Back to Journals » Journal of Asthma and Allergy » Volume 11
Rupatadine oral solution for 2–5-year-old children with allergic rhinitis: a safety, open-label, prospective study
Authors Santamaría E, Izquierdo I , Valle M , Vermeulen J , Potter P
Received 5 February 2018
Accepted for publication 4 May 2018
Published 4 September 2018 Volume 2018:11 Pages 225—231
DOI https://doi.org/10.2147/JAA.S164632
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Eva Santamaría,1,2 Iñaki Izquierdo,2 Marta Valle,1,3 Jan Vermeulen,4 Paul Potter5
1Department of Pharmacology, Therapeutics and Toxicology, Universitat Autònoma de Barcelona, Barcelona, Spain; 2Clinical Development, R&D, J. Uriach y Compañía, S.A., Barcelona, Spain; 3Pharmacokinetic/Pharmacodynamic Modeling and Simulation, Sant Pau Institute of Biomedical Research (IIB-Sant Pau), Barcelona, Spain; 4Allergic Department, Parow Research, Cape Town, South Africa; 5Allergy Diagnostic and Clinical Research Unit, Department of Medicine, University of Cape Town Lung Institute, Cape Town, South Africa
Background: There are few clinical trials that assess the efficacy of antihistamines in very young children. Rupatadine is a second-generation antihistamine indicated for the treatment of allergic rhinitis (AR) and urticaria. In this study, AR symptoms were evaluated before and after daily 1 mg/mL rupatadine oral solution administration in 2–5-year-old children.
Methods: A multicenter open-label study was carried out in 2–5-year-old children with AR. Safety assessments were collected during the study including spontaneous adverse events, vital signs, and electrocardiogram (QTc interval). Additionally, evaluations of Total Five Symptoms Score (T5SS, including: nasal congestion; sneezing; rhinorrhoea; itchy nose, mouth, throat, and/or ears; and itchy, watery, and red eyes) were analyzed. Symptoms were evaluated by parents/legal guardian before and after 4 weeks of rupatadine administration, dosed according to body weight.
Results: A total of 44 children received the study treatment. Only 15 adverse events were reported. All of them were of mild intensity and considered not related to the study treatment. No patient exceeded the standard parameter of >450 ms in the last visit, for the QTc interval on their electrocardiograms. From a maximum score value of 15, T5SS values at Day 14 (6.35) and Day 28 (5.42) were both statistically significant different (p<0.001) from the baseline T5SS value (mean 8.65), with a reduction of 26.6% and 37.4%, respectively. All individual symptoms, including nasal congestion, showed also a decrease from baseline at both 14 and 28 days.
Conclusion: Rupatadine 1 mg/mL oral solution was found to be safe in 2–5-year-old children, correlating with an improvement of AR symptoms, overall and each individually, after a daily dose administration. With this study, we enlarge the available information in this very young pediatric patients’ group, in which there is a general lack of clinical evidence.
Keywords: allergic rhinitis, children, antihistamines, safety, rupatadine
Introduction
Allergic rhinitis (AR) is increasing worldwide, particularly in industrialized regions. Although it is not a severe disease, it can cause a large impact in quality of life and, especially in children, impair their performance in learning activities.1,2 AR is the most prevalent chronic allergic disease in children, and although it is most prevalent in school-age children, its prevalence and importance in younger children are significant.3,4 In a birth cohort study in the Isle of Wight, it was shown that AR symptoms appear early in life, and increase over the age, from 3.4% at 4 years to 27.3% at 18 years.5 Prevalence of rhinitis with itchy watery eyes (ie, allergic rhinoconjunctivitis) in children 6–7 and 13–14 years old has been extensively studied in the ISAAC study, with a prevalence of 8.5% and 14.6%, respectively.6 However, prevalence of AR in preschool children is difficult to evaluate, as symptoms may be confused with upper airway infectious diseases and clinical endpoints are difficult to be evaluated although they are the ones recommended in the guidelines. Some cross-sectional studies in early childhood demonstrated that AR was diagnosed by physicians in 2.8% of 2–6-year-old children in People’s Republic of China and 3.9% in 5–6-year-old children in Germany.7,8 PARIS, a prospective birth cohort study, was implemented in 2003 to assess environmental/behavioral factors associated with respiratory and allergic disorder occurrence in early childhood in 5 Paris maternity hospitals.9 Data on AR-like symptoms (runny nose, blocked nose, and sneezing apart from a cold) were collected using a standardized questionnaire administered during the health examination at age 18 months and was included in the follow-up of the PARIS birth cohort to assess the prevalence of AR-like symptoms in the past year, which was found to be 9.1% of the 1,850 toddlers of the study cohort. AR-like symptoms and dry cough apart from a cold were frequent comorbid conditions. The results of this study support the hypothesis that AR could begin as early as 18 months of life.10
