Back to Journals » International Journal of General Medicine » Volume 15
Safely Modified Laparoscopic Liver Resection for Segment VI and/or VII Hepatic Lesions Using the Left Lateral Decubitus Position
Authors Xiao M, Wang D, Lin GL, Lin X, Tao LY, Li QY
Received 1 June 2022
Accepted for publication 10 August 2022
Published 20 August 2022 Volume 2022:15 Pages 6691—6699
DOI https://doi.org/10.2147/IJGM.S376919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Min Xiao,1,* Di Wang,2,* Guo-Ling Lin,1 Xin Lin,3 Li-Yan Tao,4 Qi-Yong Li1,5
1Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, Hangzhou, People’s Republic of China; 2Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 3Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 4Division of Hepatobiliary and Pancreatic Surgery, Department of Nursing, Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, Hangzhou, People’s Republic of China; 5Jinan Microecological Biomedicine Shandong Laboratory, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qi-Yong Li, Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, #848 Dongxin Road, Hangzhou, 310000, People’s Republic of China, Email [email protected]
Purpose: To explore the feasibility and safety of using the left lateral decubitus position (LLDP) to perform laparoscopic liver resection (LLR) for the treatment of hepatic lesions in segment VI and/or VII.
Patients and Methods: Clinical data concerning 50 patients underwent LLR including 25 patients in the LLDP and the other 25 patients in the routine operative position (ROP) at Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College (Hangzhou, China) and Shulan (Quzhou) Hospital between March 2019 and May 2022 were retrospectively analyzed. All of the patients underwent LLR while in the LLDP or the ROP for the treatment of hepatic lesions located in segment VI and/or VII.
Results: The preoperative clinical and laboratory parameters were comparable between the two groups (P > 0.05). All patients completed the surgery successfully. There were two patients required conversion to open resection in the ROP comparing with zero in the LLDP. The mean operative time was 256.9 ± 132.7 minutes in LLDP and 255.7 ± 92.1 minutes in ROP, while the median perioperative blood loss was 100 mL (range: 50– 300 mL) in LLDP and 200 mL (range: 50– 425 mL), respectively. The postoperative pathological examination showed that margin-negative resection was achieved all of the cases. The important postoperative parameters all returned to normal within five days after the LLR. The mean postoperative hospital stay (15.6 vs 19.3 days; p < 0.05) and the extraction of the drainage tube time (7.8 vs 10.4 days; p < 0.05) were shorter for patients in LLDP.
Conclusion: The LLDP represents a safe and feasible position for performing LLR in selected patients with lesions in segment VI and/or VII. LLR in the LLDP is helpful in terms of the exposure of the surgical field and the recovery of the patient.
Keywords: laparoscopic, liver resection, left lateral decubitus position
Introduction
Primary hepatic lesions can be divided into three types, namely tumor-like masses, benign tumors, and malignant tumors.1 Among them, the benign tumors and tumor-like masses of the adult liver include hemangioma, hepatocellular adenoma, focal nodular hyperplasia (FNH), macro-regenerative nodule, bile duct adenoma, peribiliary gland hamartoma, biliary adenofibroma, biliary cystadenoma, and adrenal rest tumors.2,3 Globally, liver cancer is the most common fatal malignancy, and among all the liver cancer cases worldwide, more than 90% involve hepatocellular carcinomas (HCC).4–6
According to guidelines from countries around the world, surgical resection should be considered the primary treatment modality for patients with resectable tumors.7–9 Due to its low degree of invasiveness, laparoscopic liver resection (LLR) has become increasingly commonly used in this regard.10 However, segments VI and VII of the liver are located in the deepest part of the diaphragmatic surface of the liver, which results in a narrow field of view that increases the degree of surgical difficulty. Based on clinical observations, we recognized the potential value of changing the patient’s position in order to better expose tumors located in segments VI and VII. In light of our prior experience of thoracoscopic right lung resection,11 we hypothesized that the left lateral decubitus position (LLDP) could be suitable for abdominal surgery in which it is necessary to expose segment VI and/or VII of the liver and the inferior vena cava. To test this hypothesis, we explored the safety and efficacy of LLR performed in the LLDP for the treatment of tumors in segment VI and/or VII.
