Back to Journals » International Journal of General Medicine » Volume 13
Safety and Biopharmaceutical Challenges of Excipients in Off-Label Pediatric Formulations
Authors Belayneh A , Tadese E, Molla F
Received 3 September 2020
Accepted for publication 14 October 2020
Published 9 November 2020 Volume 2020:13 Pages 1051—1066
DOI https://doi.org/10.2147/IJGM.S280330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Anteneh Belayneh,1 Ebisa Tadese,2 Fantahun Molla2
1Department of Pharmacy, College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia; 2Department of Pharmaceutics, School of Pharmacy, College of Health Sciences, Mekelle University, Mekelle, Ethiopia
Correspondence: Anteneh Belayneh Email [email protected]
Background: One of the major challenges in pediatric treatment is the lack of suitable drug preparations specifically designed and marketed for children. Most of the FDA approved drug formulations for adults have not been approved for use in pediatric patients. Shortage of suitable pediatric dosage information often leads health professionals to use adult formulations in an off-label manner. The aim of this work was to review the safety and biopharmaceutical challenges of commonly found excipients in off-label pediatric formulations as well as to show the current progress to alleviate pediatric toxicity related to excipients.
Methods: Research findings and medical case reports were searched from credible sources including Scopus, PubMed, OVID, Google Scholar, Embase, Cochrane Library, and Web of Science.
Results: As several studies and clinical case reports have revealed, off-label adult formulations usage causes pediatric patients to become exposed to potentially harmful excipients, which are essential components of drug products. In addition to their toxicities, some of the excipients affect the biopharmaceutical property of different drugs. Immature organ and body composition, large body surface area and slower metabolism and elimination capabilities of pediatrics are the main causes of toxicities associated with different excipients. Recent studies have also shown that good progress is being made to develop safe and suitable excipients for pediatric use.
Conclusion: A risk and benefit assessment should be done before using off-label formulation as excipients cause mild to severe toxicities and biopharmaceutical problems to pediatric patients.
Keywords: pediatrics, excipients, safety, biopharmaceutics, off-label formulations
Introduction
Exposure of Pediatric Patients to Off-Label Medications
The development of pharmaceutical products for pediatric use often requires age-appropriate formulations. Due to methodological and ethical prerequisites for pediatric trials, expensive formulation costs, and a small and fragmented market, pediatric drug development is related to various challenges.1 Because of these difficulties, there have been very restricted study efforts to develop medications according to pediatric requirements. This challenge (lack of suitable pediatric formulations) frequently leads health care professionals to give adult medications in an off-label or unlicensed manner to pediatric patients.2
Prescribing or administering of medications outside the terms of the market authorization is referred to as off-label (unlabeled or unapproved) medications. It differs from label medications since off-label drugs are prescribed/dispensed out of the recommended route, dose and indication which is written on the label of the package.3 On the other hand, unlicensed (unregistered) medications are formulations or dosages that have not been approved in the country in which it is prescribed or administered.4 There are various types of off-label medications such as prescribing medication out of approved dosage form, strength, frequency and route of administration, or administering contra-indications or out of age range. Most commonly, pediatric patients are frequently exposed to severe overdose and incorrect dose problems due to using off-label medications.5 Off-label medications are administered by different techniques such as cutting tablets, segmenting transdermal patches, formation of solution or suspension by using solid dosage forms and diluting liquid dosage forms.2 Although off-label use of medications is common for all age groups, it is most frequently used for pediatric populations. From the total pediatric formulations, about 60% medications are prescribed in an off-label manner.6
Pediatric patients may be uniquely vulnerable to adverse events related to excipients, due to immature absorption, distribution, metabolism, and elimination pathways. Off-label use of drugs formulated for older populations remains prevalent, as clinical studies that meet regulatory approval standards are scarce.7 The off-label medication usage of adult formulations may expose pediatric patients to potentially harmful excipients, important components of pharmaceutical formulations. Potentially harmful means, the excipients which are safe for adults but may be toxic to children since there is no evidenced safety data on pediatric doses. The toxicity not due to the dose, age or route, but because of the physiological and pharmacokinetic difference between children and adults. This might be due to the immature physiological condition of pediatric patients makes them highly sensitive to any chemicals including excipients.8,9 Therefore, a wide-ranging safety evaluation of excipients in a pediatric pharmaceutical preparation is necessary before use; referring to accessible safety data from adult human and animals as well as safety data from pediatric use and juvenile toxicity studies.10
Physiological and Pharmacokinetic Considerations of Pediatrics
It has been well recognized that pediatric patients are not small adults but rather a different and heterogeneous group with regard to pharmacotherapy.6 The internationally agreed classification of the pediatric population is as follows:
- Preterm newborn infants.
- Term newborn infants (0 to 28 days).
- Infants (> 28 days to 23 months).
- Children (2 to 11 years).
- Adolescents (12 to 16 or18 years).11
Pediatrics differs from adults in various aspects of physiology and pharmacokinetics responses of any chemicals including drugs and excipients. The main physiologic and pharmacokinetic variation between pediatrics and adults include the rate of gastric emptying and pH of the GIT, gastrointestinal permeability, the surface area available for drug absorption and distribution, hepatic and renal function, and the elimination process.12 The largest difference from adult pharmacokinetics occurrs during the first 12–18 months since organ functions are still developing.13
A number of anatomical and physiological factors determine the pharmacokinetic profile of a drug. Differences in physiology in pediatric populations compared with adults can influence the concentration of a drug within the plasma or tissue. Healthcare professionals need to be aware of anatomical and physiological changes that affect pharmacokinetic profiles of drugs to understand consequences of dose adjustments in infants and children. For example, the vital organs such as liver, kidney, heart and brain are highly immature compared to adults. This immaturity leads to a toxic response in pediatric patients. Due to the immaturity of their liver, they are not able to metabolize excipients and drugs properly. That means, excipients stay for a long time in the body which results in toxicity. Their immature kidneys are also unable to excrete toxic metabolites from their bodies.14
The proportions of body fat, protein, and extracellular water content which significantly affect the distribution of drugs and excipients also change significantly during early childhood. The body water decreases from about 80% in the newborn to 60% by the age of 5 months. The percentage of body fat is double by 4–5 months.9 Moreover, the liver and kidney size relative to the body weight also changes during growth and development. The liver, the most important organ for drug metabolism, constitutes around 5% of the body weight at birth but only 2% in adults. The drug-metabolizing enzymes are also reduced in children.15 Total cytochrome P450 content in the fetal liver is between 30 and 60% of adult values.16 Both these organs, liver and kidney, reach a maximum relative weight in 1- and 2-year-old children during the period of life when the capacity for drug metabolism and elimination is greatest. Likewise, body surface area relative to the body mass which highly affect absorption is greater in infants and children than adults.11 In addition to these developmental changes in body composition and proportions, there are other specific changes in organ function such as hepatic and renal function during growth and maturation, which affect the pharmacokinetic characteristics of medicines at different ages.8
Experimental studies have also shown that rapid growth at the receptor level for prostanoids, immunoglobulin E (IgE) antibodies, mast cells, and angiotensin II occurs during the childhood.13 Furthermore, catecholamines including dopamine, serotonin or gamma-aminobutyric acid (GABA) receptor complex functions significantly changes in a variety of organ systems such as the cardiovascular, renal and neuronal systems during this age. This rapid growth of cell receptors leads pediatric patients to give toxic and fast responses to different excipients.17,18
Pharmaceutical excipients are necessary to maintain the quality and improve patient acceptability of medicines. Excipients ideally have limited pharmacological activity; however, some are associated with toxicity in neonates. The pharmacokinetics of excipients are different in neonates than in adults or older children and may additionally be affected by underlying medical conditions.19
Excipients in Pediatric Formulations
Pediatric pharmaceutical formulations are more complex as they contain a broader range of excipients than adult dosage forms. Excipients are one of the vital components of drug formulations commonly added to ensure stability over a given shelf life, to improve palatability, to facilitate solubility, to bulk up formulations that contain highly potent active ingredients and to preserve from microbiological contamination.18,20 However, excipients that are mostly used in adult formulations may not be equally safe when used in paediatrics, even when used in very small concentrations.21 Consequently, the use of excipients in pediatric formulations should be justified through a risk-based assessment.22 In the development of pediatric medicines, the number of excipients and their associated amount in a formulation should be as minimum as possible to reduce the risk of toxicity.23,24
In 2011, the food and drug administration (FDA) changed the drug label of Kaletra® (lopinavir/ritonavir) because of some serious health problems that arose in premature newborns related to the propylene glycol and ethanol contained in the oral solution.25 The pan-European survey also showed that two-thirds of neonates received at least one potentially harmful excipient, such as ethanol and benzoates.26,27
In addition to safety challenges, some excipients also cause biopharmaceutical interactions with body physiological systems in children as some excipients influence the rate of absorption and elimination of concomitantly used medications.28,29
There are several gaps in the available study. Some studies depend only on a limited number of excipients, some studies focused only on toxicity issues, some studies focused only on neonates or infants, and some studies were not focused on the progress to solve excipient toxicities related to off-label pediatric medications. So, the aim of this work was to make a thorough review on the safety and biopharmaceutical challenges of commonly found excipients in off-label pediatric formulations as well as to show the current progress to alleviate pediatric toxicity related to excipients.
