Back to Journals » Drug Design, Development and Therapy » Volume 13
Rutaecarpine may improve neuronal injury, inhibits apoptosis, inflammation and oxidative stress by regulating the expression of ERK1/2 and Nrf2/HO-1 pathway in rats with cerebral ischemia-reperfusion injury
Received 17 May 2019
Accepted for publication 31 July 2019
Published 20 August 2019 Volume 2019:13 Pages 2923—2931
DOI https://doi.org/10.2147/DDDT.S216156
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Meiyu Han,1,* Lin Hu,2,* Yang Chen3
1Department of Internal Medicine, The Second People’s Hospital of Dongying City, Dongying City, Shandong Province 257335, People’s Republic of China; 2Department of Critical Care Medicine ICU, Zoucheng People’s Hospital, Zoucheng, Shandong Province 273500, People’s Republic of China; 3Department of Internal Neurology, Shanghai Pudong Hospital, Fudan University Pudong Medical Center, Pudong, Shanghai 201399, People’s Republic of China
Correspondence: Yang Chen
Department of Internal Neurology, Shanghai Pudong Hospital, Fudan University Pudong Medical Center, 2800 Gongwei Road, Huinan Town, Pudong, Shanghai 201399, People’s Republic of China
Tel +86 1 356 895 4215
Email [email protected]
*These authors contributed equally to this work
Background: Cerebral ischemia-reperfusion (CI/R) injury is a more serious brain injury caused by the recovery of blood supply after cerebral ischemia for a certain period of time. Rutaecarpine (Rut) is an alkaloid isolated from Evodia officinalis with various biological activities. Previous studies have shown that Rut has a certain protective effect on ischemic brain injury, but the specific molecular mechanism is still unknown.
Methods: In this study, a rat model of CI/R was established to explore the effects and potential molecular mechanisms of Rut on CI/R injury in rats.
Results: The results showed that Rut alleviated neuronal injury induced by CI/R in a dose-dependent manner. Besides, Rut inhibited neuronal apoptosis by inhibiting the activation of caspase 3 and the expression of Bax. In addition, Rut alleviated the inflammatory response and oxidative stress caused by CI/R through inhibiting the production of pro-inflammatory factors (IL-6 and IL-1β), lactate dehydrogenase (LDH), malondialdehyde (MDA) and ROS, and increased the levels of anti-inflammatory factors (IL-4 and IL-10) and superoxide dismutase (SOD). Biochemically, Western blot analyses showed that Rut inhibited the phosphorylation of ERK1/2 and promoted the expression of nuclear factor-erythroid 2 related factor 2 (Nrf2) pathway-related proteins (Nrf2, heme oxygenase 1 (HO-1) and NAD (P) H-quinone oxidoreductase 1) in a dose-dependent manner. These results show that Rut may alleviate brain injury induced by CI/R by regulating the expression of ERK1/2 and the activation of Nrf2/HO-1 pathway.
Conclusion: In conclusion, these results suggest that Rut may be used as an effective therapeutic agent for damage caused by CI/R.
Keywords: cerebral ischemia-reperfusion, rutaecarpine, nuclear factor-erythroid 2 related factor 2, extracellular regulated protein kinases
Introduction
Stroke is a kind of brain injury caused by cerebral vascular rupture or vascular obstruction, which can cause 44 million disabilities worldwide each year.1 The incidence of ischemic stroke is higher than that of hemorrhagic stroke, which is one of the main causes of disability in adults.2 The most effective treatment for ischemic stroke is to restore the blood supply quickly. However, restoring blood flow can aggravate brain injury and functional damage, resulting in cerebral ischemia-reperfusion injury (CI/R).3 At present, the treatment of ischemic stroke mainly includes surgical treatment and drug therapy. Among them, the application of neuroprotective agent is the main method of specific treatment of acute ischemic stroke.4 However, the mechanism of CI/R injury is complex, involving a variety of signal pathways and biological processes.5 Therefore, improving the functional recovery after CI/R injury is a key problem to be solved urgently.