Patients and Methods
Study Population
Patients were enrolled in the present study if they met the following inclusion criteria: their preoperative liver function was classified as Child-Pugh A, they exhibited normal cardiopulmonary function, they had resectable hepatic lesions in segment VI and/or VII, there were no contraindications for anesthesia, and all operations were wedge hepatectomy. From March 2019 to May 2022, a total of 25 adult patients (15 males and 10 females) underwent LLR in the LLDP and 25 adult patients (16 males and 9 females) underwent LLR in the routine operative position (ROP) at Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College (Hangzhou, China) or Shulan (Quzhou) Hospital. Because the study was retrospective and the data were analyzed anonymously, the need for consent was waived.
Surgical Procedure
Each patient underwent LLR in the LLDP according to the following standardized procedure. After receiving general anesthesia and endotracheal intubation, the patient was transferred from the supine position into the vertical LLDP and a towel was laid over the abdominal area following routine disinfection. Five trocars were placed into the abdominal cavity while the patient was in the LLDP to allow for the completion of the LLR (Figure 1). A 10 mm incision was made at the junction 3 cm below the right costal arch and the right anterior axillary line. Each layer was gradually cut into using an electric knife until the peritoneum was cut, with special attention being paid to protecting the internal organs during the opening of the peritoneum. Next, a 10 mm trocar without a core was easily placed into the abdominal cavity via laparoscopy to obtain a clear view into the abdomen. The remaining four trocars were located at the junctions 3 cm below the costal arch and the midline, axillary midline, posterior axillary line, and subscapular line. They were also easily and successfully placed by means of laparoscopy. If the trocar placement was blocked by the colon within the abdominal cavity, the right paracolonic sulcus could be dissociated if necessary.
Following the successful placement of the trocars, the abdominal cavity was inflated to a pressure of 12–15 mmHg, and the surgery was begun. Using an ultrasonic scalpel to dissociate the ligaments around the liver, especially between the liver and the diaphragm, was relatively easy in this position. Then, the liver parenchyma was cut using an ultrasonic scalpel after the resection area was outlined on the surface of the liver. The small blood vessels and bile ducts were clipped using a titanium clip, a hem-o-lok clip, and ligation. To ensure proper hemostasis and check that there was no obvious active bleeding, bile leakage, or gastrointestinal perforation, a liver section drainage tube was indwelled.
If the operation needed to be converted to open due to bleeding affecting the operation field or other reasons, the gauze could be placed under the endoscope to compress the operation area. Then cut the abdominal wall layer by layer along the arc at 2–3 fingers under the costal arch, pulled the hook to fully expose the operation area, and performed open surgery. The transfer process was convenient and fast without changing the body position.
All of the patients were admitted to the enhanced recovery after surgery pathway, which had been demonstrated to be safe and effective by a number of prior studies.12,13
As for the patients underwent LLR in the ROP, after receiving general anesthesia and endotracheal intubation, a sub-umbilical open technique was used to insert a 10 mm port, and pneumoperitoneum was established with carbon dioxide insufflation to a maximum pressure of 12–15 mmHg. Then other trocars were placed into the abdominal cavity depended on the location of the hepatic lesion. The subsequent surgical procedure was the same as that of patients in LLDP group.
Statistical Parameters
The examined data included preoperative, surgical, and pathological factors. The investigated preoperative factors were each patient’s age, sex, viral infection status, albumin level, total bilirubin (TB) level, prothrombin time (PT), international normalized ratio (INR), and Child-Pugh classification. The examined surgical parameters included the surgical duration, intraoperative blood loss, and blood transfusion requirements. Finally, the evaluated pathological factors included the size of the largest tumor, and surgical margin status.
In addition, the following parameters were also evaluated: aspartate aminotransferase (AST) level, alanine aminotransferase (ALT) level, pathological margins, postoperative drainage tube stay, postoperative hospital stay, and postoperative complications. The postoperative complications were assessed on the basis of the Clavien-Dindo complication classification system.14
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) version 19.0 (SPSS, Chicago, IL, USA) statistical software was used for all the data processing and statistical analyses in the present study. The Shapiro–Wilk test was used to determine whether the statistical data conformed to the normal distribution. If the statistical data did conform to the normal distribution, the results were expressed as the mean ± standard deviation (SD); otherwise, they were expressed as the median (range: 25–75%). The characteristics of patients were compared between two datasets using Student’s t-tests or Kruskal–Wallis tests for continuous variables where appropriate, χ2 or Fisher exact tests for categorical variables and the Mann–Whitney U-test to compare medians.