Materials and Methods
Search Strategy
The main source for this review was electronic databases of published scientific literature. Research findings and medical case reports were searched from credible sources including Scopus, PubMed, OVID, Google Scholar, Embase, Cochrane Library, and Web of Science. Some studies were also identified with a manual Google search. No restriction was applied to the year of publication, methodology, or study subjects. Primary search terms were “Pediatrics,” “Excipients,” “Safety,” “Biopharmaceutics,” and “Off-label formulations.”
Inclusion/Exclusion Criteria
Studies and medical case reports which do not contain information about safety and biopharmaceutical challenges of commonly found excipients in off-label pediatric formulations were excluded. Articles which are published in predator journals were also excluded from this review.
Toxicities and Biopharmaceutical Problems of Common Excipients
The safety and biopharmaceutical problems of common preservatives, solvents, antioxidants, sweeteners, fillers, coloring agents (dyes), flavorants and surfactants which are frequently found in pediatric off-label formulations are discussed below.
Antimicrobial Preservatives
Among the different formulations available, liquid oral dosage forms represent an interesting alternative in children, providing increased dose flexibility and ease of swallowing compared to solid oral forms. However, liquid dosage forms may require ingredients that are not child-friendly such as preservatives.30 The addition of preservatives is very crucial for pharmaceutical products in controlling mold, inhibiting yeast growth, and protecting from bacterial propagation.8
Parabens (Methyl, Ethyl, Propyl, and Butyl)
Parabens are one of the commonly used preservatives in pharmaceutical formulations, foods and cosmetics. However, the use of methylparaben, ethylparaben and propyl para1bens in pediatric parenteral formulations is risky as it strongly affects the bilirubin-albumin binding. It might cause potentially hazardous problems in hyperbilirubinemic neonates.31 Paraben-containing drugs, injectable saline and water for injections should be contraindicated in jaundiced newborn infants when the high-affinity albumin-binding sites approaches saturation. A study by Andersen32 showed that butyl parabens causes reproductive cell damage, and severe skin sensitization in study animals. Cross-sensitization among the parabens groups is also a common problem in pediatric patients.32 By considering the above facts, the World Health Organization (WHO) has set an estimated total acceptable daily intake for methyl-, ethyl-, butyl- and propyl-parabens at no more than 10 mg/kg/day.33
Benzyl Alcohol
Benzyl alcohol is commonly used as a preservative in many injectable drugs and solutions due to useful local anesthetic properties.2 Several neonatal deaths and severe respiratory and metabolic disorders in underweight premature infants have been related with the use of benzyl alcohol in saline intravascular flush and endotracheal tube lavage preparations. Use of benzoic acid and benzyl alcohol commonly via intravascular (IV) and intramuscular (IM) methods causes intraventricular hemorrhage, metabolic acidosis and increased mortality in neonates.9 The occurrence of early infant mortality, kernicterus and intraventricular hemorrhage decreased significantly after changing these preservatives by other alternatives. Benzyl alcohol is also found to be associated with morbidity, including cerebral palsy and developmental delay.34
Following at least a minimal exposure to 130 mg/kg per day of benzyl alcohol, neonates developed fatal gasping syndrome in premature neonates, characterized by hypotension, bradycardia, cardiovascular collapse, and metabolic acidosis.2
A medical case report showed that deaths in neonates (1-year-olds) have been related with the IV administration of 99–234 mg/kg/day benzyl alcohol in large-volume parenteral or endotracheal preparations. The toxic effects of benzyl alcohol, which include respiratory vasodilatation, convulsions, and paralysis, have been known for years.23 However, little is known about the toxic effects or levels of benzyl alcohol and the metabolic acidosis caused by an accumulation of the metabolite, benzoic acid, in neonates, especially in ill premature infants. A possible explanation could be that a high load of this metabolite may exceed the detoxification capacity of the immature liver and kidneys because of glycine deficiency which is responsible for converting toxic benzoic acid to safe hippuric acid as shown in Figure 1.24
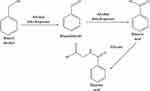 |
Figure 1 Benzyl alcohol metabolism pathway in the body. |
Also, in another study, a number of neonatal deaths, severe respiratory and metabolic complications (32–105 mg/kg/d) such as bronchitis, hemoptysis, and hypersensitivity reactions occur due to IM administration of benzoic acid. The WHO has set the estimated acceptable daily intake of benzyl/benzoic moiety up to 5 mg/kg body weight, but its daily limit in pediatric patients has not been established.2,35 Both the FDA and the American Academy of Pediatrics (AAP) recommend against prescribing preparations which are preserved using benzyl alcohol in infants.2
Benzalkonium Chloride
Benzalkonium chloride is the core preservative used in ophthalmic dosage forms. About 74% of ophthalmic preparations contain benzalkonium chloride as a preservative.2 It is also used as a preservative in nebulized solutions of anti-asthma drugs. However, the use of benzalkonium chloride in the pediatric population has been reported to cause dose-related bronchoconstriction especially in pediatrics who have asthmatic conditions and has been related with the precipitation of respiratory arrest. Ototoxicity can also occur when applied topically to the ear and prolonged exposure with skin occasionally causes irritation and hypersensitivity. Specific to pediatrics, it was reported that ingestion of benzalkonium chloride caused severe respiratory insufficiency in a newborn baby.6
Sodium Benzoate
It is used as a preservative in pharmaceutical products (including injectable and oral liquids) and cosmetics. Injectable preparations which contain sodium benzoate should not be used in neonates as it causes severe non-immunological contact reactions, urticaria, anaphylaxis and atopic dermatitis when it is administered via IV and IM routes.36,37 It might increase the risk of jaundice to neonates when used in parenteral preparations.24 The WHO recommended daily consumption of total benzoates up to 5 mg/kg.33,35
Solvents
Although water is the most commonly preferred solvent during the formulation of liquid preparations, most active pharmaceutical ingredients (APIs) have poor aqueous solubility which limits the achievable concentration in formulated solutions.38 In many cases, an acceptable solution requires the use of solubility enhancing methods including surfactants and co-solvents such as glycerol, polyethylene glycol (PEG) and ethanol. However, the use of these methods requires safety considerations as they significantly affect the pediatric health. Risk of irritation, damage of intestinal tissues, hyperosmolality and local administration site toxicity are common problems due to exposure of solvents and solubility enhancing agents. The risks are severe when these agents are included in parenteral formulations rather than oral formulations.39
Ethanol
Ethanol is one of the most commonly used solvents in oral liquid preparations. It is also used as a preservative agent. There are severe acute and chronic adverse effects with the use of ethanol-containing medications in the pediatric population.40,41 Acutely, the co-administration of ethanol may alter the drug absorption or metabolism and may result in drug interaction. In addition, ethanol causes severe adverse effects on the central nervous system when its concentration in blood arising in the range of 1–100 mg/100 mL. Depression and confusion are the commonly occurring central nerve system (CNS) side effects. Gastrointestinal (GI) upset which caused by ethanol is also more pronounced in children than other populations.12