Very young children need safe and efficacious drugs that treat AR symptoms, enabling them to keep up with the learning requirements of that time of life. In this regard, major AR guidelines have been developed from evidence obtained in adults, not in children, although the same therapeutic approach is recommended, with antihistamines being the cornerstone of treatment for children suffering from this condition.3,11 However, there are a few clinical studies with second-generation antihistamines evaluating the efficacy in very young children.12–14
Rupatadine is a H1 receptor antagonist and platelet antagonist factor (PAF) antagonist indicated for the treatment of AR and urticaria in adults and children >2 years. Several mediators are implied in the inflammatory cascade, with histamine the most relevant of them. PAF has a role in the inflammatory cascade, and so blocking PAF might have additional effects to the antihistamine action of rupatadine.15
In a previous, double-blind, randomized, placebo-controlled trial in persistent AR, rupatadine was the first anti-H1 compound to assess its efficacy and safety following Allergic rhinitis and its Impact on Asthma (ARIA) recommendations in pediatric patients aged 6–11 years.16 This current paper adds clinical information in 2–5-year-old children, assessing AR symptoms.
Methods
This was a 28-day open-label, multicenter study in children aged 2–5 years, conducted in Hungary and South Africa. To be enrolled in this study, children must weigh ≥10 kg and must have a history of mild to moderate AR defined as either intermittent or persistent according to ARIA guideline.3 Children had to be symptomatic with a baseline of 5 symptoms score: Total Five Symptoms Score (T5SS: nasal congestion; sneezing; rhinorrhea; itchy nose, mouth, throat, and/or ears; and itchy, watery, and red eyes score) ≥6 during each of the last 2 days before of inclusion, and allergen skin prick test positive wheal of 3 mm greater than the diluent control, or a positive (class 3 of positivity; ≥3.5–17.5 kU/L) on ImmunoCAP® test (Phadia, Uppsala, Sweden). They were required to have normal results for standard laboratory tests and a 12-lead electrocardiogram (ECG) obtained at screening (Day –7 to 0) within acceptable limits. QTc interval values (ms) after Fridericia’s correction had to be normal (<450 ms). Prolongation of the QTc interval on the ECG and the development of torsades de pointes-type arrhythmias were reported in the literature for astemizole and terfenadine during the 1990s, and this has led to wider concern regarding the cardiotoxic potential of the second generation of antihistamines. For this reason, cardiac safety is closely monitored in these types of products. Informed consent was obtained from the patient’s parents or legal guardians, before inclusion in the study.
Children with a history of chronic sinusitis or severe bronchial asthma, nonallergic rhinitis, chronic nasal or upper respiratory symptoms/disorders, nasal polyps, and significant deviation of nasal septum were not eligible. None of the children had suffered any ear, nose, or throat infection in the 15 days prior to the baseline. Patients were not permitted to take systemic or topical medication for AR for a wash-out period prior to inclusion, as follows: oral and nasal corticosteroids (28 days), nasal decongestants (3 days), cromones (14 days), leukotriene inhibitors (3 days); oral/topical H1-receptor antagonists (7 days); H1- and H2-receptor antagonists: doxepin (7 days); leukotriene antagonists (4 days), anticholinergics (3 days); ophthalmic nonsteroidal anti-inflammatory drugs (3 days); nasal– ophthalmic wash solutions (12 h); tricyclic antidepressants (30 days); and/or any drug interacting with CYP3A4. Inhaled β2 bronchodilators were permitted. Children with mild asthma treated with inhaled corticosteroids of ≤250 µg/d for fluticasone, ≤400 µg/d for budesonide, or ≤160 µg/d for ciclesonide were allowed in the study.
The study had a duration of 28 days scheduled in 3 visits: baseline (Visit 0), treatment day 14 (Visit 1), and a final visit after 28 days (Visit 2). Rupatadine 1 mg/mL oral solution (J Uriach y Compañía, S.A., Spain) was given at a dose of 2.5 mL in children with a body weight ≥10 kg up to <25 kg and at a dose of 5 mL in children ≥25 kg. The medication was dispensed using a graduated syringe of 5 mL.