Rutaecarpine (Rut) is an alkaloid isolated from Evodia rutaecarpa. Rut has obvious antioxidant6 and anti-inflammatory effects.7 Besides, Rut also has a certain cardiovascular protective effect,8 specifically in preventing hypertensive myocardial hypertrophy9 and preventing the dysfunction of vascular smooth muscle cells.10 It is reported that Rut can prevent myocardial ischemia-reperfusion injury in rats by activating capsaicin-sensitive sensory nerves.11 Besides, Rut has a significant protective effect on ischemic brain injury by increasing the level of calcitonin gene-related peptide (CGRP) in the brain.12 In addition, Rut can improve the neural function after CI/R injury, and its mechanism may be related to oxidative stress.13
Nuclear factor-erythroid 2 related factor 2 (Nrf2) is an important antioxidant-regulated transcription factor. The binding of Nrf2 to the antioxidant response element (ARE) regulates the expression of heme oxygenase 1 (HO-1) and NAD (P) H-quinone oxidoreductase 1 (NQO1).14 Nrf2/HO-1 is an important antioxidant system. The Nrf2/HO-1 pathway has been reported to play an important role in I/R injury.15 Activation of the Nrf2/HO-1 pathway attenuates oxidative stress and inflammation caused by I/R injury, restores neurological deficits and reduces cerebral infarction volume.16 Extracellular regulated protein kinases (ERK), as part of the MAPK signaling molecule, is also involved in the regulation of neural function. López-Morales et al found that atrial natriuretic peptide improved neurological status and reduced infarct volume by reversing the expression of ischemia-upregulated pERK2/ERK2.17 G-Rg1 attenuates phosphorylation of p38 and ERK-1/2 in cortical neurons and hippocampal slices caused by hypoxia.18 Interestingly, Rut inhibited excessive activation of the ERK1/2 pathway.9 However, whether Rut can improve the damage caused by CI/R by inactivating ERK1/2 and Nrf2/HO-1 pathways is still unknown.
In this study, a rat model of CI/R was constructed to investigate the effects of Rut on apoptosis, inflammation and oxidative stress in rats with CI/R and the underlying molecular mechanisms. These results suggest that Rut may be used as an effective therapeutic agent for damage caused by CI/R.
Materials and methods
Animal model
All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Medical Ethics Committee of The Second People’s Hospital of Dongying City. A total of 50 Sprague-Dawleyrats (male, weight 250–280 g) were obtained from the Animal Center of The Second People’s Hospital of Dongying City and housed in a controlled environment at 25±3°C, humidity 60%, in a 12-hr light/dark cycle with free access to food and water. A middle cerebral artery occlusion (MCAO) rat model was prepared by the longa’s suture-occluded method.19 The rats were anesthetized with chloral hydrate (0.3 mL/100 g). After blocking the blood flow for 2 hrs, the thrombus was removed for reperfusion. After successful modeling, the rats were grouped into five groups (ten in each group): control group, the rats were received similar surgical treatment but no screws were inserted; CI/R model; CI/R+Rut (5 mg/kg, ≥98% PureOne Biotechnology, Shanghai, People's Republic of China), the CI/R rats were treated with 5 mg/kg for 4 weeks; CI/R+Rut (10 mg/kg), the CI/R rats were treated with 10 mg/kg for 4 weeks; CI/R+Rut (20 mg/kg), the CI/R rats were treated with 20 mg/kg for 4 weeks.
Neurobehavioral score
After MCAO rats were awake for 24 hrs, the neurological deficit score was scored by classical Zea Longa method,20 and the average neurological deficit score was measured.
H&E staining
The brain tissue was fixed with 4% paraformaldehyde for 24 hrs and embedded in paraffin. Then, the tissue was cut into slices, dewaxed in xylene and dehydrated in alcohol. Subsequently, the slices were stained with Hematoxylin and eosin staining, respectively. Finally, the slices were observed under an optical microscope (Olympus, Japan).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
The brain tissue was dewaxed with xylene for 20 mins, rehydrated with a gradient of ethanol (from a high concentration to a low concentration) and permeabilized with proteinase K. The TUNEL kit was used to detect apoptosis in the cerebral cortex according to the manufacturer’s instructions (Beyotime, Shanghai, People's Republic of China). Apoptosis index (AI)=(TUNEL positive cells/total cells)×100%.
