and diabetes, chronic kidney disease, or chronic cardiovascular disease: a subanalysis of the multicenter, randomized, double-blind, parallel-group TRINITY study. Cardiovasc Diabetol. 2012;11:134. | |
Punzi HA. Efficacy and safety of olmesartan/amlodipine/hydrochlorothiazide in patients with hypertension not at goal with mono, dual or triple drug therapy: results of the CHAMPiOn study. Ther Adv Cardiovasc Dis. 2014;8:12–21. | |
Volpe M, de la Sierra A, Ammentorp B, Laeis P. Open-label study assessing the long-term efficacy and safety of triple olmesartan/amlodipine/hydrochlorothiazide combination therapy for hypertension. Adv Ther. 2014;31:561–574. | |
Weir MR, Shojaee A, Maa JF. Efficacy of amlodipine/olmesartan medoxomil ± hydrochlorothiazide in patients aged ≥65 or <65 years with uncontrolled hypertension on prior monotherapy. Postgrad Med. 2013;125:124–134. | |
Ogihara T, Rakugi H, Ikegami H, Mikami H, Masuo K. Enhancement of insulin sensitivity by troglitazone lowers blood pressure in diabetic hypertensives. Am J Hypertens. 1995;8:316–320. | |
Wang OJ, Krumholz HM. Clinical trial participation: are we studying the patients we are trying to treat? Eur J Heart Failure. 2009;11:1021–1022. | |
Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294–1303. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.