Pediatric patients are more vulnerable to the effects of ethanol, especially in the younger age groups of less than 6 years. Higher peak ethanol blood concentrations are observed in children compared to adults for similar intake. Toxicity on brain maturation in young children is highly probable and also supported by non-clinical data.39 Chronic exposure to medications which contain ethanol (>1week) even at small doses is contraindicated in children below 6 years and limited to 2 weeks above 6 years. Ethanol must also be avoided in pediatric patients taking other medications that would cause a disulfiram-like reaction (such as metronidazole) when combined with ethanol.2 The effect of high ethanol concentration in long-term management on liver and kidney treatment is not well studied in the pediatric population. The toxicities of ethanol are related to the deficiency of alcohol dehydrogenase enzyme (ADH) due to the immature livers of pediatric patients which leads to the accumulation of toxic acetaldehyde as shown in Figure 2.24,42
 |
Figure 2 Alcohol metabolism pathway in the body. |
In one study in Philadelphia, the ethanol concentration in the blood was measured following ethanol-containing oral phenobarbitone and dexamethasone administration in 15 neonates. The blood levels of ethanol for about 30% of neonates were above the recommended level of the European Medicines Agency (EMA) guideline.13,34
Neonates may be more susceptible to drug–alcohol pharmacokinetic interactions when compared with adults because of their immature and variable metabolic activity.2 Due to multiple post-marketing life-threatening events on premature babies until 14 days of birth or in full-term babies younger than age 14 days, the FDA recommended avoiding Kaletra (lopinavir/ritonavir) oral solution in 2011. The oral solution of this medication contains 42.4% (v/v) of ethanol and 15.3% (w/v) of propylene glycol. But, both ethanol and PEG are metabolized by the ADH in the liver.43
As the WHO and FDA recommended, the maximum amount of ethanol in pediatric medications should not be more than 0.5% v/v for children under 6 years, 5% for children 6–12 years, 10% for children over 12 years.17 The American Academy of Pediatrics (AAP) also established that the quantity of ethanol in pediatric formulations should not produce a blood concentration higher than 25 mg/100 mL after the administration of a single suggested therapeutic dose.44
Propylene Glycol and Polyethylene Glycol
Propylene glycol (PEG) is a dihydroxy alcohol used as a pharmaceutical solvent, preservative, and humectant.4 It is commonly used as a solvent for substances which are not soluble in water such as phenobarbital, glibenclamide, phenytoin, diazepam and injectable multivitamin preparations. Its metabolism depends on the ADH and aldehyde dehydrogenase enzymes. But, these enzymes are not available in adequate amounts for the elimination of PEG in infants and children younger than 4 years, which leads to different adverse effects such as hypotension, arrhythmia, or hemolysis when administered to infants/children.2
A study by Kaplan et al27 found that the application of dexamethasone cream containing 8% (w/w) PEG on the wounded body of infants has resulted in serum hyperosmolality which is associated with cardio-respiratory arrest. Hyperosmolality occurred in 5.2% of the pediatric patients who took this topical preparation.27,45 Another study also showed that administration of an intravenous multivitamin product, which contained more than 3 g/dl of PEG, for at least 5 consecutive days, was related to high toxicity in infants. These toxicities are both biochemical (such as hyperosmolality, lactic acidosis, plasma creatinine and bilirubin) and clinical such as seizures.46,47
A study done by Kristine et al17 on 3-year-old children showed that PEG 400 has an accelerating effect on pediatric gastrointestinal transit which leads to reduced time for absorption of drugs such as oral ranitidine and cimetidine. Due to this, the bioavailability of both drugs is decreased by 20%.17 The study done on healthy male volunteers in the UK showed that the absolute bioavailability of ranitidine from the pellet formulation was significantly reduced by 31% (from 51% to 35%) and small intestinal liquid transit time was significantly shortened by 37% (from 226 minutes to 143 minutes) as a consequence of oral PEG 400 in the test preparation. These results clearly demonstrate that PEG 400 adversely influences the gastrointestinal absorption of ranitidine. The area under the curve as well as the peak maxima of oral ranitidine were significantly changed due to the presence of PEG 400.48
Similarly, another study conducted on healthy male volunteers in the UK showed that the presence of 1, 2.5, and 5 g PEG 400 reduced the mean small intestinal transit times of the ranitidine solutions by 9, 20, and 23%, respectively. The mean cumulative amount of ranitidine was reduced by 38% in the presence of both 2.5 and 5 g PEG 400 but increased by 41% in the presence of 1 g PEG 400. The author concluded that low concentrations of PEG 400 enhance the absorption of ranitidine, while high concentrations have a detrimental effect on ranitidine absorption by affecting the small intestinal transit time.49
In addition, PEG can also affect the metabolism of concomitantly administered drugs. An in vivo study in the People's Republic of China showed that PEG-40 stearate decreased the total AUC of midazolam by 74% due to the fact that PEG-40 induced the enzyme (CYP450 3A4) which metabolized the drug.50 This result is corroborated with an in vivo study which found that PEG 400 increased the AUC of midazolam by 2-fold and decreased the CL/F from 8.86 to 5.25 L/h/kg (p<0.05).51 The WHO recommended a maximum acceptable daily intake of 1mg/kg for infants and 25 mg/kg for children.23
Glycerol
Glycerol is one of the important solvents employed to prepare suspensions due to its good suspending activity and preferable taste. However, administration of high concentration of glycerol, more than 40%, contributes to severe adverse effects such as mucositis, diarrhea, electrolyte disturbances, headache, and stomach upset.25 These adverse effects of glycerol might be due to its strong osmotic properties. Severe diarrhea caused by the glycerol could lead to decreased drug absorption by shorting the transit time of drugs in the gastrointestinal system for pediatrics.33 A study done by Rene et al,52 showed that concomitant use of oral glycerol significantly decreased the absorption of cimetidine, omeprazole and loperamide in 13-year-old children.52,53
Cyclodextrin
Cyclodextrin is commonly used as a solubilizing agent in insoluble drugs. Most of the drugs administered through the oral route have poor aqueous solubility and dissolution rate. Cyclodextrin and its derivates represent pharmaceutical adjuvants used to overcome these challenges and help in the development of stable formulations with enhanced bioavailability. Cyclodextrins are unique structures with versatile physicochemical properties which aid the pharmaceutical scientists to overcome drug delivery challenges for poorly aqueous soluble drugs. Cyclodextrin and its derivates are widely useful as solubilizers, assisting in the preparation of various dosage forms such as liquid oral, solid, and parenteral preparations. Cyclodextrins interact with appropriately sized guest molecules to form an inclusion complex and enhance the aqueous solubility, physical chemical stability, and bioavailability of drugs. Through the various reported literatures, the review highlights the concept of cyclodextrin and its derivatives in enhancing solubility and bioavailability of poorly aqueous soluble drugs.54
Antioxidants
Oxidation is one of the major causes of stability problems in pharmaceutical preparations. To protect pharmaceutical formulations from oxidation, antioxidants are added.6 Sulfites and propyl gallate are the most commonly available excipients in off-label pediatric formulations.
