Back to Journals » Cancer Management and Research » Volume 12
Ropivacaine Inhibits Cell Proliferation, Migration and Invasion, Whereas Induces Oxidative Stress and Cell Apoptosis by circSCAF11/miR-145-5p Axis in Glioma
Authors Yin D, Liu L, Shi Z, Zhang L, Yang Y
Received 31 July 2020
Accepted for publication 23 September 2020
Published 3 November 2020 Volume 2020:12 Pages 11145—11155
DOI https://doi.org/10.2147/CMAR.S274975
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Danqin Yin,1,* Li Liu,2,* Zhengyuan Shi,1 Lihui Zhang,3 Yan Yang4
1Department of Anesthesiology, Danyang People’s Hospital of Jiangsu, Danyang City, Jiangsu Province, People’s Republic of China; 2Department of Anesthesiology, Tianjin Fourth Central Hospital, Tianjin City, People’s Republic of China; 3Department of Anesthesiology, Hulunbeier Municipal People’s Hospital (Hulunbuir Hospital Affiliated to Suzhou University), Hulunbeier City, Inner Mongolia Province, People’s Republic of China; 4Department of Anesthesiology, The First People’s Hospital of Jiangxia District, Wuhan City, Hubei Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Yang
Department of Anesthesiology, The First People’s Hospital of Jiangxia District, No. 1 Cultural Avenue, Jiangxia District, Wuhan 430200, Hubei Province, People’s Republic of China
Tel\Fax +86 13775889005
Email [email protected]
Background: Glioma is a heterogeneous aggressive tumor. Ropivacaine, a widely used anesthetic, has been shown to repress the progression of multiple cancers, including glioma. In this study, the effects of ropivacaine on cell proliferation, migration, invasion and apoptosis in glioma were revealed.
Methods: The expression levels of circSCAF11 and miR-145-5p were detected by quantitative real-time polymerase chain reaction (qRT-PCR) in glioma tissues and cells. The expression levels of epithelial–mesenchymal transition (EMT)-related proteins were determined by Western blot. Oxidative stress was evaluated by the measurement of reactive oxygen species (ROS) and determination of mitochondrial 8-hydroxy-2-deoxyguanosine (8-OHdG) assay in glioma cells. Cell proliferation was determined by cell counting kit-8 (CCK-8) assay and cell colony formation assay. Cell apoptosis and metastasis were detected by flow cytometry analysis and transwell assay, respectively. The binding relationship between circSCAF11 and miR-145-5p was predicted by circular RNA Interactome and identified by dual-luciferase reporter assay and RNA immunoprecipitation assay. In vivo tumor formation assay was performed to reveal the effects between ropivacaine and circSCAF11 overexpression on tumorigenesis in vivo.
Results: CircSCAF11 expression was obviously upregulated and miR-145-5p was significantly downregulated in glioma tissues and cells compared with control groups. Ropivacaine treatment upregulated E-cadherin protein expression and repressed the protein expression of Vimentin. Functionally, ropivacaine exposure promoted ROS and 8-OHdG production and cell apoptosis, whereas inhibited cell proliferation, migration and invasion; however, these effects were hindered by circSCAF11 overexpression. Mechanistically, circSCAF11 was a sponge of miR-145-5p. In addition, ropivacaine was revealed to inhibit tumor growth in vivo by regulating circSCAF11 and miR-145-5p expression.
Conclusion: Ropivacaine suppressed glioma progression by regulating circSCAF11 and miR-145-5p, which might provide a theoretical foundation in glioma treatment.
Keywords: glioma, ropivacaine, circular RNA, circSCAF11, miR-145-5p
Introduction
Glioma, an angiogenic malignant solid tumour, mainly occurs in central nervous system.1 Glioma is recognized as high proliferation and metastasis and is resistant to therapy because of the existence of brain barrier.2,3 Although much attempt has been focused on the glioma treatment, the 5 years survival rate is still unsatisfactory.4
Ropivacaine, one of the safest local anesthetics, is widely used in cancer therapy.5,6 Ropivacaine works via repressing the voltage-gated sodium channels.7 It has been reported that ropivacaine inhibited the cell metastasis of esophageal cancer.8 Yang et al indicated that the combination usage of ropivacaine and lidocaine suppressed cell proliferation in gastric cancer.9 Ropivacaine was revealed to induce cell apoptosis by regulating caspase-3 activity in hepatocellular carcinoma.10 However, the effects of ropivacaine on glioma progression and underneath regulatory mechanism are unclear.
Circular RNAs (circRNAs) are >200 nts in size and mainly serve by sponging microRNAs (miRNAs) and binding proteins.11 Multiple studies investigate that circRNAs participate in cell proliferation, metastasis, apoptosis and cell cycle in glioma. For example, Shi et al indicated circ_0014359 silencing repressed cell proliferation and metastasis in glioma.12 CircHIPK3 also promoted tumor growth in glioma.13 Additionally, Meng et al revealed circSCAF11 knockdown suppressed cell proliferation, invasion and induced cell cycle arrest in glioma.14 But the studies on the ropivacaine-mediated glioma progression regulated by circSCAF11 are unknown.
