Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Safe and Effective Lip Enhancement with VYC-15L in Chinese Adults
Authors Li D, Gao Z, Sun J, Li Q, Jiang P, Zhang L, Chawla S
Received 13 July 2022
Accepted for publication 4 November 2022
Published 10 November 2022 Volume 2022:15 Pages 2427—2436
DOI https://doi.org/10.2147/CCID.S382194
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Dong Li,1 Zhanwei Gao,2 Jiaming Sun,3 Qin Li,4 Ping Jiang,5 Lijuan Zhang,6 Smita Chawla7
1Peking University Third Hospital, Beijing, People’s Republic of China; 2Japan Friendship Hospital, Beijing, People’s Republic of China; 3Union Hospital Tongji Medical College Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 4General Hospital of Guangzhou Military Command of PLA, Guangzhou, People’s Republic of China; 5Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China; 6Allergan Aesthetics, an AbbVie Company, Beijing, People’s Republic of China; 7Clinical Development, Allergan Aesthetics, an AbbVie Company, Irvine, CA, USA
Correspondence: Smita Chawla, Clinical Development, Allergan Aesthetics, an AbbVie Company, 2525 Dupont Drive, Irvine, CA, 92612, USA, Tel +1 714 246-2213, Email [email protected]
Background: Hyaluronic acid (HA) soft tissue fillers are used to restore volume loss to the lips and perioral area.
Objective: Evaluate the safety and effectiveness of the HA filler Juvéderm® Volbella® (VYC-15L) for lip enhancement in Chinese adults.
Methods & Materials: In this multicenter, evaluator-blind study, subjects seeking lip enhancement were randomized 3:1 to VYC-15L or no-treatment control group (with optional treatment delayed by 3 months). Effectiveness endpoints included 1-point improvement on the 5-point Lip Fullness Scale (LFS) assessed by evaluating investigators (EIs) and subjects, and overall volume of lips and change in lip surface area measured from 3-dimensional images. Safety assessments included procedural pain, injection site reactions (ISRs), and adverse events (AEs).
Results: The modified intention-to-treat population included 163 subjects (median age, 34 years; 96.9% female). More VYC-15L-treated subjects had a 1-point reduction in EI-reported LFS score at month 3 versus untreated controls (84.7% vs 0%; p < 0.0001), and 65% of the treated subjects self-reported a 1-point LFS improvement at month 3. Procedural pain during injection was mild. The most common ISRs were swelling, tenderness to touch, and firmness. One treatment-related serious AE (injection site compression arterial ischemia) resolved in 16 days.
Conclusion: VYC-15L treatment was superior to no treatment at 3 months and was well tolerated by subjects.
Clinical Trial Registration Number: NCT03519204.
Keywords: dermal fillers, hyaluronic acid, China
Introduction
Across different cultures, the lips are a focal aesthetic feature associated with youth, health, and beauty.1,2 However, aging can lead to loss of definition in the Cupid’s bow, vermilion border, and philtral column, thinning lips, loss of lip volume, and vertical rhytids and marionette lines surrounding the lips resulting from repetitive muscle activity.1,3,4 Treatment with temporary hyaluronic acid (HA) fillers can provide volume to the lips and perioral area, restoring fullness and the natural contour of the region.3–5 As with any dermal treatment injection, pain during administration is a possible adverse effect.6 This is a concern for both individuals undergoing the procedure and physicians performing it.
Juvéderm® Volbella® (VYC-15L; Allergan Aesthetics, an AbbVie company, Dublin, Ireland) is a temporary HA soft tissue filler that utilizes Vycross technology (Allergan Aesthetics, an AbbVie company), combining lower- and higher-molecular weight HA to produce a higher-viscosity gel with better lift capacity.7 Compared with other HA fillers, VYC-15L is a smoother and softer gel.7–9 Similar to other soft tissue fillers from the same product family, VYC-15L contains 0.3% lidocaine to reduce injection-related pain and to provide consistency in anesthetic dosing (instead of separately mixing lidocaine into the product, which may affect sterility and the overall quality of the product).6,10
VYC-15L has been approved for lip augmentation in the United States. In the US pivotal study, 80.3% of the subjects treated with VYC-15L showed a ≥1-point improvement in the validated Lip Fullness Scale (LFS) 3 months post-treatment.11 At the 12-month follow-up, the majority of subjects (61.8%) of subjects continued to demonstrate a response. The most frequently reported adverse events (AEs) were typical reactions to injection of soft tissue fillers, such as injection site mass (lumps/bumps) and bruising.11 A number of other studies have demonstrated the safety and effectiveness of VYC-15L for lip augmentation in populations from the US and Europe.8,11–15 In Asia, the number of subjects who request facial injectable treatments has rapidly grown in the last decade.16 The objective of this study was to evaluate the safety and effectiveness of VYC-15L for lip enhancement in a Chinese population.