Sulfites
Sulfites are commonly used antioxidants and preservatives in pharmaceutical, cosmetics and food industries. Although sulfites are frequently used, they cause several clinical adverse effects in children. These adverse effects range from slight flushing, dermatitis, hypotension, diarrhea urticaria and abdominal pain to life-threatening asthma and anaphylactic reactions.35 The prevalence of sulfite sensitivity reaction is about 3 to 10% from the asthmatic pediatrics who take these excipients. The severity of hypersensitivity reactions of sulfites varies among children; asthmatic pediatric patients having a steroid-dependent hyperresponsiveness and children with chronic asthma may have a greater risk. The exact mechanisms of sulfite sensitivity remain unclear.47
According to the FDA, six sulfite compounds (sodium sulfite, sulfur dioxide, potassium bisulfite, sodium bisulfate, potassium metabisulfite and sodium metabisulfite) have been labeled as “Generally Recognized as Safe” for use in pharmaceuticals, cosmetics, and foods products.28 Contradictions to this statement include; there have been different toxic effects of sulfites for pediatric populations. A report by Yang and Purchase (1985)33 showed that about 250 cases of adverse reactions including six deaths in USA and 10 adverse reactions with one death in Canada occurred due to sulfite-containing medications and foods. Metabisulfite hypersensitivity is a major problem in children compared to other types of sulfite compounds.33
Propyl Gallate
Propyl gallate is a white to creamy-white crystalline powder and it is also found in amorphous form. It is used as an antioxidant for pharmaceuticals and food products prepared as fats, oils, emulsions, and waxes.26 Propyl gallate is the commonly used antioxidant in the formulation of synthetic vitamin A. A report on the safety assessment of propyl gallate showed that it causes different adverse effect in children such as pruritus and erythema. This report also showed that allergy, hypersensitivity, hyperactivity, asthma, neurological damage and cancer have sometimes occurred in pediatric patients due to propyl gallate.24
Butylated Hydroxytoluene and Butylated Hydroxyanisole
Butylated Hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) are the commonly used antioxidant in cosmetic product and some drug formulations. BHT is generally recognized as a safe preservative and antioxidant for food, pharmaceutical, and cosmetics products.52 But, different clinical case reports showed that both BHT and BHA cause mild to severe health problems in pediatric populations when taken with medications. The most frequently reported toxicity of BHT was liver injury in pediatric patients.28 A clinical case report in New York city showed that nine of 82 infants in a pediatric ward who took BHT as an oral food additive suffered from acute gastroenteritis and signs of methemoglobinemia particularly cyanosis. A large number of long-term toxicity and carcinogenicity studies with BHA have also been also reported in animal studies.55
Sweeteners
Sweeteners play a significant role in the acceptability of oral pediatric formulations as it enhances the taste of the pharmaceutical product. The choice and concentration of sweetening agents depend on the properties of the APIs and the presence of flavoring agents.20 The basis for the use of a specific sweetener in a pediatric formulation must be clearly described and justified. As there are different safety concerns on the use of some sugars and other sweeteners particularly in the case of diabetes and severe renal insufficiency, the risk benefit assessment should be done.56 Sweeteners can be grouped into natural and artificial sweeteners.
Natural Sweeteners
There are several natural sweeteners used in oral pharmaceutical preparations. They contain high calories and metabolize and change when passing through the body. Sucrose and fructose are the most commonly used natural sweetening agents during formulation of pharmaceutical products.56
From nature, sucrose is the most commonly used sweetener. It is a disaccharide sugar and hydrolyzed in the small intestine into fructose and glucose to be absorbed. Sucrose is not suggested for use in children as well as adult medicinal formulations due to its toxicities. It must be removed from pediatric formulations as it causes a decrease in dental plaque pH, dissolves tooth enamel and encourages dental caries in this age group.13 Tooth decay and decreasing of dental pH could cause degradation of acid sensitive APIs which may lead to treatment failure. Sucrose may also cause severe dental allergic reactions in children when it is administered orally.57
As sucrose and other natural sweeteners have cariogenic effects in pediatric populations, using them as sweetening agent needs strong justification. The potential laxative ability of poorly absorbable sucrose combined with its osmotic properties leads to decreased bioavailability of some drugs.13,58 Special attention should be given to the safety of the sweeteners in relation to specific conditions of pediatrics such as diabetes and fructose intolerance.57 In the United States, the FDA recommends pharmaceutical industries not to use natural sweeteners, specifically sucrose, in pediatric medications.59
Fructose is also another monosaccharide used as a sweetening agent. It causes a rise in blood glucose levels and should be removed from youngsters suffering from diabetes.57 It may also cause laxative effects when administered orally at high doses; this might decrease the absorption of APIs.24
Artificial Sweeteners
Artificial sweeteners are prepared either by completely synthesized or derived chemicals from naturally existing materials. They have an intense sweetening ability and better safety profile, hence are used to replace natural sweetening agents such as sucrose in several pharmaceutical formulations.13,34 Aspartame, saccharin, sucralose, and fructose are the most commonly used artificial sweeteners in pediatric formulations.
Aspartame is one of the most commonly used synthetic sweetening agents for both adult and pediatric medical products as well as food products. It is chemically a dipeptide of methyl ester of phenylalanine and aspartic acid. The consumption of aspartame increases in chewable tablets, oral disintegrating tablets and sugar-free solutions and suspensions. Its sweetening ability is more than 150–200 times of sucrose.2 As aspartame contains phenylalanine, a harmful chemical for some pediatric populations, the list of labels for both prescription and non-prescription medications should include phenylalanine content. Phenylalanine causes severe adverse events on pediatric patients who have phenylketonuria and epileptogenic disorders. It may also reduce the neonate’s insulin sensitivity. The cross-reactivity between sulfonamides and aspartame is another problem. The WHO recommended the acceptable consumption of aspartame should be 40 mg/kg/day for all age groups.33
Saccharin is also another important artificial sweetener in pharmaceutical products and toothpastes. Its sweetening nature is about 300 times more than sucrose. It is one of the potential carcinogen agents especially in pediatric populations.45 Bladder cancer in pediatrics is common during intake of high amounts of saccharin. There are also other different toxicities in pediatric populations such as hypertonia, insomnia, irritability, strabismus, opisthotonus and cross-sensitivity with drugs from the sulfonamide groups. Due to these reasons, it is recommended to use only for children greater than 3 years. Its suitable daily ingestion is 5 mg/kg/day for general populations.13
Sucralose is a chlorinated sugar which was discovered by the British scientists and Tate & Lyle in 1976. From all sweeteners, it is the only non-caloric sweetener and widely known as the international Zero-Calorie sugar substitute and 600 times as sweet as sucrose. Sucralose is minimally absorbed by the body and most of it passes out of the body unchanged. As sucralose belongs to the class of harmful chemicals called organic chlorides, safety concerns should be considered.24 A study done by Goldsmith57 on juvenile animals concluded that sucralose may cause several toxic effects, even carcinogenic, for children. As it is sugar free, sucralose is a safe sweetener for diabetes patients. According to the WHO and FDA recommendation, the maximum daily intake of sucralose should be 15 mg/kg body weight.58
Sorbitol is a mono-saccharide sweetening agent and it is not absorbed from the gastrointestinal tract. Because of its poor absorption ability, sorbitol is considered safe for diabetes patients. It commonly causes abdominal pain, flatulence and osmotic diarrhea as it has a high osmotic nature.13 Since sorbitol is metabolized to fructose, it is contraindicated in pediatric patients with hereditary fructose intolerance and hypoglycemia. It can also cause severe liver damage and coma which may lead to patient deaths. Particularly, intravenous administration of sorbitol should be avoided especially for pediatric patients.24
The absorption of active drugs in pediatrics is highly decreased when sorbitol is concomitantly used as an excipient.35 Decreasing of drug absorption might be due to shortening of the small intestinal transit time because of the high osmotic nature of sorbitol. For instance, a study by Abraham and Mathew61 showed that sorbitol has a dose-dependent effect on the absorption of lamivudine (3TC). The study revealed that with higher doses of oral sorbitol (3.2 g, 10.2 g, and 13.4 g), the Cmax of 3 TC was decreased by 28%, 52%, and 55%, respectively. Likewise, the AUC (0–24) of 3TC was decreased by 20%, 39%, and 44% and the AUC (0-∞) by 14%, 32%, and 36%. Decreasing effect of the absorption of this drug may lead to a rise in the viral load and also drug resistance may occur. To manage this drug interaction, chronic co-administration of sorbitol-containing medicines and 3TC should be avoided. Specifically, with regard to children, 3TC given concomitantly with sorbitol-containing medicines should be used to treat human immunodeficiency virus (HIV) only when an all-tablet regimen cannot be used and the benefits of treatment outweigh possible risks, including lower virologic suppression.60 Furthermore, a conference on retroviruses and opportunistic infections which was held in Seattle in 2001 also showed that concomitant administration of 3TC with sorbitol-containing medications should be avoided as it can highly reduce the viral load reduction ability of 3TC.61
A case study on healthy volunteers in the USA showed that the Cmax and AUC of ranitidine were decreased by 50% and 45%, respectively, in the presence of sorbitol. Similarly, sorbitol reduced the metoprolol Cmax by 23%, but did not exhibit a significant effect on the AUC. Sorbitol decreased the systemic exposure of ranitidine in a dose-dependent manner and affected bioavailability.62
Flavoring Agents
Flavorants plays an important role in patient acceptability especially for pediatric oral liquid formulations. The selection of flavorants should be clearly described and justified as some flavorants have toxic effects in pediatric populations. The safety concerns must be recognized including the risk of allergies and sensitization.2 From several flavorants, peppermint oil is known for its toxic effect in the pediatric population.