Safety was evaluated by means of data collection on adverse events and by assessing clinically relevant changes in physical examination and vital signs at each visit. ECG (QTc/QTcF) and laboratory tests were done at screening and final visit (Visit 2). A paired Student’s t-test was used in order to compare values between the 2 visits.
Referring to efficacy assessment, daily evaluation was made by the parents/legal guardians in a reflective way by means of a “Patient Diary Card.” Symptoms were assessed using a 4-grade scale ranging as follows: (0) = absent; (1) = mild: (symptom is present but not annoying); (2) = moderate: (symptom is annoying but does not interfere with daily activity); and (3) = severe: (symptom interferes with daily activity or sleep). The maximum possible value for T5SS would be 15. Symptoms were collected from baseline and during the whole treatment period. Parents/legal guardians were advised to fill in the diary cards at the same time of the day to ensure homogeneity of the reflective assessment.
The efficacy endpoint consisted in evaluating the change from baseline in the T5SS after 14 and 28 days of treatment. The time to beginning of action was considered as the first day in which any significant difference was observed for T5SS.
Overall impression of efficacy was also assessed based on investigator’s criteria on days 14 and 28. The following scale was used: (0): increase in symptom intensity; (1): no changes in symptom intensity; (2): slight improvement in symptom intensity; (3): significant symptom improvement; and (4): excellent improvement or complete disappearance of symptoms.
Blood samples were also taken to perform a population pharmacokinetic analysis, which will be reported in a separate publication.
Drug compliance was checked at Visit 1 and Visit 2 by questioning the parents/legal guardians on how many days they forgot to administer the dose. Patients who missed less than or equal to 4 doses (either consecutive or not) were considered to have good compliance.
A paired Student’s t-test (significant level: α=0.05) was used to detect differences. Linear interpolation was used to estimate missing data from diary missing values (for each symptom) and last observation carried forward was applied in withdrawn patients. The safety and efficacy analysis were performed using SAS software (v.8.2) (SAS Institute Inc., Cary, NC, USA).
The study (EudraCT: 2012-004900-37) was performed in compliance with the Declaration of Helsinki and was a part of the completed Paediatric Investigation Plan (EMEA-000582-PIP01-09). The study was approved by Regulatory Authorities and the central Ethics Committee in Hungary (National Institute of Pharmacy) and local Ethics Committees in South Africa (The University of Cape Town and Pharma Ethics)
Results
A total of 49 patients were screened, from which 5 were did not fulfill all the inclusion/exclusion criteria: 1 patient had T5SS <6 and 4 had an infection. Thus, 44 children were allocated to the study treatment and considered for safety, and in 43 of them efficacy analysis was done; this was because one child discontinued the study due to intake of prohibited concomitant medication. The study was carried out in 5 centers distributed between South Africa (3) and Hungary (2). The parents or legal guardians provided written informed consent before the study beginning. The majority of patients were sensitive to house dust mite. The sample was recruited very shortly and the total number of subjects was selected between January and February, which meant that it was summer in South Africa and winter in Hungary. The main demographic and baseline characteristics are shown in Table 1.
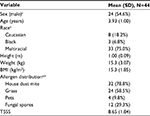  | Table 1 Demographic and other baseline characteristics Note: an (%); b1 patient may be positive for 1 or more allergen. Abbreviations: BMI, body mass index; T5SS, Total Five Symptoms Score. |
The 44 included children were generally in good health. The most frequent previous or concurrent diseases were eczema (40.9% of patients) and asthma (36.4% of patients).
Baseline symptom values were of mild to moderate intensity, with a mean (SD) T5SS of 8.65 (1.84). The mean of individual symptoms scores at baseline was 1.88 (0.67) for nasal congestion; 1.92 (0.52) for sneezing; 1.81 (0.71) for rhinorrhea; 1.87 (0.72) for itchy nose, mouth, throat, and/or ears; and 1.16 (0.73) for itchy, watery, and red eyes.
Assessing the adherence to treatment, it was found that all of them (100.0%) did not miss 4 doses throughout treatment period.