Methods
Subjects
Eligible subjects met the following criteria: male or female aged ≥18 years, an overall baseline score of ≤2 on the 5-point LFS (0 = minimal, 1 = mild, 2 = moderate, 3 = marked, 4 = very marked)17 as assessed by the evaluating investigator (EI), and the desire to achieve at least a 1-point improvement in the overall LFS after treatment. Subjects were excluded from the study if they received any of the following treatments: oral surgery (within 6 weeks of study); facial plastic surgery or facial implants, semipermanent soft tissue filler treatment below the inferior orbital rim (within 24 months of study); temporary soft tissue filler treatment below the inferior orbital rim (within 12 months of study); facial tissue augmentation with fat injections, botulinum toxin injections below the inferior orbital rim, mesotherapy, or cosmetic procedures in the face (within 6 months of study); and/or anticoagulation therapy (ongoing) or medications (within 10 days of study).
Study Design
This was a prospective, multicenter, randomized, no-treatment–controlled study of the safety and effectiveness of VYC-15L for lip enhancement in a Chinese population. Subjects were enrolled from five different study centers, and clinical trial approval was obtained from each institution’s Ethics Committee at the Nanfang Hospital, Southern Medical University; Peking University Third Hospital; General Hospital of Guangzhou Military Command of PLA; Union Hospital Tongji Medical College Huazhong University of Science and Technology; and China Japan Friendship Hospital. The study was conducted in compliance with the Declaration of Helsinki. All subjects provided written informed consent.
Randomization was stratified by baseline LFS score. Based on a central randomization schedule, subjects were assigned 3:1 to the VYC-15L treatment group or the no-treatment control group (subjects in the no-treatment control group had the option of receiving VYC-15L delayed by 3 months). EIs, but not treating investigators (TIs) or subjects, were blinded to groups throughout the duration of the study. In total, there were 7 TIs (1 TI each at 3 sites and 2 TIs each at 2 sites). To minimize bias among the TIs, they administered VYC-15L injections according to the manufacturer’s investigational directions for use.
At randomization (and prior to receiving treatment for the VYC-15L group), assessments were made for baseline EI- and subject-reported LFS, as well as philtral column definition (0 = not defined, 1 = barely defined, 2 = somewhat defined, 3 = well-defined, 4 = very well-defined). Facial 3-dimensional (3D) imaging (VECTRA M3-Lip System, Canfield Scientific, Inc., Fairfield, NJ) was also performed. Prior to VYC-15L injections, aseptic skin preparation was used. Allowable VYC-15L treatment areas included the vermilion body, vermilion border (including Cupid’s bow), and the philtral columns. VYC-15L was injected subdermally to the vermilion body and intradermally to the vermilion border and philtral columns using a 30-gauge, ½-inch needle. Injection volume was determined by the TI, but volumes did not exceed 4.0 mL for initial and touch-up treatments combined. Ice and topical anesthesia were permitted to reduce injection discomfort, with injectable anesthesia being limited to the treatment area only.
Follow-up visits occurred 1, 3, and 6 months post-treatment. For the VYC-15L treatment group, facial 3D imaging was performed at these visits. EI-assessed philtral column definition, as well as EI- and subject-assessed LFS and Global Aesthetic Improvement Scale (GAIS; 2 = much improved, 1 = improved, 0 = no change, –1 = worse, –2 = much worse) measurements of the lips and philtral column, was collected. For the control group, EI-assessed philtral column definition, LFS, and GAIS, as well as facial 3D imaging measurements were collected at months 1 and 3, subject-assessed LFS was collected at month 3 only, and subject-assessed GAIS was not evaluated. Subjects from the VYC-15L treatment group received an optional touch-up 30 days after initial treatment if the TI assessed that optimal correction was not achieved. Long-term safety data were collected at 9 and 12 months after the last treatment for the VYC-15L treatment group and 9 months after the last optional treatment for the control group.