RT-qPCR
Total RNAs were extracted from brain tissue and reversed transcribed into cDNA using FastKing One Step RT-PCR Kit (Tiangen, Beijing, People's Republic of China). SYBR Green (TAKARA, Japan) were applied to qPCR. Amplification condition in this study was as follows: 95°C for 15 s, followed by 40 cycles at 58°C for 46 s, and 72°C for 60 s. The primers of caspase 3 were as follows: S, 5ʹ- TCGATCGTAGTAGCTGATCG-3ʹ and AS, 5ʹ-CGCTACTACATAGCTGCAC-3ʹ
Western blotting
Total proteins were extracted from the brain tissue with lysis buffer (Beyotime). The proteins were then separated by SDS-PAGE and moved onto PVDF membrane. After blocking with 5% skim milk powder for 1 hr, proteins were hybridized with primary antibodies: caspase 3 (#9662, 1: 1000, Cell Signaling Technology, Beverly, MA, USA), GAPDH (#5174, 1: 1000, CST), ERK1/2 (ab17942, 1: 5000, Abcam, Cambridge, UK), Nrf2 (#12721, 1: 1000, CST), HO-1 (#86806, 1: 1000, CST, Beverly), NQO1 (ab80588, 1: 5000, Abcam) and Actin (ab179467, 1: 5000, Abcam) overnight at 4°C. Subsequently, the membrane was washed with TBST buffer and incubated with Anti-rabbit IgG (#7074, CST, Beverly, ) for 2 hrs at room temperature, and treated with ECL reagent (ThermoFisher, USA) to detect protein expression levels. Protein bands were quantified using Image J software (Rawak Software Inc., Germany).
ELISA assay
Pro-inflammatory factors (IL-6 and IL-1β) and anti-inflammatory factors (IL-4 and IL-10) in serum were measured by ELISA kit (Beyotime) according to the manufacturer’s instructions.
Determination of oxidative stress
ROS levels were measured by 2ʹ7’-dichrolorohydrofluorescein diacetate (DCHFDA). LDH was measured using LDH Cytotoxicity Assay Kit (Beyotime). SOD was measured using Superoxide Assay Kit (Beyotime), while MDA was measured by Lipid Peroxidation MDA Assay Kit (Beyotime). All operations are carried out in accordance with the manufacturer’s instructions.
Statistical analysis
All data are expressed as mean±SD. Analysis among multiple groups was carried out by ANOVA. Student’s t-test was used for two-group comparison. Statistical analyses were conducted with SPSS 19.0 software and P<0.05 was considered to be statistically significant.
Results
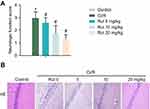
Rut relieves neurological damage in a MCAO rat model
As shown in Figure 1A, the results of Zea Longa nerve score showed that there was no abnormal behavior in the healthy group. The rats in the model group had a certain degree of behavior disorder. In addition, the symptoms of neurological deficits in the model group were significantly increased compared with the healthy group. However, Rut treatment dose-dependently inhibited behavioral disorders and neurological deficit symptoms in the model group. Therefore, the neurobehavioral score of Rut treatment group was significantly lower than that of model group. Similarly, the results of H&E staining showed that the neurons in the healthy group had complete morphology, clear structure, clear nuclear membrane, obvious nucleolus. There are no obvious pathological changes in the health group. In the model group, the structure of neurons was blurred with a loose. The cell body was swollen, and the nucleus was stained and pyknotic. The cytoplasm was loose, and the tissue around the infarction area was edema. However, Rut treatment improved the neuronal injury in a dose-dependent manner (Figure 1B). Taken together, these results demonstrated that Rut relieves neurological damage in a MCAO rat model.