Peppermint Oil
Peppermint oil is an important flavoring agent used for different oral liquid preparations especially for pediatrics as it has a favorable taste and odor for them.28 But, different unwanted effects such as atrial fibrillation, muscle pain and cooling or burning sensations have occurred commonly in pediatric populations due to the peppermint oil.43 A clinical case report by Benjamin (2007) showed that taking medication which contains peppermint oil as a flavoring agent caused toxic effects such as digestive disorders and atrial fibrillation in children.63
Coloring Agents and Dyes
Coloring agents have different roles in the pharmaceutical as well as the cosmetics and food industries. Attraction of consumers, identification of products and protection of light sensitive products are some of the functions of coloring agents.29 Most coloring agents used in pharmaceutical oral preparations are grouped into one of the following groups: azo dyes (tartrazine), triphenylmethane dyes, xanthene dyes (erythrosine) and quinoline dyes (quinoline yellow). The numbers of coloring agents that are globally acceptable from a regulatory perspective are limited as many coloring agents have been associated with hypersensitivity and other adverse reactions for pediatrics population.64
Anaphylactic reactions, asthma, urticaria, angioedema and hyperkinesis are the common complications due to most dyes. Cross-sensitivity with acetylsalicylic acid, sodium benzoate and indomethacin is also common in youngsters when they take azo dyes.39 Due to this, azo-dyes are recommended to be avoided from pediatric medications or risk benefit assessments should be done before including them in the formulation. Contact dermatitis is common because of quinoline dyes. Triphenylmethane dyes also might cause bronchoconstriction, skin rash, erythema, angioedema and anaphylaxis. Xanthine dyes might also cause photosensitization and carcinogenicity on children.65
Fillers (Diluents)
Diluents are chemically inactive excipients, mostly used to make up the required bulk of solid dosage form and used up to 80% in a formulation.35 Many studies have reported that fillers used in the solid dosage forms such as tablets and hard gelatin capsules show several unwanted effects to pediatric patients on long-term as well as short-term durations of therapy. So, it is necessary to categorize the safety of diluents before preparing a pharmaceutical formulation to reduce the adverse effects.66 Lactose and mannitol are the most frequently reported toxic fillers in pediatric off-label formulations.
Lactose
More than 20% of all solid dosage forms found in the market contain lactose as a binder and filler. It is a disaccharide of glucose and galactose and absorbed after hydrolysis by intestinal lactase. It can cause hypersensitivity reactions in children and young infants when used in pediatric formulations.67 Because of immature intestine, preterm infants have reduced levels of lactase enzyme to hydrolyze lactose. In infants with lactose intolerance (lactase deficiency), lactose is not readily metabolized by lactase leading to the buildup of lactic acid, hydrogen and carbon dioxide. Abdominal pain, distention or bloating, flatulence, diarrhea, muscle and joint pain and eczema are the common toxicities of lactose in infants and children.29
Sometimes children have serious and prolonged reactions to lactose and might lead to complications such as metabolic acidosis, jaundice, dehydration and bacterial proliferation.6 Moreover, the unabsorbed lactose may raise the osmotic pressure in the colon which may prevent water and other important nutrients and drugs from absorption. The WHO recommended using no more than 3 g per day for children.36
Mannitol
Mannitol is widely used as a filler in several pharmaceutical formulations especially in chewable and orodispersible tablets as it has a good mouth filling property. Compared to sugars, it is less cariogenic. In some cases, hypersensitivity and anaphylactic reactions are reported after intravenous infusion of 10% or 20% (w/v) solutions containing mannitol.66 Direct action of mannitol on mast cells is responsible for the anaphylactic reactions. Its absorption is not more than 20% when taken orally. Due to its high osmotic pressure creating ability, it leads to severe diarrhea which in turn decreases the small intestine transit time, resulting in decreased absorption of some drugs.68
Surfactants
Surfactants are one of the major components of liquid and semisolid pharmaceutical preparations and are added to enhance the solubility of APIs in aqueous media by lowering the surface tension between solutes and solvents, and to enhance the dissolution of active substances in aqueous gastrointestinal fluid or to facilitate absorption of active substances.17 It is also used as detergents, foaming agents, dispersants and wetting agents. However, some surfactants, polysorbates and carrageenan, are known for causing different toxic effects in pediatric patients.43
Polysorbates
Polysorbates 20 and 80 are the most frequently used excipients in biotherapeutics, the safety data for which have been well documented in adults. The polysorbate content in therapeutic formulations that are administered to pediatric patients, however, has been less clearly regulated or defined with regard to safety. In pediatric patients, excessive amounts of polysorbate in biotherapeutics have been linked to hypersensitivity and other toxicity-related effects. Neonates and infants are the most susceptible, because their hepatic and renal functions do not develop fully until 6–12 months and 2 years of age, respectively. In Belgium, an initially healthy newborn experienced acute cardiogenic shock and multiple organ failure after receiving intravenous amiodarone with polysorbate excipient at a loading dose (47 mg/kg) that was intended for oral administration.69
Polysorbate 20 and 80 are also surfactants commonly used in protein parenteral formulations to minimize denaturation at the air-water interface. It is also sometimes used in injectable solutions of small molecules for the purpose of solubility enhancement due to its ability to form micelle.66 However, the polysorbate content in pediatric therapeutic formulations has been less clearly regulated or defined with regard to safety. Parenteral administration of more than 50.6 g polysorbate 80 and 3.37 g polysorbate 20 in biotherapeutics have been linked to hypersensitivity and other toxicity-related effects such as thrombocytopenia, cholestasis, ascites, hypotension, renal dysfunction, hepatomegaly, and severe metabolic acidosis which may lead to death.69
Polysorbates are also found to inhibit the activity of P-glycoproteins (P-gp) which are efflux transporters of absorbed drugs.68 For instance, a study done by Zhang et al,70 showed that the blood concentration of orally administered digoxin was increased by 61% when polysorbate was used concomitantly. According to the authors, the increased concentration of digoxin was due to the inhibition of P-gp in the gut by polysorbate.70 An in vivo study conducted on pigs also showed that both Tween 20 and Tween 80 enhanced the transfer of digoxin across the intestinal mucosa by 2.1-fold and 1.8-fold, respectively, due to their inhibition effect on P-gp. Another in vivo study on rats showed that Tween® 80 enhanced the bioavailability of etoposide by 2.5-fold. It also enhanced the permeability of etoposide by 2.7-fold in an in situ single-pass intestinal perfusion model.70,71
Carrageenan
Carrageenan is used as a surfactant, suspending and wetting agent for suspension and cream preparations. As it is identified as causing severe inflammatory reactions in animal trials, it is not recommended to include carrageenan in pediatric preparations.23 The American Academy of Pediatrics put out an urgent call in 2018 to conduct animal test studies as there is no sufficient safety and toxicity data of carrageenan in pediatric medications and foods.72
Generally the summary of the main excipients found in pediatric off-label medications and their major uses as well as their toxicities are presented in Table 1.