Effectiveness Endpoints
The primary effectiveness endpoint was the EI-assessed LFS responder rate at month 3. LFS response was defined as ≥1-point improvement in overall lip fullness compared with baseline. Secondary endpoints included the subject-assessed LFS responder rate at month 3, as well as the overall change in lip volume and surface area as measured from 3D images at month 3. Other effectiveness endpoints included EI- and subject-assessed GAIS responder rates, and EI-assessed philtral column definition response. GAIS response was defined as a score of “improved” or “much improved” on the GAIS. Philtral column response was defined as ≥1-point improvement from baseline.
Safety Endpoints
Incidence, severity, and duration of injection site responses (ISRs) were reported by subjects in a 30-day diary after initial and touch-up treatment. Procedural pain (pain during injection) was assessed by subjects after initial treatment on an 11-point scale ranging from 0 (no pain) to 10 (worst pain imaginable). AEs, including serious AEs (SAEs), were recorded by the investigators.
Statistical Analyses
Effectiveness analyses were performed on the modified intention-to-treat (mITT) population, which comprised all randomized subjects who received ≥1 VYC-15L treatment (treatment group) and had a baseline and ≥1 post-treatment assessment of the primary effectiveness endpoint (treatment and control groups). Superiority in the primary effectiveness endpoint (responder rate for EI-assessed LFS at month 3) of VYC-15L treatment over no-treatment control was determined based on a two-sided Fisher’s exact test with 5% significance level. For the secondary effectiveness endpoints, the subject-assessed LFS responder rate at month 3 was analyzed using a responder exact test within group for the treatment group only. Change from baseline in overall lip volume and percentage change from baseline in lip surface area at month 3 were analyzed using a 2-sample t-test or Wilcoxon test as appropriate. Other effectiveness endpoints (EI- and subject-assessed GAIS, EI-assessed philtral column definition) at months 1, 3, and 6 were presented as descriptive statistics. Statistical analyses were performed using SAS version 9.3 or newer (SAS Institute, Cary, NC).
Results
Subjects
Of 212 enrolled subjects, 176 were randomized and 148 completed the study. The analysis populations were as follows: mITT (N=163; n=130 for treatment group and n=33 for control group) and safety (N=176; n=136 for treatment group and n=40 for control group). In the treatment group, 136 subjects received initial treatment, and 34 subjects received touch-up treatment. In the control group, 29 subjects received optional treatment at the end of the 3-month, no-treatment control period, and 6 subjects received touch-up treatment. Of the 163 subjects in the mITT population, most were female (96.9%) with a median age at study entry of 34 years (range, 19–63 years) and a median body mass index of 20.6 kg/m2 (range, 15–34 kg/m2; Table 1). The most common Fitzpatrick skin type was II (44.8%), followed by III (39.9%) and IV (14.1%).
 |
Table 1 Demographic and Baseline Characteristics (mITT Population) |
Three subjects randomized to the no-treatment control group were treated in error on the day of randomization and included in the treatment group during analysis. Twenty-eight subjects did not complete the study for the following reasons: study withdrawal (n=16), lost to follow-up (n=11), and other reasons (subject assigned to the control group and refused randomization result; n=1). No subjects discontinued the study due to AEs.
Volumes Injected
The total mean injection volume (combined initial and touch-up treatments) for the VYC-15L treatment group was 1.7 mL (minimum, 0.55; maximum, 3.90). When analyzed by treatment site, the total mean injection volumes (combined initial and touch-up treatments) were as follows: upper lip (0.9 mL), lower lip (0.7 mL), and philtral columns (0.2 mL). Injection volumes were similar for the treated control group.
Injection Planes and Technique
During initial treatment, the most common plane of injection for the VYC-15L treatment group was the lips mucosa for the upper lip (99/136 [72.8%]) and lower lip (94/131 [71.8%]), whereas the mid-dermis was the most common plane of injection for the philtral columns (35/63 [55.6%]). Injection planes were comparable for the treated control group; touch-up treatments also followed a similar pattern.
During initial treatment, the most common injection technique was tunneling (linear threading) for all treatment areas in the VYC-15L treatment group: upper lip (87/136 [64.0%]), lower lip (78/131 [59.5%]), and philtral columns (57/63 [90.5%]). During touch-up treatment, serial puncture (multiple injections of small aliquots) was most frequently used for the upper lip (23/32 [71.9%]) and lower lip (24/30 [80.0%]), whereas tunneling was most frequently used for the philtral columns (4/5 [80%]). Injection techniques were similar for the treated control group.