Safety
There were only 15 adverse events reported in 11 patients (25%). The list of the reported adverse events is summarized in Table 2. Out of the 15 events, 10 (66.7%) were of mild, 4 (26.7%) of moderate, and 1 (6.7%) was of severe intensity. Only viral infection (20.0%) and conjunctivitis (13.3%) were reported in >1 patient. None of these adverse events were considered related to the study medication, and there were no serious adverse events reported in this study.
  | Table 2 Adverse events Note: aNumber of episodes; bnumber of patients. |
One patient showed a biochemistry with clinically relevant abnormal value for creatine kinase, 718.0 U/L. This patient suffered a viral infection considered mild during this period of time. The creatine kinase increase was considered not serious and not related to the study drug by the investigator. No action was taken, and the patient recovered.
There were no statistically significant differences for any of the vital signs, except for weight, where a statistically significant increase was observed (p<0.001) with a mean (SD) value at the end of study of 15.68 (2.95) kg. This mean difference of <300 g cannot be considered as clinically relevant, as children in the growing age were the target population of the study.
No abnormalities were found when QT and QTcF >450 ms were assessed at Visit 2 with respect to screening visit.
Efficacy
T5SS values at Day 14 (6.35) and Day 28 (5.42) were both statistically significant lower (p<0.001) than the baseline value of 8.56 (1.84), as presented in Figure 1. These values represented a considerable decrease from baseline T5SS with a percentage of 26.6% and 37.4% of reduction at 14 and 28 days, respectively.
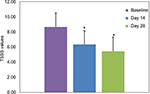  | Figure 1 Mean (SD) absolute T5SS values at baseline and Days 14 and 28. Note: *p<0.001 compared to baseline. Abbreviation: T5SS, Total Five Symptoms Score. |
Mean absolute values of each individual daily symptom score measured at baseline and Days 14 and 28 are presented for each of the symptoms in Figure 2. Each individual symptom’s daily symptom score showed a statistically significant decrease (p<0.01) after both 14 and 28 days of treatment. Nasal congestion showed a reduction of 20.2% and 32.3%; sneezing 32.4% and 42.9%; rhinorrhoea 20.9% and 30.7%; itchy nose, mouth, throat, and/or ears symptoms 32.3% and 43.1%; and itchy, watery, and red eyes 26.9% and 37.6%, at Days 14 and 28, respectively.
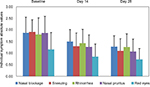  | Figure 2 Mean (SD) absolute individual values in each symptom score at baseline and Days 14 and 28. |
The mean daily evolution of T5SS during 28 study days is presented in Figure 3, which shows a decrease in T5SS maintained during the whole treatment period. In terms of onset of action, statistically significant differences in T5SS values were found from the first day on, when compared with the baseline value (Day 1: p=0.001), and this was maintained until the end of the study.
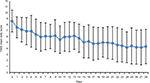  | Figure 3 Mean (SD) daily T5SS absolute values during 28 days. Abbreviation: T5SS, Total Five Symptoms Score. |
According to the investigator, on Day 28, approximately 45% of patients had significant or excellent improvement/complete disappearance of symptoms, while 39.5% showed a slight improvement, and the rest of the patients showed no change or an increase.
No patient used rescue medication due to unacceptable severity of symptoms.
Discussion
The aim and strength of this study was to provide information regarding the safety and efficacy of rupatadine in very young children (2–5 years) suffering from AR. There is very little published research on children diagnosed according with ARIA classification and treated with second-generation anti-H1 compounds.16,17 Most of the available pediatric literature with antihistamines are published with seasonal or perennial AR in older children over 6 year old.18–22 In the other few published studies in this very young population, old compounds that were launched in the market in the 1980s (eg, cetirizine and loratadine) were evaluated.12–14
Despite the lack of placebo, the percentage of improvement in symptoms after 4 weeks of treatment (37.4%) is close to a major pivotal trial using the same endpoint with a placebo-controlled design, and this similarity gives external validation of the present data in very young children, thus enabling the conclusion that rupatadine oral solution may also be effective in this population of young children.16 In addition, in the current study, all symptoms improved individually, at least an average 20% of improvement, even for nasal congestion. Moreover, the improvement obtained in T5SS after 28 days was somewhat higher than after 14 days (mean value 26.6%), showing that there was no tachyphylaxis.
The majority of the patients included in our study were polysensitive, and most of them sensitive to house dust mite. This means that the allergies that affected this group of patients were less dependent on seasonality, and this confounding factor is not likely to have played a relevant role in the improvement of symptoms in our study. In addition, the study was performed over a short duration, during few weeks when no season changes occurred.