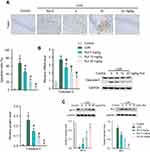
Rut inhibits the apoptosis of nerve cells in MCAO rats
TUNEL analysis is employed to detect the apoptosis of nerve cells. As shown in Figure 2A, there are a large number of brown nerve cells in the MCAO model group compared with the healthy group, while Rut treatment dose-dependently reduced the number of brown nerve cells, indicating that Rut treatment significantly inhibited the apoptosis of nerve cells in MCAO rats (Figure 2A). Similarly, RT-qPCR and Western blotting results showed that the mRNA level and protein level of apoptosis-related protein caspase 3 were significantly increased in MCAO rats compared with the control group, while Rut treatment decreased the expression of caspase 3 in mRNA and protein (Figure 2B). In addition, Western blotting assay also showed that compared to the model group, Rut treatment significantly increased Bcl-2 level, while decreased Bax level in a dose-dependent manner (Figure 2C). In total, these results suggested that Rut inhibits the apoptosis of nerve cells in MCAO rats.
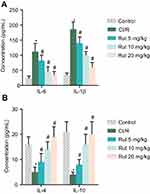
Rut regulates inflammatory response in MCAO rats
In addition, we explored the effect of Rut on the inflammatory response. As shown in Figure 3A and B, the levels of pro-inflammatory factors (IL-6 and IL-1β) were significantly increased in the model group compared to the control group, while the levels of anti-inflammatory factors (IL-4 and IL-10) were significantly reduced. Interestingly, Rut treatment dose-dependently inhibited levels of IL-6 and IL-1β, while increasing the levels of IL-4 and IL-10. Collectively, these results indicated that Rut regulates inflammatory response in MCAO rats.
Rut improves oxidative stress in MCAO rats
To investigate the effects of Rut on oxidative stress, the levels of SOD, MDA, LDH and ROS were determined using an ELISA kit. As shown in Figure 4B–D, the levels of MDA, LDH and ROS in the serum of the model group were significantly increased compared with the control group, while the level of SOD was decreased (Figure 4A). After treatment with different doses of Rut (5, 10 and 20 mg/kg), the levels of MDA, LDH and ROS were significantly reduced, while the levels of SOD were increased.
Rut inhibits the ERK pathway and activates the Nrf2 pathway
Western blotting analysis was used to explore the potential molecular mechanisms of Rut in MCAO rats. As shown in Figure 5A, phosphorylation of ERK1/2 was activated in MCAO rats compared to the control group. However, different doses of Rut treatment (5, 10 and 20 mg/kg) dose-dependently inhibited phosphorylation of ERK1/2. In addition, Western blotting results also showed that the expression of Nrf2 pathway-associated proteins (Nrf2, HO-1 and NQO1) in MCAO rats was significantly inhibited, while different doses of Rut treatment (5, 10 and 20 mg/kg) dose-dependently promoted the expression of Nrf2, HO-1 and NQO1 (Figure 5B). These results indicate that Rut inhibits the ERK pathway and activates the Nrf2 pathway in MCAO rats.
Discussion
Ischemic stroke is the main subtype of stroke, accounting for 80–90% of stroke cases. Cerebral ischemia causes excessive secretion of glutamate, triggering a large amount of calcium ions to enter nerve cells, thereby activating various proteases, which ultimately leads to neurotoxicity, neuronal damage and even apoptosis.19 Previous studies have shown that ischemia slows mitochondrial respiration and depletes ATP and creatine phosphate levels. After blood reperfusion, it will also cause secondary energy failure, which will aggravate brain damage and functional damage.3 It was found that 3,14,19-triacetyl andrographolide (CX-10) has a good neuroprotective effect on ischemic brain damage by increasing anti-inflammatory and anti-oxidative activities.21 Besides, Mohamed et al found that zearalenol-activated ERK signaling pathway can reduce brain cell apoptosis and inflammatory response, and has protective effects on CI/R injury in OVX rats.22 Similarly, current studies have shown that Rut improves neuronal damage, inhibits neuronal apoptosis, reduces inflammatory responses, and improves oxidative stress in rats with cerebral ischemia-reperfusion by activating ERK1/2-mediated Nrf2/HO-1 signaling pathway.