Effectiveness
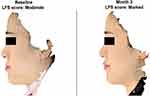
For the primary effectiveness endpoint, the VYC-15L treatment group had a significantly higher EI-assessed LFS responder rate compared with the no-treatment control group at 3 months (84.7% vs 0%; Table 2). The difference between the EI-assessed responder rate for the VYC-15L treatment and no-treatment control group was 84.7% (95% CI, 68.2–94.2%; p < 0.0001; Table 2). For the VYC-15L treatment group, EI-assessed LFS responder rates were high at 97.6% as early as 1 month after treatment, then it was 84.7% at month 3 and 64.5% by 6 months (Table 2). Figure 1 illustrates representative results of treatment in a 28-year-old female subject.
 |
Table 2 EI-Assessed LFS Responder Rates (mITT Population) |
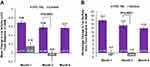
The subject-assessed LFS responder rate at month 3 for the VYC-15L treatment group was 65.0% (95% CI, 55.6–73.5%). For the VYC-15L treatment group, subject-assessed LFS responder rates were 87.1% as early as 1 month after treatment with a majority (57.5%) remaining responders for up to 6 months (Table 3). Compared with the no-treatment control group, the VYC-15L treatment group also showed significantly higher differences in the mean change in lip volume and mean percentage change in overall lip surface area from baseline to month 3 (Figure 2). Similar results were observed as early as 1 month after treatment until 6 months.
 |
Table 3 Subject-Assessed LFS Responder Rates (mITT Population) |
At months 1, 3, and 6, more than 90% of VYC-15L-treated subjects showed “improved” or “much improved” lips on the EI-assessed GAIS, and more than 80% of VYC-15L treated-subjects reported “improved” or “much improved” lips on the subject-assessed GAIS (Figure 3). The majority (≥80%) of VYC-15L-treated subjects also demonstrated ≥1-point improvements from baseline on the EI-assessed philtral column definition at months 1, 3, and 6 (Table 4). Similar results were seen in the treated control group (data not shown).
 |
Table 4 EI-Assessed Philtral Column Definition (mITT Population) |
Safety
The VYC-15L treatment and treated control groups both reported mild procedure pain after initial treatment (mean, 2.6; range, 0–7 for both groups). The majority of ISRs reported for all treated subjects were mild to moderate; the most common ISRs during the initial and touch-up treatments were swelling and tenderness to touch (Table 5). During the initial treatment, the majority of ISRs reported by treated subjects in their diaries (n=158) resolved within 2 weeks. Some subjects experienced incidences of firmness (49/158 subjects [31.0%]), lumps/bumps (33/158 [20.9%]), tenderness to touch (18/158 [11.4%]), swelling (14/158 [8.9%]), bruising (5/158 [3.2%]), redness (5/158 [3.2%]), pain after injection (4/158 [2.5%]), discoloration (4/158 [2.5%]), and itching (2/158 [1.3%]) that resolved within 1 month. During the touch-up period (n=38 subjects), fewer subjects experienced ISRs that took >2 weeks to resolve (firmness [2/38 subjects; 5.3%] and swelling [1/38; 2.6%]).
 |
Table 5 Incidences of Injection Site Responses (ISRs) During Initial and Touch-Up Treatment for the VYC-15L Treatment Group and Treated Control Subjects |
During the entire study period, 32 of 165 (19.4%) treated subjects had treatment-related AEs, all of which were at the injection site except for 1 subject who experienced 2 mild AEs not at the injection site (abnormal electrocardiogram [ECG] ST segment and ECG T wave, which returned to normal at month 1). The most common events were injection site induration (24/165 subjects [14.5%]) and injection site mass (10/165 [6.1%]). One (0.6%) subject had a treatment-related SAE (compression arterial ischemia of moderate severity at the lower lip injection site). This subject received treatment of hyaluronidase, cefradine, loratadine, and other medications; the AE resolved in 16 days. There were no adverse device effects reported in the study.