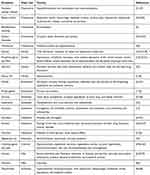 |
Table 1 Summary of Excipients with Their Major Uses and Adverse Effect on Pediatrics |
Current Progress to Minimize Excipient Toxicity for Pediatrics
Although the progress has been slow, clinical trials for pediatrics have undergone a renaissance with international recognition of the importance for new and existing excipients. However, still the deficiencies are continued including inadequate funding and conflicts of interest with trials still being driven by financial and political incentives.73,74 There are greater advocacy and collaboration between all major stakeholders including regulatory authorities, pharmaceutical industries, the scientific community, clinicians and the public at international level. As the future health of children depends on the success of pediatric trials, good investment into better evidence-based treatments and trials for children has a crucial value for a better future for the world.75
FDA recommended that it is very important to support the development and maintenance of scientific and organizational facilities that can plan, start-up, conduct and close out pediatric clinical investigations. They decided to issue awards to pediatric trial-related research to facilitate pediatric clinical trials. Each award will provide one million dollars for each year under the Global Pediatric Clinical Trials Network Cooperative Agreement.76 The good newsis that sponsors are accepting the approach to new and safe excipient developments and quality tests for existing excipients. This is a supporting factor by reducing the financial limit for new excipient development.77 The International Pharmaceutical Excipients Council (IPEC) is doing several assessment procedures for pediatric excipients including tiered toxicology testing.78,79 Pharmaceutical industries and companies also provide excessive demands for juvenile animal testing, mainly for excipients used in pediatric formulations.80
Even though the demand for pediatric data on the safety of excipients has grown rapidly, there is very limited and uncompiled pediatric excipient safety data.2 There is a collaborative effort by the European (EU) and United States (US) Pediatric Formulation Initiatives (PFIs) to develop a STEP (Safety and Toxicity of Excipients for Pediatrics) database.81 Similarly, the European Study of Neonatal Exposure to Excipients (ESNEE) has developed methodologies to provide an integrated assessment of exposure among neonates in Europe to potentially toxic excipients contained in medicines.10 The aim of both STEP and ESNEE is to gather available toxicity and safety information and arranging this information in a systematic way as a searchable system. These systems allow data mining, visualization and analysis to discover useful information from the collected data sets. As they are freely and publicly accessible sources, they will help to build up an evidence base of safety and toxicity information of excipients for the pharmaceutical industry, academic institutions, pharmacists, clinicians and regulators to make informed decisions during pediatric drug development.77,82 The Excipients Safety workshop between scientists representing the diversity of involved disciplines (formulators, nonclinical scientists, clinicians, and regulators) have also had practical and meaningful discussions to provide specific and key recommendations for defining paths in the future. These scientists recommend the leverage of orthogonal sources of data, share data collaboratively, and increase awareness of the existing sources such as the STEP database.7
The information available on excipient acceptability for pediatric age groups is sparse and distributed over various sources. Hence, European (EU) and United States (US) Paediatric Formulation Initiatives (PFIs) are collaboratively creating a STEP database. Because the development of the database is a costly and time-consuming venture, it is important to capture the requirements from the potential users and identify them at an early stage.81 The purposes of the STEP database are: 1) to serve as a freely/publicly accessible evidence base for safety and toxicity of excipients for the pharmaceutical industry, academics, pharmacists, clinicians and regulators to make informed decisions; 2) to enhance the prospects for identifying potential safety issues at the initial stages of the development process, when excipients are being screened and selected; 3) to help highlight any relationship between exposure and evidence of clinically significant toxicity in the pediatric age group as a whole, or in pediatric subpopulations; 4) to identify possible differences in expression, types or patterns of toxicity in children compared to adults5) to provide a basis for assessing the need for generating new data for pediatric medicines (eg, bridging studies, juvenile toxicity studies, etc.), in order to clarify what kind of new data, gaps in knowledge or studies may be required; 6) to support companies with their regulatory filings with readily available information; and 7) to support and enhance research activities by providing a platform to share the unpublished data and data available with corporate entities.77,83
A recently published worldwide survey of studies showed that excipients are not sufficiently and properly labeled in pediatric formulations. Therefore, the voluntary labeling system is clearly inadequate. To solve this problem, the American Academy of Pediatrics (AAP) urgently decided obligatory labeling of all excipients for all prescription and over-the-counter pediatric products.36
Currently using toxic coloring agents for identification purposes of pediatric formulations are going to be replaced by other methods. Where there is a need to differentiate between similar preparations to avoid accidental dosing errors, the use of other strategies such as shape size, and embossing are considered prior to the use of coloring agents.2 Pharmaceutical industries have started to use coating, complex formulations and suitable vehicles instead of using toxic sweetening agents and flavorants in pediatric medications.67
At the present time, new findings are emerging as options to prepare pediatric oral liquid dosage forms free of potentially harmful excipients. One study has shown that there is a safe stability agent called Syrspend®SF PH4 to prepare dexamethasone, hydrochlorothiazide, phenytoin, and spironolactone pediatric oral liquid formulations. This agent provides a reliable solution to reduce the exposure of children, especially newborns, to potentially harmful preservatives, such as parabens.30 Multiarticulate solid dosage forms could also partly replace the liquids and provide more stable and cheaper alternatives to existing liquid drug products or new developments. Dispersible solid drug dosage forms like orodispersible tablets, mini-tablets and films are also another better opportunity for efficient and safe use in pediatrics. These above formulation strategies can solve the problems which is related to harmful excipients including toxic preservatives, antioxidants and solubilizing agents in liquid pediatric formulations.84
Conclusion
Excipients developed for the purpose of adults are not always appropriate for use in pediatric populations as they affect children differently to adults. Therefore, extensive safety evaluation of the excipients should be done when off-label preparations are used for pediatrics. In addition to referring the existing safety data from adult human and animal studies, juvenile toxicity study and pediatric trials should be done extensively to assess toxicities or sensitivities of excipients to pediatrics.
Generally, because of the toxicity and biopharmaceutical problem for pediatrics associated with excipients involved in off-label formulations, development of age-appropriate pediatric drug formulations as well as new and safe excipients are the current major interests in the field of pediatric therapy. The safety information of excipients which has been found from evidenced researches and medical case reports should be included into the STEP repository database to enable easy access for all stakeholders for better decision making during choosing of excipients for pediatric formulations.
Data Sharing Statement
The data used to support the findings of this study are included within the article.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Diana A, Van-Riet N, Piotr K, Brian A, Rutger de V. Paediatric drug development and formulation design—a European perspective. PharmSciTech. 2017;18(2):17.
2. Ivanovska V, Rademaker M, van Dijk L, Mantel-Teeuwisse K. Pediatric drug formulations: a review of challenges and progress. Pediatrics. 2014;134(2):361–372. doi:10.1542/peds.2013-3225
3. Quaas AM, Weedin EA, Hansen KR. On-label and off-label drug use in the treatment of endometriosis. Fertil Steril. 2015;103(3):612–625. doi:10.1016/j.fertnstert.2015.01.006
4. Mason J, Pirmohamed M, Nunn T. Off-label and unlicensed medicine use and adverse drug reactions in children: a narrative review of the literature. Eur J Clin Pharmacol. 2012;68:21–28. doi:10.1007/s00228-011-1097-1
5. Yewale VN, Dharmapalan D. Promoting appropriate use of drugs in children. Int J Pediatr. 2012;2012:1–5. doi:10.1155/2012/906570
6. Yochana S, Yu M, Alvi M, Varenya S, Chatterjee A. Pharmaceutical excipients and pediatric formulations. Chim Oggi. 2012;30:56–61.