As an alkaloid, Rut has various biological activities. There is plenty of evidence that Rut plays a positive role in the treatment of cardiovascular disease.8 Rut stimulates the synthesis and release of CGRP by activating TRPV1 and plays an effective role in vasodilation and hypertension.23 Besides, Xue et al found that Rut (1.1 mg/mL) could reduce the myocardial infarction size of myocardial I/R injury, improve the disorder of fatty acid and glucose metabolism, and increase the content of ATP.24 In addition, Dai et al found that Rut inhibited H/R-induced hippocampal neuronal apoptosis by inhibiting oxidative stress and caspase 3 activation.25 In addition, Yan et al found that Rut can improve learning and memory ability and neurological symptoms, reduce cerebral infarction and brain water content, reduce oxidative stress and improve neurological function induced by CI/R in rats.13 Compared with the above results, the current study found that Rut can significantly improve neuronal damage induced by CI/R in rats, and inhibit the apoptosis of nerve cells by regulating the activation of caspase 3. Besides, Rut was also found to improve inflammation and oxidative stress induced by CI/R by modulating the levels of IL-6 and IL-1β, IL-4, IL-10, SOD, MDA, LDH and ROS. It is well known that Rut stimulates the synthesis and release of CGRP. Furthermore, CGRP exerts protective effects in several cardiovascular diseases, including cardiac ischemia-reperfusion injury.26 Interestingly, CGRP treatment significantly inhibited the activation of ERK1/2, p38 and JNK proteins in bone marrow-derived endothelial progenitor cells.27 Similarly, Zeng et al indicated that CGRP inhibited inflammation and reduced the expression of ADAM17 and the activation of EGFR and ERK1/2 in Ang II-stimulated VSMCs.28 Collectively, the repair effect of Rut on CI/R injury may be achieved by promoting the expression of CGRP and activation of Nrf2 pathway, and inhibiting the activation of ERK1/2 pathway.It has been reported that the Nrf2/HO-1 pathway has a significant protective effect on I/R injury.18 Jin et al found that IL-1ra inhibits oxidative stress and inflammation caused by I/R injury by promoting the expression of Nrf2 and HO-1 and the phosphorylation level of Nrf2.15 Similarly, Li et al found that icariside II can improve oxidative stress in brain tissue of rats with CIR injury by activating Nrf2/HO-1 pathway.16 In addition, upregulation of Nrf2 and HO-1 protein levels improved neurological deficits, cerebral infarction volume and body weight after CI/R. The MAPK/ERK pathway is involved in cell proliferation, differentiation and apoptosis, and MAPK/p-38 can be activated by a variety of inflammatory extracellular mediators. Hypoxia promotes phosphorylation of p38 and ERK1/2 in cortical neurons and hippocampal slices, whereas G-Rg1 inhibits phosphorylation of cortical neurons and hippocampal slices p38 and ERK1/2.18 Besides, Fan et al found that oxidative stress induced by CI/R can cause lung damage. The expression levels of PI3K, ERK and p-ERK1/2 were higher in the CI/R-model group.29 Interestingly, Rut inhibited excessive activation of the ERK1/2 pathway.9 On this basis, current studies have shown that Rut can inhibit CI/R-induced ERK1/2 phosphorylation and activate Nrf2/HO-1 signaling pathway in a dose-dependent manner. The inactivation of ERK1/2 and activation of Nrf2/HO-1 pathway induced by Rut treatment further improved neuronal injury and inhibited neuronal apoptosis, inflammation and oxidative stress.