Results
All the results were showed in the Table 1.
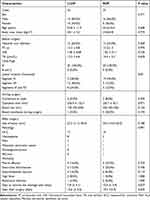 |
Table 1 Patient Demographics and Surgical Outcomes |
In the LLDP there were 15 males and 10 females (mean age: 52.8 ± 11.9 years) participated in the present study, and in the ROP there were 16 males and 9 females (mean age: 54.4 ± 10.6 years) (p > 0.05). In the LLDP, the numbers of patients with hepatic lesions located in segment VI, VII, or both were seven (28.0%), 12 (48.0%), and six (24%), respectively, While the numbers in the ROP were 19 (76.0%), three (12.0%), and three (12%).
All patients completed the surgery successfully. There were two patients required conversion to open resection in the ROP comparing with zero in the LLDP (p > 0.05). In the LLDP the operative time was 256.9 ± 132.7 minutes, and 255.7 ± 92.1 minutes in the ROP (p > 0.05). In the LLDP the median perioperative blood loss was 100 mL (range: 50–250 mL), and 200 mL (range: 50–425 mL) in the ROP (p > 0.05). The median size of the largest resected tumor was 22.3 mm (range: 11.5–56.6 mm) in the LLDP, and 33.0 mm (range: 16.0–52.0 mm) (p > 0.05). One patient received an intraoperative blood transfusion during the surgery in the LLDP and the number in the ROP was five (p > 0.05). The average time until the extraction of the drainage tube from the relevant liver section was 7.8 ± 4.1 days in the LLDP, and 10.4 ± 3.9 days in the ROP (p < 0.05). Moreover, the average time from surgery to discharge was 15.6 ± 5.2 days in the LLDP, and 19.3 ± 5.9 days in the ROP (p < 0.05).
The frozen section pathological results concerning all of the patients revealed that the cutting edge was negative. Furthermore, all of the patients were diagnosed by means of postoperative pathology. In the LLDP, the pathological results of 15 patients indicated the presence of HCC, eight patients had hemangioma, one patient had FNH, and one patient had metastatic pancreatic cancer. In the ROP, 16 patients had HCC, five patients had hemangioma, one patient had FNH, one patient had metastatic pancreatic cancer, one patient had cholangiocarcinoma, and one patient had PEComa.
Postoperative complications occurred in 14 patients in the LLDP. More specifically, four patients (16.0%) experienced pleural effusion, which was resolved through conservative management. In addition, three patients (12.0%) experienced electrolyte disturbances, while four patients (16.0%) exhibited gastrointestinal reactions, which were all treated using conventional medications. Two of these cases (8.0%) was complicated by a high fever, which was treated with an upgrading antibiotic. The only major (4.0%) complication was an abdominal infection in one patient, which required abdominal puncture. And in the ROP, six (24.0%) patients experienced pleural effusion, five (20.0%) experienced electrolyte disturbances, and one patient had high fever, the treatment method was the same as above.
The postoperative examination indexes of the patients are presented in Figure 2. After the operation, the biochemical standards of the patients tended to gradually return to normal within five days both in the LLDP and ROP.