The absence of a placebo comparator and the open-label design are limitations of the study that could cause bias and be a confounding factor. It is very difficult to perform placebo comparative clinical trials in young children, as they usually face reticence from both the ethic committees or local health authorities, and this is even more so when the product has shown efficacy in a previous trial in children of a different age group.16 In the past, studies with placebo in infant children were accepted for other indications rather than allergic rhinitis, such as in the ETAC (atopic dermatitis) and EPAC (prevention of asthma) studies with cetirizine.23,24 In AR, there is only 1 previous study that included placebo in AR in young children (2–6 years), along with cetirizine and montelukast.13 Another aspect that made the inclusion of a placebo group difficult was that the study objective was also pharmacokinetic, and the ethics committees considered that it was not appropriate to draw blood samples in very young children who underwent placebo treatment. Due to the difficulties in performing clinical trials in children, in the past, doses have been extrapolated from those used in adults. Recently, a concept paper by the European Medicines Agency (EMA) has been issued in order to establish the basic principles of the scientific validity of the extrapolation concept (EMA/129698/2012). Although it is an accepted practice, it can lead to infra- or supra-dosification in these pediatric populations. Thus, well-designed efficacy trials remain the gold standard for demonstrating the efficacy of a drug in children.
This study shows that rupatadine 1 mg/mL oral solution was safe for administration in children aged 2–5 years. An overall 25% of the 44 involved children suffered an adverse event, but none of them was considered related to the study medication.
Conclusion
This study showed that once-daily rupatadine oral solution was found to be safe and was correlated with an improvement of AR symptoms in young children (2–5 years old) with a magnitude similar to that observed in older ones (6–11 years old). Only very few second-generation antihistamines provide such consistency of data in very young pediatric patients, with a very low incidence of side effects.
Acknowledgments
We would like to thank Josep Giralt for statistical assistance and analysis of results and figure production. We would like to thank Dr Leon Fouché (Limpopo Clinical Research Inititative, Limpopo, South Africa), Dr Lajos Kósa (Svábhegyi Országos, Budapest, Hungary), and Dr Zoltán Novák (Szegedi Tudományegyetem, Szeged, Hungary) for their participation in the study. The study coordinator was Prof Paul Potter (Allergy Diagnostic and Clinical Research Unit, Department of Medicine, University of Cape Town Lung Institute, South Africa). The principal investigators that participated in this study were Dr Jan Vermeulen (Parow Research, Cape Town, South Africa), Dr Leon Fouché (Limpopo Clinical Research Initiative, Limpopo, South Africa), Dr Lajos Kósa (Svábhegyi Országos, Budapest, Hungary), and Dr Zoltán Novák (Szegedi Tudományegyetem, Szeged, Hungary).
Disclosure
Eva Santamaría is currently affiliated with Novella Clinical Ltd. Paul Potter has been a speaker for J Uriach y Compañía, S.A., Iñaki Izquierdo is an employee of J Uriach y Compañía, S.A., Eva Santamaría has been an employee of J Uriach y Compañía, and S.A. Marta Valle and Jan Vermeulen declare no financial or nonfinancial competing interests. The authors report no other conflicts of interest in this work.