7. Buckley LA, Salunke S, Thompson K, Baer G, Fegley D, Turner MA. Challenges and strategies to facilitate formulation development of pediatric drug products: safety qualification of excipients. Int J Pharm. 2018;536(2):563–569. doi:10.1016/j.ijpharm.2017.07.042
8. Dresser R, Frader J. Off-label prescribing: a call for heightened professional and government oversight. J Law Med Ethics. 2009;37:476–486.
9. Burgess A. How to identify and manage ‘problem’ excipients in medicines for children. Stroke. 2018;13:57.
10. Georg S. Safety of excipients in pediatric formulations—a call for toxicity studies in juvenile animals. Children. 2015;2:191–197. doi:10.3390/children2020191
11. WHO, 2007. Promoting safety of medicines for children. Available from: https://apps.who.int/medicinedocs/en/m/abstract/Js14235e/.
12. Mei M, Hong X, Libo W, Guoying H, Yonghao G, Xiaobo Z. Current practice and awareness of pediatric off-label drug use in Shanghai, China –a questionnaire-based study. Pediatrics. 2019;19:281.
13. Fatima B, Ladislav N. Artificial sweeteners and sugar substitutes: some properties and potential health benefits and risks. Res J Pharm Biol Chem Sci. 2014;5:638–647.
14. Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol. 2015;79(3):395–404. doi:10.1111/bcp.12267
15. Anderson GD, Lynn AM. Optimizing pediatric dosing: a developmental pharmacologic approach. Pharmacotherapy. 2009;29(6):680–690. doi:10.1592/phco.29.6.680
16. Fernandez E, Perez R, Hernandez A, Tejada P, Arteta M, Ramos JT. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics. 2011;3(1):53–72. doi:10.3390/pharmaceutics3010053
17. Kristine S, Helle H, Karel A. Excipients in neonatal medicinal products: never prescribed, commonly administered. Pharm Med. 2018;32:251–258. doi:10.1007/s40290-018-0243-9
18. Allen D. Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Advan Ped. 2006;53:101–110.
19. Saito J, Akabane M, Ishikawa Y, Nakamura H, Yamatani A. Potentially harmful excipients in neonatal medications: an observational and cross-regional comparison of Japan and Europe. Neonat Pediatr Med. 2018;4(172):2. doi:10.4172/2572-4983.1000172
20. Narang A, Boddu H. Excipient Applications in Formulation Design and Drug Delivery. Vol. 3. Spring Int Pub; 2015:1–10.
21. Allegaert K. Neonates need tailored drug formulations. World J Clin Pediatr. 2013;2:11.
22. Vanriet-Nales D, Kozarewicz P, Aylward B, de Vries R, Egberts T, Rademaker C. Pediatric drug development and formulation design—a European perspective. Pharm Sci Tech. 2017;18:241–249. doi:10.1208/s12249-016-0558-3
23. WHO. Development of Pediatric Medicines: Pharmaceutical Development. Denmark: Expert Committee on Specifications for Pharmaceutical Preparations; 2008. Available from: http://www.who.int/medicines/services/expertcommittees/pharmprep/PaediatricMedicinesPharmDevelopment_QAS08_257_29022008.pdf.
24. Gold S. Reflection paper: formulations of choice for the pediatric population. Eur Medic Agen. 2006. Available from: https://www.ema.europa.eu/en/formulations-choice-paediatric-population. Accessed October 16, 2020.
25. Parnali C, Mohammed A. Excipients and active pharmaceutical ingredients. Int Week J Sci. 2014;11:347–361.
26. Alkhattawi A, Mohammed R. Excipients in medicines for children: scientific and regulatory paradigms. Eur Pharm Rev. 2014;4:48–62.
27. Kaplan W, Wirtz V, Mantel A, Beatrice S. Priority medicines for Europe and the world update report. Methodology. 2013;2:7.
28. Shilpa P, Pradeep S. Pharmaceutical excipients: a review. Int J Anal Pharm Biomed Sci. 2012;3:57–64.
29. Aracy P, Silveira B, Lucilena B, Cortez M. Pharmaceutical excipients and the information on drug labels. Braz J Otorhinolaryngol. 2006;72:400–406. doi:10.1016/S1808-8694(15)30976-9
30. Binson G, Beuzit K, Migeot V, et al. Preparation and physicochemical stability of liquid oral dosage forms free of potentially harmful excipient designed for pediatric patients. Pharmaceutics. 2019;11(4):190. doi:10.3390/pharmaceutics11040190
31. Ohio Northern University. Pharmacist Role in Managing Special Patient Needs Related to Excipients. ohio: Medical Association; 2009. Available from: https://apps.who.int/medicinedocs/en/d/Jh2995e/1.6.2.html.
32. Andersen A. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2014;27(4):1–82.
33. Yang W, Purchase E. Adverse reactions to sulfites. CMAJ. 1985;133:865–867.
34. Elizabeth M, Walter K. Ethanol pharmacokinetics in neonates and infants. Curr Ther Res Clin Exp. 2014;76:90–97. doi:10.1016/j.curtheres.2014.09.002
35. Rubio-Bonilla M, Londono R, Rubio A. Liquid dosage forms. Pharm Sci Ency. 2008. Available from: https://formulation.pharmaceuticalconferences.com/events-list/liquid-dosage-forms. Accessed October 16, 2020.
36. Milap C, Nahata M. Pediatric drug formulations: challenges and potential solutions. Ann Pharmacother. 1999;33:247. doi:10.1345/aph.18154
37. Walsh J, Mills S. Conference report: formulating better medicines for children: 4th European paediatric formulation initiative conference. Ther Deliv. 2013;4:21–25. doi:10.4155/tde.12.135
38. WHO. Development of Pediatric Medicines: Points to Consider in the Formulation. Geneva: WHO technical report series; 2012. Available from: https://apps.who.int/medicinedocs/en/m/abstract/Js19833en/.
39. Hannah K, Jhon F. Formulations for children: problems and solutions. Br J Clin Pharmacol. 2015;79:405–418. doi:10.1111/bcp.12268
40. Soremekun R, Ogbuefi I, Aderemi-Williams R. Prevalence of ethanol and other potentially harmful excipients in pediatric oral medicines: survey of community pharmacies in a Nigerian City. BMC Res Notes. 2019;12(1):460. doi:10.1186/s13104-019-4486-7
41. Samir Z. How is alcohol metabolized by the body: overview. Alcohol Res Health. 2006;29:246–253.
42. Robert S. Solubilizing excipients in oral and injectable formulations: review article. Pharm Res J. 2004;21:201–230.
43. Schulze J, Peters E, Vickers A. Excipient effects on gastrointestinal transit and drug absorption in beagle dogs. Int J Pharm. 2005;300:67–75. doi:10.1016/j.ijpharm.2005.05.004
44. Minna H. Compounding of Paediatric Oral Formulations: Extemporaneous Nifedipine Capsules, Powders and Suspensions in the Hospital Pharmacy. Vol. 99. Publications of the University of Eastern Finland Dissertations in Health Sciences; 2013:81.
45. Mahendra S, Maunab P, Meenakshi P. Excipients and its variation in pharmaceutical aerosol formulation: a review. Innovat Int J Med Pharm Sci. 2016;22:1.
46. European Medicines Agency (EMA). Excipients in the Labeling and Package Leaflet of Medicinal Products for Human Use. Sante: EMA Committee for Medicinal Products for Human Use; 2017. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/annex-european-commission-guideline-excipients-labelling-package-leaflet-medicinal-products-human_en-0.pdf.