Discussion
Prior to the LLR procedure first being described in 1991, open hepatectomy was considered the best approach for the surgical treatment of liver tumors.15 Now, LLR is widely accepted as a minimally invasive way to treat resectable liver lesions.16 In fact, LLR has been performed for the treatment of HCC,10,17,18 metastatic liver tumors,19,20 hepatic cysts,21,22 and benign tumors.15,23 When compared with the open hepatectomy approach, laparoscopic liver surgery is associated with lower complication rates, reduced intraoperative blood loss, and a shorter hospital stay.24
Although recent studies have demonstrated both the safety and the reproducibility of LLR,25 as well as the favorable surgical outcomes associated with it, when compared with open surgery, the procedure requires a steep learning curve on the part of surgeons due to the technical complexity of performing major resections.26 In recent years, several studies have sought to improve the surgical technique in an effort to render LLR safer and more convenient. For instance, Liu et al proposed the liver parenchyma transection approach––the first step toward the laparoscopic left hemihepatectomy procedure––to avoid the need for the laparoscopic dissection of the hilar plate and reduce the risk of injury to the hepatic veins. The proposed approach also had a shorter operative time (210 vs 235 minutes, p = 0.035) than the conventional technique.27 Siddiqi et al posited that a surgical approach should be defined and that each patient should be considered separately, taking into account the lesion’s location, size, and nature as well as the patient’s liver anatomy.24
A few studies have investigated the potential for improving surgical outcomes by changing the patient’s body position. The routine patient position involves lying on the spine, although there are various possible alternative patient positions depending on the location of the tumor and/or the type of surgical procedure, for example, a semi-prone position (median operative time: 373 minutes; median blood loss: 146 mL)28,29 and a supine position with approximately 30 degrees of right-sided (median operative time: 240 minutes; median blood loss: 600 mL).24 All of the body positions are intended to fully expose the surgical field and, therefore, to facilitate surgical procedures. The present study sought to identify a new body position for performing LLR to treat segment VI and/or VII tumors.
Laparoscopic right posterior sectionectomy (LRPS) is considered one of the most complex procedures that can be completed laparoscopically, scoring nine or ten on the difficulty scoring system for laparoscopic liver surgery developed by Ban et al (eg, a laparoscopic right hemihepatectomy scores seven). The difficultly associated with accessing segment VII, the potential for a close relationship between the lesion and the right hepatic vein, the lack of anatomical delineation on the part of the right posterior section, the need for complex inflow control, and the requirement for a resection margin with a large surface area all pose significant challenges to the procedure. Moreover, LRPS can be associated with a long operative time, a high degree of blood loss, and a prolonged postoperative hospital stay.
The resection of segments VI and VII is known to be more demanding and dangerous than other segmentectomies, resulting in a longer operative time and increased blood loss.30 To reduce the technical challenges associated with the procedure, Morise et al used the left lateral position to perform posterior segmentectomy and the semi-prone position to perform both segment VII segmentectomy and partial resections of segment VII and deep segment VI.31 Similarly, Inoue et al placed patients into a moderate LLDP to complete the surgery.16 To a certain extent, the use of these positions widened the surgical field of view and made it easy to operate. In addition, Siddiqi et al introduced three different techniques for LRPS: resection following hilar inflow control, resection following inflow control at Rouviere’s sulcus, and resection with intra-parenchymal control.24 On the basis of the above-mentioned studies, our hospital adopted the use of the LLDP for the LLR of segment VI or VII hepatic lesions. The use of this position allows for the weight of the liver to facilitate its own mobilization, which ultimately provides a good and stable surgical space above the liver. Moreover, in the LLDP, segments VI and VII, which are difficult to expose in the standard laparoscopic field of vision, appear at the top of the laparoscopic field of vision, which results in a larger field of vision and operating space.
The use of the LLDP can also reduce the operative difficulty to a certain extent. The LLDP allows the weight of the right lobe of the liver to expand the surgical field of view, thereby facilitating the mobilization of the liver. After mobilization, an adequate space is available above the liver and it is possible to ensure both the stable handling of surgical instruments and the removal of tumors in regions up to the root of the right hepatic vein.
As has previously been established, the prevention and control of bleeding under laparoscopic vision is critical for hepatectomy because massive hemorrhage may increase postoperative morbidity and mortality.32,33 When patients are placed in the LLDP, the decreased venous pressure in the right hepatic vein, which is positioned vertically upwards from the inferior vena cava, results in decreased intraoperative bleeding. In addition, because the surgical field of vision and the operating space are wider, bleeding points can be quickly identified and hemostasis can be more easily achieved, which may also lead to less intraoperative bleeding. However, if there is a problem that cannot be solved under the endoscope, you can refer to the method described above to transfer to open surgery without changing the body position. The transfer process is convenient and fast.
The routine position used to perform LLR is the supine position, and the use of trocars often accompanies the use of the supine approach. As the patient is in a neutral position, there is no need for intercostal trocar placement.34 However, when the patient is in the LLDP, it is important to strictly follow the steps outlined in the present article when it comes to placing the trocars, especially the first one. By using an electric knife to cut layer by layer and placing a trocar without a core, it is possible to avoid damaging the internal organs.