References
Mir E, Panjabi C, Shah A. Impact of allergic rhinitis in school going children. Asia Pac Allergy. 2012;2(2):93–100. | ||
Sanchez J, Estarita J, Salemi C. Efecto de la rinitis y el asma en el ausentismo y rendimiento laboral y escolar en una población del trópico latinoamericano [Rhinitis and asthma as a cause of absenteeism and poor work/school performance in a population from Latin-American tropic]. Rev Alerg Mex. 2016;63(1):32–40. Spanish. | ||
Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA[2]LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160. | ||
Hardjojo A, Shek LP, van Bever HP, Lee BW. Rhinitis in children less than 6 years of age: current knowledge and challenges. Asia Pac Allergy. 2011;1(3):115–122. | ||
Kurukulaaratchy RJ, Karmaus W, Raza A, Matthews S, Roberts G, Arshad SH. The influence of gender and atopy on the natural history of rhinitis in the first 18 years of life. Clin Exp Allergy. 2011;41:851–859. | ||
Aït-Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy. 2009;64(1):123–148. | ||
Dong GH, Ren WH, Wang D, et al. Exposure to secondhand tobacco smoke enhances respiratory symptoms and responses to animals in 8,819 children in kindergarten: results from 25 districts in northeast China. Respiration. 2011;81(3):179–185. | ||
Schäfer T, Stieger B, Polzius R, Krauspe A. Associations between cat keeping, allergen exposure, allergic sensitization and atopic diseases: results from the Children of Lübeck Allergy and Environment Study (KLAUS). Pediatr Allergy Immunol. 2009;20(4):353–357. | ||
Clarisse B, Nikasinovic L, Poinsard R, Just J, Momas I. The Paris prospective birth cohort study: which design and who participates? Eur J Epidemiol. 2007;22(3):203–210. | ||
Herr M, Clarisse B, Nikasinovic L, et al. Does allergic rhinitis exist in infancy? Findings from the PARIS birth cohort. Allergy. 2011;66(2):214–221. | ||
Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38(1):19–42. | ||
Sienra-Monge JJ, Gazca-Aguilar A, Del Rio-Navarro B. Double-blind comparison of cetirizine and loratadine in children ages 2 to 6 years with perennial allergic rhinitis. Am J Ther. 1999;6(3):149–155. | ||
Chen ST, Lu KH, Sun HL, Chang WT, Lue KH, Chou MC. Randomized placebo-controlled trial comparing montelukast and cetirizine for treating perennial allergic rhinitis in children aged 2–6 yr. Pediatr Allergy Immunol. 2006;17(1):49–54. | ||
Lutsky BN, Klöse P, Melon J, et al. A comparative study of the efficacy and safety of loratadine syrup and terfenadine suspension in the treatment of 3- to 6-year-old children with seasonal allergic rhinitis. Clin Ther. 1993;15(5):855–865. | ||
Mullol J, Bousquet J, Bachert C, et al. Update on rupatadine in the management of allergic disorders. Allergy. 2015;70(Suppl 100):1–24. | ||
Potter P, Maspero JF, Vermeulen J, et al. Rupatadine oral solution in children with persistent allergic rhinitis: a randomized, double-blind, placebo-controlled study. Pediatr Allergy Immunol. 2013;24(2):144–150. | ||
Marcucci F, Sensi LG, Abate P, et al. Anti-inflammatory activity and clinical efficacy of a 3-month levocetirizine therapy in mite-allergic children. Inflamm Allergy Drug Targets. 2011;10(1):32–38. | ||
Pearlman DS, Lumry WR, Winder JA, Noonan MJ. Once-daily cetirizine effective in the treatment of seasonal allergic rhinitis in children aged 6 to 11 years: a randomized, double-blind, placebo-controlled study. Clin Pediatr (Phila). 1997;36(4):209–215. | ||
de Blic J, Wahn U, Billard E, Alt R, Pujazon MC. Levocetirizine in children: evidenced efficacy and safety in a 6-week randomized seasonal allergic rhinitis trial. Pediatr Allergy Immunol. 2005;16(3):267–275. | ||
Meltzer EO, Scheinmann P, Rosado Pinto JE, et al. Safety and efficacy of oral fexofenadine in children with seasonal allergic rhinitis – a pooled analysis of three studies. Pediatr Allergy Immunol. 2004;15(3):253–260. | ||
Jobst S, van den Wijngaart W, Schubert A, van de Venne H. Assessment of the efficacy and safety of three dose levels of cetirizine given once daily in children with perennial allergic rhinitis. Allergy. 1994;49(8):598–604. | ||
Ngamphaiboon J, Direkwattanachai C, Visitsunthorn N, Vangveeravong M, Tiensuwan M. The efficacy and safety of 30 mg fexofenadine HCl bid in pediatric patients with allergic rhinitis. Asian Pac J Allergy Immunol. 2005;23(4):169–174. | ||
Diepgen TL; Early Treatment of the Atopic Child Study Group. Long-term treatment with cetirizine of infants with atopic dermatitis: a multi-country, double-blind, randomized, placebo-controlled trial (the ETAC trial) over 18 months. Pediatr Allergy Immunol. 2002;13(4):278–286. | ||
Simons FE; Early Prevention of Asthma in Atopic Children (EPAAC) Study Group. Safety of levocetirizine treatment in young atopic children: an 18-month study. Pediatr Allergy Immunol. 2007;18(6):535–542. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