47. Hassan V, Neil L. Adverse reactions to the sulfite additives. Gastroenterol Hepatol Bed Bench. 2012;5(1):16–23.
48. Basit AW, Podczeck F, Newton JM, Waddington WA, Ell PJ, Lacey LF. Influence of polyethylene glycol 400 on the gastrointestinal absorption of ranitidine. Pharm Res. 2002;19(9):1368–1374. doi:10.1023/A:1020315228237
49. Schulze JDR, Waddington WA, Ell PJ, Parsons GE, Coffin MD, Basit AW. Concentration-dependent effects of polyethylene glycol 400 on gastrointestinal transit and drug absorption. Pharm Res. 2003;20(12):1984–1988. doi:10.1023/B:PHAM.0000008046.64409.bd
50. Ren X, Mao X, Cao L, et al. Nonionic surfactants are strong inhibitors of cytochrome P450 3A biotransformation activity in vitro and in vivo. Eur J Pharm Sci. 2009;36(4–5):401–411. doi:10.1016/j.ejps.2008.11.002
51. Ren X, Mao X, Si L, et al. Pharmaceutical excipients inhibit cytochrome P450 activity in cell free systems and after systemic administration. Eur J Pharm Biopharm. 2008;70(1):279–288. doi:10.1016/j.ejpb.2008.03.019
52. Rene S, Nazila B, Hans B, Jules T, Ger B. Effect of glycerol on the properties of drugs. Clin Pharmacokinet. 2004;43(14):951–962.
53. Rebecca S, Lanigan D, Torill A. Final report on the safety assessment of BHT. Int J Toxicol. 2002;21(2):19–94.
54. Maheriya PM. Cyclodextrin: a promising candidate in enhancing oral bioavailability of poorly water soluble drugs. MOJ Bioequiv Availab. 2017;3(3):00034.
55. Ayse B, Osman K, Neslihan C. Effect of butylated hydroxytoluene (E321) pretreatment versus L-arginine on liver injury after sub-lethal dose of endotoxin administration. J Environ Toxicol Pharmacol. 2015;32(10):89–97.
56. Kirtida T. Sugar substitutes: health controversy over perceived benefits. J Pharmacol Pharmacother. 2011;2:236–243. doi:10.4103/0976-500X.85936
57. Minna T. Extemporaneous preparation of paediatric oral formulations studies conducted in nifedipine powders, capsules and suspensions in a hospital pharmacy. Lice Thes Pharm J. 2008;59:42–49.
58. Kimberly A, Cynthia M, Allen W, Yu L, Zhiping Z, Amy E. Effect of sorbitol on lamivudine pharmacokinetics following administration of EPIVIR® solution in adults. Conference on retroviruses and opportunistic infections. Clin Pharmacol Ther. 2017;103(3):402–408.
59. Passos I, Sampaio F, Martínez C, Freitas C. Sucrose concentration and pH in liquid oral pediatric medicines of long-term use for children. Rev Panam Salud Publica. 2010;27(2):132–137. doi:10.1590/S1020-49892010000200007
60. Adkison K, Wolstenholme A, Lou Y, et al. Effect of sorbitol on the pharmacokinetic profile of lamivudine oral solution in adults: an open‐label, randomized study. Clin Pharmacol Ther. 2018;103(3):402–408. doi:10.1002/cpt.943
61. Abraham J, Mathew F. Taste masking of pediatric formulation: a review on technologies, recent trends and regulatory aspects. Int J Pharm Sci. 2001;46:12–19.
62. Chen ML, Straughn AB, Sadrieh N, et al. A modern view of excipient effects on bioequivalence: case study of sorbitol. Pharm Res. 2007;24(1):73–80. doi:10.1007/s11095-006-9120-4
63. Benjamin K. Peppermint oil. Am Fam Physician. 2007;75(7):1027–1030.
64. Khanal D. Helping ingredients (excipient) in pharmaceutical formulation: coloring agents–use and health concern. J Manmohan Mem Inst Health Sci. 2014;1:40–48. doi:10.3126/jmmihs.v1i1.9900
65. Krishna V, Gannu K. Colorants: the cosmetics for the pharmaceutical dosage forms. Int J Pharm Sci. 2013;3:13–21.
66. Nagpal N, KaurP, KumarR, RaharS, DhawanR, AroraM. Pharmaceutical diluents and their unwanted effects: a review.Bull Pharm Res. 2016;6(2):45–49. doi:10.21276/bpr.2016.6.2.2
67. EFSA. Panel on Dietetic products, nutrition and allergies (NDA); scientific opinion on lactose thresholds in lactose intolerance and galactosaemia. EFSA J. 2010;8(9):1777. doi:10.2903/j.efsa.2010.1777
68. Ajit N. Excipients impacting bioavailability: a case for simplicity in formulation. AAPS Blog Curr Prog. 2013;6:28–32.
69. Kriegel C, Festag M, Kishore RS, Roethlisberger D, Schmitt G. Pediatric safety of polysorbates in drug formulations. Children. 2020;7(1):1. doi:10.3390/children7010001
70. Zhang H, Yao M, Morrison R, Chong S. Commonly used surfactant, tween 80, improves absorption of P-glycoprotein substrate, digoxin, in rats. Arch Pharm Res. 2003;26(9):768–772. doi:10.1007/BF02976689
71. Cornaire G, Woodley J, Hermann P, Cloarec A, Arellano C, Houin G. Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int J Pharm. 2004;278(1):119–131. doi:10.1016/j.ijpharm.2004.03.001
72. Tom N. American Academy of Pediatrics Calls for “Urgently Needed Reforms” to Fix Broken Food Additive Regulatory System. Environmental Defense Fund (EDF) for Health: New York; 2018.
73. Sandhya R, Pritam J, Madhur K. Development and evaluation of the chronomodulated delivery system of metoclopramide hydrochloride. Int J App Pharm. 2016;8:38–42.
74. Pathma D, Jonathan C, Craig PH. Clinical trials in children. Br J Clin Pharmacol. 2015;79:357–369. doi:10.1111/bcp.12305
75. Adusumilli P, Adepu R. Drug related problems: an over view of various classification systems. Asian J Pharm Clin Res. 2014;7:7–10.
76. Susan M. FDA awards funding to support pediatric clinical trials research, FDA voice. 2017.
77. Smita S, Barbara B, George G, Catherine T. The STEP (Safety and Toxicity of Excipients for Paediatrics) database: part 2 – the pilot version. Int J Pharm. 2013;457:310–322. doi:10.1016/j.ijpharm.2013.09.013
78. European Medicines Agency. Guideline on Pharmaceutical Development of Medicines for Paediatric Use. London: Committee for Medicinal Products for Human Use; 2013.
79. Sara A, Anna B. How to identify and manage problem excipients in medicines for children. Pharm J. 2017;1:34–38.
80. Christopher D, Jay G, Ranga V, William B, Robert O. The IPEC novel excipient safety evaluation procedure: regulatory update. Pharm Technol. 2009;33:72–82.
81. Salunke S, Liu F, Batchelor H, Walsh J, Turner R, Ju TR; European Paediatric Formulation Initiative. European paediatric formulation initiative (EuPFI)—formulating ideas for better medicines for children. AAPS PharmSciTech. 2017;18(2):257–262. doi:10.1208/s12249-016-0584-1
82. Bonifazi D, Lupo M, Pignataro V, et al. EPTRI-European Paediatric Translational Research Infrastructure. Bridging the gaps of the paediatric excellence medicine. Br J Pharmacol. 2019;4(1):S20.
83. Salunke S, Giacoia G, Tuleu C. The STEP (safety and toxicity of excipients for paediatrics) database. Part 1—a need assessment study. Int J Pharm. 2012;435(2):101–111. doi:10.1016/j.ijpharm.2012.05.004
84. Thabet Y, Klingmann V, Breitkreutz J. Drug formulations: standards and novel strategies for drug administration in pediatrics. J Clin Pharmacol. 2018;58:26–35. doi:10.1002/jcph.1138
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