Rut may achieve synergistic effects of neuroprotection by modulating multiple signaling pathways (such as BMP, SHH, Wnt, Notch) or neurogenic niches or microenvironments in the brain. Adult brains with impaired stroke are self-repairing by creating new neurons in areas without neurogenesis. Studies by Lindvall et al have shown that stroke-damaged brains were effectively repaired by increasing the survival and differentiation of the resulting neuroblasts and by combining with other endogenous neural regenerative responses.30 Strict regulation of BMP signaling was critical for balancing proliferating and non-proliferating cells. The microenvironment of growth factors in the subventricular zone (SEZ) of the adult brain provided a guiding environment for neurogenesis. Gajera et al found that LRP2, a clearing receptor for BMP4, was specifically expressed in the ependymal cells of the lateral ventricle of adult brain. Lack of LRP2 expression in adult mice resulted in impaired proliferation of neural progenitor cells in SEZ, resulting in a decrease in the number of neuroblasts reaching the olfactory bulb. It is suggested that LRP2-mediated ependymal BMP4 catabolism played an important role in the regulation of SEZ microenvironment and the continuation of adult nerve development.31 Armenteros et al pointed out that adult rat hippocampal neural stem cells and progenitor cells (AH-NSPCs) expressed the receptor of BMPs, and the BMP/P-Smad pathway was activated in the differentiation of neural stem cells and progenitor cells into neuronal lineage. BMP2/4 and WNT3A were the key regulators of hippocampal neurogenesis in adults. They cooperate to further enhance the production of neurons.32 In addition, Bunatyan et al suggested that the loss of ependymal LRP2 in mice resulted in a significant decrease in the proliferation of SVZ. Consistent with neurological damage in SVZ, the number of newly generated neurons reaching the olfactory bulb was significantly reduced, indicating the role of LRP2 in controlling adult neurogenesis in SVZ, which may involve balancing the morphogenetic signals provided by SHH and BMP4.33
In the study, Western blots shown and quantified the activation of ERK 1/2 and Nrf2 pathways. However, this evidence is just one component of numerous orthogonal evidences. Understandably, there is other evidence outside of our scope that involves the role of Rut in CI/R. So, next, we will delve deeper into the role of Rut in CI/R injury. In addition to Rut, previous studies have shown that several herbal constituents drugs like Curcumin, Icariin and Ginger Ashwagandha possessed the effects of neuroprotective, antioxidant, neurogenic and immunomodulatory.34–38 Therefore, these herbal ingredients can be combined to bring synergistic effect for functional therapeutic effect.
Conclusion
In conclusion, the present study indicates that Rut improves neuronal injury, inhibits apoptosis, inflammation and oxidative stress by regulating the expression of ERK1/2 and Nrf2/HO-1 pathway in rats with CI/R. These results suggest that Rut may be used as an effective therapeutic drug for CI/R injury.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38(7):1167–1186. doi:10.1007/s10072-017-2938-1
2. Li Z, Chen H, Lv J, Zhao R. The application and neuroprotective mechanisms of cerebral ischemic post-conditioning: a review. Brain Res Bull. 2017;131:39–46. doi:10.1016/j.brainresbull.2017.03.002
3. Chomova M, Zitnanova I. Look into brain energy crisis and membrane pathophysiology in ischemia and reperfusion. Stress. 2016;19(4):341–348. doi:10.1080/10253890.2016.1174848
4. Lancelot S, Zimmer L. Small-animal positron emission tomography as a tool for neuropharmacology. Trends Pharmacol Sci. 2010;31(9):411–417. doi:10.1016/j.tips.2010.06.002
5. Cui HX, Chen JH, Li JW, Cheng FR, Yuan K. Protection of anthocyanin from myrica rubra against cerebral ischemia-reperfusion injury via modulation of the TLR4/NF-kappaB and NLRP3 pathways. Molecules. 2018;23(7):E1788. doi:10.3390/molecules23071788
6. Jin SW, Hwang YP, Choi CY, et al. Protective effect of rutaecarpine against t-BHP-induced hepatotoxicity by upregulating antioxidant enzymes via the CaMKII-Akt and Nrf2/ARE pathways. Food Chem Toxicol. 2017;100:138–148. doi:10.1016/j.fct.2016.12.031
7. Yan L, Li QF, Rong YT, et al. The protective effects of rutaecarpine on acute pancreatitis. Oncol Lett. 2018;15(3):3121–3126. doi:10.3892/ol.2017.7659
8. Tian KM, Li JJ, Xu SW. Rutaecarpine: a promising cardiovascular protective alkaloid from Evodia rutaecarpa (Wu Zhu Yu). Pharmacol Res. 2019;141:541–550. doi:10.1016/j.phrs.2018.12.019
9. Zeng SY, Yang L, Lu HQ, Yan QJ, Gao L, Qin XP. Rutaecarpine prevents hypertensive cardiac hypertrophy involving the inhibition of Nox4-ROS-ADAM17 pathway. J Cell Mol Med. 2019;23(6):4196–4207. doi:10.1111/jcmm.14308
10. Wang M, Wu Y, Yu Y, et al. Rutaecarpine prevented ox-LDL-induced VSMCs dysfunction through inhibiting overexpression of connexin 43. Eur J Pharmacol. 2019;853:84–92. doi:10.1016/j.ejphar.2019.03.028
11. Hu CP, Li NS, Xiao L, Deng HW, Li YJ. Involvement of capsaicin-sensitive sensory nerves in cardioprotection of rutaecarpine in rats. Regul Pept. 2003;114(1):45–49.