As far as we have known, this study was the first study to describe in detail the surgical procedure of a vertical left lateral decubitus position in liver resection, introducing a new surgical option for the resection of segment VI and/or VII hepatic lesions. Laparoscopic non-anatomical right posterior lobe liver resection in the LLDP offers the advantages of a wider field of vision, a simpler procedure, and reduced bleeding. The perioperative results for patients in the LLDP were comparable to those of the routine position, with a faster postoperative recovery and less hospital stay. However, is the present study was also associated with a number of limitations. For instance, trocar placement was more difficult than in the supine position. Other limitations of the present study included the limited size of the enrolled patient population and relatively short follow-up duration. The findings need to be validated by prospective randomized controlled trial study. Moreover, the use of the LLDP increases the difficulty of hepatic portal vein dissociation, which means that only non-anatomical resection can presently be performed.
Conclusion
The LLDP is a feasible and effective position for performing LLR in selected patients with lesions in segment VI and/or VII. In addition, LLR in the LLDP is helpful in terms of the exposure of the surgical field and the recovery of the patient.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki. The studies involving human participants were reviewed and approved by Shulan Hangzhou Hospital. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Acknowledgments
The authors would like to thank the Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College and the Shulan (Quzhou) Hospital and the Jinan Microecological Biomedicine Shandong Laboratory for sponsoring this work. We also thank all study participants.
Author Contributions
Min Xiao and Di Wang are co first authors. All authors contributed to data collection, analysis, drafting, or revising the article, have agreed on the journal to which the article will be submitted, gave final approval to the version to be published, and agree to be responsible for all aspects of the work.
Funding
This work was supported by the Grant from Health Commission of Zhejiang Province (JBZX-202004) and the Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022022C).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cong WM, Dong H, Tan L, Sun XX, Wu MC. Surgicopathological classification of hepatic space-occupying lesions: a single-center experience with literature review. World J Gastroenterol. 2011;17(19):2372–2378. doi:10.3748/wjg.v17.i19.2372
2. Brunt EM. Benign tumors of the liver. Clin Liver Dis. 2001;5(1):1–15. doi:10.1016/s1089-3261(05)70151-3
3. Bajenaru N, Balaban V, Săvulescu F, Campeanu I, Patrascu T. Hepatic hemangioma -review. J Med Life. 2015;8 Spec Issue:4–11.
4. Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188314. doi:10.1016/j.bbcan.2019.188314
5. Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
6. El Jabbour T, Lagana SM, Lee H. Update on hepatocellular carcinoma: pathologists’ review. World J Gastroenterol. 2019;25(14):1653–1665. doi:10.3748/wjg.v25.i14.1653
7. Clark T, Maximin S, Meier J, Pokharel S, Bhargava P. Hepatocellular carcinoma: review of epidemiology, screening, imaging diagnosis, response assessment, and treatment. Curr Probl Diagn Radiol. 2015;44(6):479–486. doi:10.1067/j.cpradiol.2015.04.004
8. Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31(3):334–351. doi:10.1016/j.annonc.2019.12.001
9. Alvaro D, Hassan C, Cardinale V. Italian clinical practice guidelines on cholangiocarcinoma - Part II: treatment. Dig Liver Dis. 2020;52(12):1430–1442. doi:10.1016/j.dld.2020.08.030
10. Mamada Y, Yoshida H, Taniai N, et al. Usefulness of laparoscopic hepatectomy. J Nippon Med Sch. 2007;74(2):158–162. doi:10.1272/jnms.74.158
11. Chiumello D, Formenti P, Bolgiaghi L, et al. Body position alters mechanical power and respiratory mechanics during thoracic surgery. Anesth Analg. 2020;130(2):391–401. doi:10.1213/ane.0000000000004192
12. Agarwal V, Divatia JV. Enhanced recovery after surgery in liver resection: current concepts and controversies. Korean J Anesthesiol. 2019;72(2):119–129. doi:10.4097/kja.d.19.00010
13. Rouxel P, Beloeil H. Enhanced recovery after hepatectomy: a systematic review. Anaesth Crit Care Pain Med. 2019;38(1):29–34. doi:10.1016/j.accpm.2018.05.003
14. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
15. Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol. 1991;78(5 Pt 2):956–958.