Discussion
The primary objective was met in this study; VYC-15L was superior to no-treatment control in terms of the EI-assessed LFS responder rate at month 3, with a difference of 84.7% in the responder rates between groups. The majority of VYC-15L subjects showed improvements in effectiveness endpoints, including the subject-assessed LFS, EI- and subject-assessed GAIS, as well as the EI-assessed philtral column definitions. Treatment differences were supported by objective 3D imaging, which revealed significant increases in lip volume and surface area at month 3 for the VYC-15L treatment compared with the no-treatment control group. Most ISRs following initial treatment with VYC-15L were mild to moderate in severity; the number of subjects, severity, and percentage of ISRs lasting more than 2 weeks were lower for touch-up compared with initial treatments. Overall, ISR and AE incidences were as expected for a soft tissue filler study. A subset of ISRs, mostly incidences of firmness, lumps/bumps, and tenderness to touch during the initial treatment period, resolved within 1 month.
This analysis is the first to provide data on an HA-based soft tissue filler for lip augmentation in a Chinese population. The month 3 LFS responder rates in this study involving a Chinese population were comparable to previous studies investigating VYC-15L treatment for lip augmentation in populations from the United States and Europe. In studies comparing VYC-15L with the nonanimal stabilized HA with lidocaine (NASHA; Restylane-L, Q-Med AB/Galderma, Uppsala, Sweden), the EI-assessed LFS responder rate at month 3 was between 80% and 84% for VYC-15L and between 71% and 81% for Restylane-L.11,13–15 The subject-assessed LFS responder rate at month 3 was 73.8% for VYC-15L and 59.8% for Restylane-L.14 An open-label study showed a 93.2% EI-assessed LFS responder rate at month 3 for VYC-15L.8 The duration of effectiveness for VYC-15L has also been established in these studies. The EI-assessed responder rates ranged from 62% to 78% at month 9 and 48% to 62% at month 12.8,11,13 The present study evaluated effectiveness only until month 6; the EI-assessed LFS responder rate was 84.7% at month 3 and 64.5% at month 6. In contrast to previous studies, the current study complemented scale measurements with quantitative 3D imaging of lip surface area and volume, as was reported previously in a study investigating another soft tissue filler for lip augmentation.18
The current study did not have an active control group due to the absence of approved soft tissue fillers for lip augmentation in China but instead included a control (no treatment) group. At month 3, the EI-assessed response rates for the control group (0% in the LFS and GAIS, 10.3% in the philtral column definition) were similar to the response rates (10.3% and 16.2% in the upper and lower lip assessments, respectively) in a previous trial with small particle HA with lidocaine (SP-HAL; Restylane® Silk; Q-Med AB/Galderma).19,20 Throughout the duration of the current study, the EI remained blinded to treatment assignments. Several processes were implemented to maintain blinding, such as the EI not being present during the injection procedures and not having access to the study documents, proper training of site staff, and regular checks by study monitors during visits. No issues in study blinding were identified; therefore, the responder rate in the control group was unlikely due to a break in study blind.
Safety outcomes, including procedural pain, ISRs, and treatment-related AEs were similar to previous studies on VYC-15L for lip augmentation.8,11–15 During the initial and touch-up treatment, the incidence of treatment-related AEs (19.4%) for all subjects who received VYC-15L (VYC-15L treatment group and treated controls) was within the range of incidence rates in previous studies (10.1% and 50.6% in the EU and US VYC-15L pivotal studies, respectively).11,13
Conclusions
The results of this study demonstrate that VYC-15L is a safe and effective treatment for improving lip fullness in a Chinese population. VYC-15L treatment was superior to no treatment at 3 months and was well tolerated by the subjects.
Data Sharing Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Acknowledgments
The authors thank Kate Wang, MS, of AbbVie for performing statistical analysis for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Allergan Aesthetics, an AbbVie company, funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. Medical writing support was provided by Maria Lim, PhD, of Peloton Advantage, an OPEN Health company, Parsippany, NJ, and was funded by Allergan Aesthetics, an AbbVie Company.
Disclosure
Lijuan Zhang and Smita Chawla are employees of AbbVie and own AbbVie stock. The authors report no other conflicts of interest in this work.