12. Liu Y, Cui YP, Song T. Rutaecarpine ameliorates cerebral ischemia-reperfusion injury by stimulating calcitonin gene-related peptide release in the rat brain. J Chin Physician. 2005;7(5):589–591.
13. Yan C, Zhang J, Wang S, Xue G, Hou Y. Neuroprotective effects of rutaecarpine on cerebral ischemia reperfusion injury. Neural Regen Res. 2013;8(22):2030–2038. doi:10.3969/j.issn.1673-5374.2013.22.002
14. Motterlini R, Foresti R. Heme oxygenase-1 as a target for drug discovery. Antioxid Redox Signal. 2014;20(11):1810–1826. doi:10.1089/ars.2013.5658
15. Jin C, Fu WL, Zhang DD, et al. The protective role of IL-1Ra on intestinal ischemia reperfusion injury by anti-oxidative stress via Nrf2/HO-1 pathway in rat. Biomed J. 2019;42(1):36–45. doi:10.1016/j.bj.2018.11.001
16. Li Y, Meng F. Effects of icariside II on brain tissue oxidative stress and Nrf2/HO-1 expression in rats with cerebral ischemia-reperfusion injury1. Acta Cir Bras. 2019;34(2):e201900208. doi:10.1590/s0102-8650201900208
17. Lopez-Morales MA, Castello-Ruiz M, Burguete MC, et al. Molecular mechanisms underlying the neuroprotective role of atrial natriuretic peptide in experimental acute ischemic stroke. Mol Cell Endocrinol. 2018;472:1–9. doi:10.1016/j.mce.2018.05.014
18. Xie W, Zhou P, Sun Y, et al. Protective effects and target network analysis of ginsenoside Rg1 in cerebral ischemia and reperfusion injury: a comprehensive overview of experimental studies. Cells. 2018;7(12):E270. doi:10.3390/cells7120270
19. Tian L, Wang JH, Zhao M, et al. [Effect of scalp-acupuncture stimulation on neurological function and expression of ASIC 1 a and ASIC 2 b of hippocampal CA 1 region in cerebral ischemia Rats]. Zhen Ci Yan Jiu. 2016;41(5):417–422.
20. Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–476. doi:10.1161/01.str.17.3.472
21. Yang MY, Yu QL, Huang YS, Yang G. Neuroprotective effects of andrographolide derivative CX-10 in transient focal ischemia in rat: involvement of Nrf2/AE and TLR/NF-kappaB signaling. Pharmacol Res. 2019;144:227–234. doi:10.1016/j.phrs.2019.04.023
22. Mohamed SK, Ahmed AAE, Elmorsy EM, Nofal S. ERK activation by zeranol has neuroprotective effect in cerebral ischemia reperfusion. Life Sci. 2019;227:137–144. doi:10.1016/j.lfs.2019.04.035
23. Ma J, Chen L, Fan J, et al. Dual-targeting Rutaecarpine-NO donor hybrids as novel anti-hypertensive agents by promoting release of CGRP. Eur J Med Chem. 2019;168:146–153. doi:10.1016/j.ejmech.2019.02.037
24. Xue H, Cheng Y, Wang X, Yue Y, Zhang W, Li X. Rutaecarpine and evodiamine selected as beta1-AR inhibitor candidates using beta1-AR/CMC-offline-UPLC/MS prevent cardiac ischemia-reperfusion injury via energy modulation. J Pharm Biomed Anal. 2015;115:307–314. doi:10.1016/j.jpba.2015.07.022
25. Dai Z, Xiao J, Liu SY, Cui L, Hu GY, Jiang DJ. Rutaecarpine inhibits hypoxia/reoxygenation-induced apoptosis in rat hippocampal neurons. Neuropharmacology. 2008;55(8):1307–1312. doi:10.1016/j.neuropharm.2008.08.030
26. Luo D, Deng PY, Ye F, Peng WJ, Deng HW, Li YJ. Delayed preconditioning by cardiac ischemia involves endogenous calcitonin gene-related peptide via the nitric oxide pathway. Eur J Pharmacol. 2004;502(1–2):135–141. doi:10.1016/j.ejphar.2004.08.051
27. Wu J, Liu S, Wang Z, Ma S, Meng H, Hu J. Calcitonin gene-related peptide promotes proliferation and inhibits apoptosis in endothelial progenitor cells via inhibiting MAPK signaling. Proteome Sci. 2018;16:18. doi:10.1186/s12953-018-0137-5
28. Zeng SY, Yang L, Hong CL, et al. Evidence that ADAM17 mediates the protective action of CGRP against angiotensin II-induced inflammation in vascular smooth muscle cells. Mediators Inflamm. 2018;2018:2109352. doi:10.1155/2018/2109352
29. Fan J, Lv H, Li J, et al. Roles of Nrf2/HO-1 and HIF-1α/VEGF in lung tissue injury and repair following cerebral ischemia/reperfusion injury. J Cell Physiol. 2019;234(6):7695–7707. doi:10.1002/jcp.27767
30. Lindvall O, Kokaia Z. Neurogenesis following stroke affecting the adult brain. Cold Spring Harb Perspect Biol. 2015;7(11):a019034. doi:10.1101/cshperspect.a019034
31. Gajera CR, Emich H, Lioubinski O, et al. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci. 2010;123(Pt 11):1922–1930. doi:10.1242/jcs.065912
32. Armenteros T, Andreu Z, Hortiguela R, Lie DC, Mira H. BMP and WNT signalling cooperate through LEF1 in the neuronal specification of adult hippocampal neural stem and progenitor cells. Sci Rep. 2018;8(1):9241. doi:10.1038/s41598-018-27581-0
33. Bunatyan L, Christ A, Gajera C, Hammes-Lewin A, Willnow T. LRP2 in SHH-dependent adult neurogenesis. Mech Dev. 2017;145:S116. doi:10.1016/j.mod.2017.04.313
34. Vasantha R, KDPP G. Recent perspectives on the medicinal potential of ginger. Botanics: Targets and Therapy. 2015;5:55–63. doi:10.2147/BTAT.S68099
35. de Souza Tavares W, Akhtar Y, Gonçalves GLP, Zanuncio JC, Isman MB. Turmeric powder and its derivatives from Curcuma longa rhizomes: insecticidal effects on cabbage looper the role of synergists. Sci Rep. 2016;6:34093. doi:10.1038/srep34093
36. Shah N, Singh R, Sarangi U, et al. Combinations of Ashwagandha leaf extracts protect brain-derived cells against oxidative stress and induce differentiation. PLoS One. 2015;10(3):e0120554–e0120554. doi:10.1371/journal.pone.0120554
37. Rao RV, Descamps O, John V, Bredesen DE. Ayurvedic medicinal plants for Alzheimer’s disease: a review. Alzheimers Res Ther. 2012;4(3):22. doi:10.1186/alzrt125
38. Zhu T, Zhang F, Li H, et al. Long-term icariin treatment ameliorates cognitive deficits via CD4+ T cell-mediated immuno-inflammatory responses in APP/PS1 mice. Clin Interv Aging. 2019;14:817–826. doi:10.2147/CIA
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.