16. Inoue Y, Suzuki Y, Fujii K, et al. Laparoscopic liver resection using the lateral approach from intercostal ports in segments VI, VII, and VIII. J Gastrointest Surg. 2017;21(12):2135–2143. doi:10.1007/s11605-017-3516-9
17. Krenzien F, Wabitsch S, Haber P, et al. Validity of the Iwate criteria for patients with hepatocellular carcinoma undergoing minimally invasive liver resection. J Hepatobiliary Pancreat Sci. 2018;25(9):403–411. doi:10.1002/jhbp.576
18. Takahara T, Wakabayashi G, Beppu T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22(10):721–727. doi:10.1002/jhbp.276
19. Truant S, El Amrani M, Baillet C, et al. Laparoscopic partial ALPPS: much better than ALPPS! Ann Hepatol. 2019;18(1):269–273. doi:10.5604/01.3001.0012.7937
20. Beppu T, Wakabayashi G, Hasegawa K, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22(10):711–720. doi:10.1002/jhbp.261
21. Vardakostas D, Damaskos C, Garmpis N, et al. Minimally invasive management of hepatic cysts: indications and complications. Eur Rev Med Pharmacol Sci. 2018;22(5):1387–1396. doi:10.26355/eurrev_201803_14484
22. Tan YM, Chung A, Mack P, Chow P, Khin LW, Ooi LL. Role of fenestration and resection for symptomatic solitary liver cysts. ANZ J Surg. 2005;75(7):577–580. doi:10.1111/j.1445-2197.2005.03432.x
23. Mamada Y, Onda M, Tajiri T, et al. Liver cell adenoma in a 26-year-old man. J Nippon Med Sch. 2001;68(6):516–519. doi:10.1272/jnms.68.516
24. Siddiqi NN, Abuawwad M, Halls M, et al. Laparoscopic right posterior sectionectomy (LRPS): surgical techniques and clinical outcomes. Surg Endosc. 2018;32(5):2525–2532. doi:10.1007/s00464-017-5958-2
25. Buell JF, Cherqui D, Geller DA. The international position on laparoscopic liver surgery. Ann Surg. 2009;250(5):825–830. doi:10.1097/SLA.0b013e3181b3b2d8
26. Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21(10):745–753. doi:10.1002/jhbp.166
27. Liu Q, Li J, Zhou L, et al. Liver parenchyma transection-first approach for laparoscopic left hemihepatectomy: a propensity score matching analysis. World J Surg. 2021;45(2):615–623. doi:10.1007/s00268-020-05846-y
28. Ikeda T, Yonemura Y, Ueda N, et al. Pure laparoscopic right hepatectomy in the semi-prone position using the intrahepatic Glissonian approach and a modified hanging maneuver to minimize intraoperative bleeding. Surg Today. 2011;41(12):1592–1598. doi:10.1007/s00595-010-4479-6
29. Ikeda T, Toshima T, Harimoto N, et al. Laparoscopic liver resection in the semiprone position for tumors in the anterosuperior and posterior segments, using a novel dual-handling technique and bipolar irrigation system. Surg Endosc. 2014;28(8):2484–2492. doi:10.1007/s00464-014-3469-y
30. Giuliani A, Aldrighetti L, Di Benedetto F, et al. Total abdominal approach for postero-superior segments (7, 8) in laparoscopic liver surgery: a multicentric experience. Updates Surg. 2015;67(2):169–175. doi:10.1007/s13304-015-0305-4
31. Morise Z. Laparoscopic liver resection for posterosuperior tumors using caudal approach and postural changes: a new technical approach. World J Gastroenterol. 2016;22(47):10267–10274. doi:10.3748/wjg.v22.i47.10267
32. Sucher R, Seehofer D, Pratschke J. [Management of intraoperative and postoperative bleeding in liver surgery]. Management intra- und postoperativer Blutungen in der Leberchirurgie. Chirurg. 2015;86(2):114–120. doi:10.1007/s00104-014-2879-7
33. Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236(4):
34. Kose E, Kahramangil B, Aydin H, et al. Minimally invasive resection of posterosuperior liver tumors in the supine position using intra-abdominal trocars. Surg Endosc. 2020;34(2):536–543. doi:10.1007/s00464-019-06789-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.