References
1. Wollina U. Perioral rejuvenation: restoration of attractiveness in aging females by minimally invasive procedures. Clin Interv Aging. 2013;8:1149–1155. doi:10.2147/CIA.S48102
2. Samizadeh S, Wu W. Ideals of facial beauty amongst the Chinese population: results from a large national survey. Aesthetic Plast Surg. 2018;42(6):1540–1550. doi:10.1007/s00266-018-1188-9
3. Ponsky D, Guyuron B. Comprehensive surgical aesthetic enhancement and rejuvenation of the perioral region. Aesthet Surg J. 2011;31(4):382–391. doi:10.1177/1090820X11409009
4. Ali MJ, Ende K, Maas CS. Perioral rejuvenation and lip augmentation. Facial Plast Surg Clin North Am. 2007;15(4):491–500, vii. doi:10.1016/j.fsc.2007.08.008
5. Gassia V, Raspaldo H, Niforos FR, Michaud T. Global 3-dimensional approach to natural rejuvenation: recommendations for perioral, nose, and ear rejuvenation. J Cosmet Dermatol. 2013;12(2):123–136. doi:10.1111/jocd.12035
6. Weinkle SH, Bank DE, Boyd CM, Gold MH, Thomas JA, Murphy DK. A multi-center, double-blind, randomized controlled study of the safety and effectiveness of Juvéderm injectable gel with and without lidocaine. J Cosmet Dermatol. 2009;8(3):205–210. doi:10.1111/j.1473-2165.2009.00451.x
7. Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(5 Suppl):139S–148S. doi:10.1097/PRS.0000000000001734
8. Eccleston D, Murphy DK. Juvéderm (R) Volbella in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167–172. doi:10.2147/CCID.S35800
9. Borrell M, Leslie DB, Tezel A. Lift capabilities of hyaluronic acid fillers. J Cosmet Laser Ther. 2011;13(1):21–27. doi:10.3109/14764172.2011.552609
10. Smith L, Cockerham K. Hyaluronic acid dermal fillers: can adjunctive lidocaine improve patient satisfaction without decreasing efficacy or duration? Patient Prefer Adherence. 2011;5:133–139. doi:10.2147/PPA.S11251
11. Geronemus RG, Bank DE, Hardas B, Shamban A, Weichman BM, Murphy DK. Safety and effectiveness of VYC-15L, a hyaluronic acid filler for lip and perioral enhancement: one-year results from a randomized, controlled study. Dermatol Surg. 2017;43(3):396–404. doi:10.1097/DSS.0000000000001035
12. Philipp-Dormston WG, Hilton S, Nathan M. A prospective, open-label, multicenter, observational, postmarket study of the use of a 15 mg/mL hyaluronic acid dermal filler in the lips. J Cosmet Dermatol. 2014;13(2):125–134. doi:10.1111/jocd.12085
13. Raspaldo H, Chantrey J, Belhaouari L, et al. Lip and perioral enhancement: a 12-month prospective, randomized, controlled study. J Drugs Dermatol. 2015;14(12):1444–1452.
14. Raspaldo H, Chantrey J, Belhaouari L, Saleh R, Murphy DK. Juvéderm Volbella with Lidocaine for lip and perioral enhancement: a prospective, randomized, controlled trial. Plast Reconstr Surg Glob Open. 2015;3(3):e321. doi:10.1097/GOX.0000000000000266
15. Rivkin A, Weinkle SH, Hardas B, et al. Safety and effectiveness of repeat treatment with VYC-15L for lip and perioral enhancement: results from a prospective multicenter study. Aesthet Surg J. 2019;39(4):413–422. doi:10.1093/asj/sjy019
16. Liew S, Wu WT, Chan HH, et al. Consensus on changing trends, attitudes, and concepts of Asian beauty. Aesthetic Plast Surg. 2016;40(2):193–201. doi:10.1007/s00266-015-0562-0
17. Werschler WP, Fagien S, Thomas J, Paradkar-Mitragotri D, Rotunda A, Beddingfield FC. Development and validation of a photographic scale for assessment of lip fullness. Aesthet Surg J. 2015;35(3):294–307. doi:10.1093/asj/sju025
18. Dayan S, Bruce S, Kilmer S, et al. Safety and effectiveness of the hyaluronic acid filler, HYC-24L, for lip and perioral augmentation. Dermatol Surg. 2015;41(suppl1):S293–S301. doi:10.1097/DSS.0000000000000540
19. Restylane Silk [instructions for use]. Fort Worth, TX, USA: Galderma Laboratories, L.P; 2017.
20. Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(Suppl 1):S127–S136. doi:10.1097/DSS.0000000000000199
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.