MiRNA is 18 to 22 nts ncRNA. And miRNA regulates cell function by associating with mRNA. MiRNA is linked to cancer progression, including cell proliferation, migration, apoptosis and so on.15 Studies revealed that miRNA could serve as a biomarker in cancer treatment.16,17 Chen et al explained that miR-145-5p accelerated glioma development by working with circ-PTN.18 Donzelli et al revealed that miR-145-5p promoted brain metastasis.19
Herein, the effects of ropivacaine on glioma progression were studied by CCK-8 assay, measurement of reactive oxygen species (ROS) assay, determination of mitochondrial 8-hydroxy-2-deoxyguanosine (8-OHdG) assay, cell colony formation assay, flow cytometry analysis and transwell assay. CircSCAF11 and miR-145-5p expression were detected by qRT-PCR. Circular RNA Interactome, dual-luciferase reporter assay and RNA immunoprecipitation (RIP) assay were employed to explain the relationship between circSCAF11 and miR-145-5p. Furthermore, in vivo tumor formation assay was performed to study the impacts between ropivacaine and circSCAF11 overexpression on glioma growth in vivo.
Materials and Methods
Sample Collection and Cell Culture
Glioma tissues from glioma patients and normal brain tissues from the patients of traumatic brain edema during underwent partial brain resection were collected from Danyang People’s Hospital of Jiangsu. These tissues were kept in liquid nitrogen. The Ethics Committee of Danyang People’s Hospital of Jiangsu agreed with this experiment. Patients signed the written informed consents.
Glioma cells T98G and LN229 were gotten from Sciencell (Carlsbad, CA, USA). The Otwo Biotech (Shenzhen, China) provided human astrocytes NHA. Glioma cells and NHA cells were cultivated in DMEM (Thermo Fisher, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) as well as 1% penicillin-streptomycin (Thermo Fisher) at 37°C with 5% CO2.
Cell Transfection
The overexpression vector of circSCAF11 (pcDNA-circSCAF11), miR-145-5p mimic, small interfering RNA targeting circSCAF11 (si-circSCAF11), miR-145-5p inhibitor (anti-miR-145-5p) and control groups (pcDNA-NC, miR-NC mimic, si-NC and anti-miR-NC) were amplified by Ribobio Co., Ltd. (Guangzhou, China). PcDNA-circSCAF11 and pcDNA-NC were transfected into cells to determine the effects of circSCAF11 overexpression on ropivacaine-mediated glioma growth in vitro and in vivo. Si-circSCAF11 and anti-miR-145-5p were transfected into cells with their controls to illustrate that whether circSCAF11 regulated the glioma process by sponging miR-145-5p. T98G and LN229 cells were transfected with Lipofectamine 2000 (Thermo Fisher). The primer sequences were pcDNA-circSCAF11 5ʹ-CGGAATTCTAATACTTTCAGGAAACTCTTTATACAGTGAAACAGG-3ʹ (forward), and 5ʹ-CGGAATTCTAATACTTTCAGGAAACTCTTTATACAGTGAAACAGG-3ʹ (reverse); miR-145-5p mimic 5ʹ-GUCCAGUUUUCCCAGGAAUCCCU-3ʹ; miR-NC mimic 5ʹ-UUUGUACUACACAAAAGUACUG-3ʹ; miR-145-5p inhibitor 5ʹ-AGGGAUUCCUGGGAAAACUGGAC-3ʹ; anti-miR-NC 5ʹ-CAGUACUUUUGUGUAGUACAAA-3ʹ; si-circSCAF11 5ʹ-GCTGACAGATGCCCAATAT-3ʹ and si-NC 5ʹ-GCTAGACCCGTAACAGTAT-3ʹ.
Cell Counting Kit-8 Assay
T98G and LN229 cells were cultivated in a 96-well plate for 24 hrs. T98G and LN229 cells treated with 10 μM, 100 μM and 1 mM of ropivacaine were cultured for 24 hrs, 48 hrs and 72 hrs, respectively. After that, 10 μL CCK-8 reagent was added in plate and cells were cultured for 4 hrs. And the absorbance at 450 nm was detected by a microplate reader (Thermo Fisher).
Measurement of Reactive Oxygen Species (ROS)
ROS detection kit (Abcam, Cambridge, UK) was chosen to determine cellular ROS production. T98G and LN229 cells were cultivated in a 96-well plate overnight. T98G and LN229 cells were washed using PBS, followed were treated with 1 mM ropivacaine, pcDNA-circSCAF11, si-circSCAF11 or si-circSCAF11+anti-miR-145-5p with control groups, respectively. Twenty-microliter 2.7-Dichlorofluorescin diacetate (DCFDA) was incubated with cells for 45 min after 24 h. The results were assessed by flow cytometry (BD Biosciences, San Diego, CA, USA).
Determination of Mitochondrial 8-Hydroxy-2-Deoxyguanosine (8-OHdG)
Genomic DNA was extracted from T98G and LN229 cells. The DNA was incubated with DNase I and Nuclease P1, followed was incubated with alkaline phosphatase. Then sample was boiled. The results were assessed using an 8-OHdG ELIZA kit (Abcam).
Cell Colony Formation Assay
T98G and LN229 cells were cultivated in a 6-well plate for 2 weeks. After that, the colonies were incubated with crystal violet. Colony numbers were counted and photographed.
Flow Cytometry Analysis
Apoptosis detection kit (Solarbio, Beijing, China) was chosen to determine the apoptosis rate of T98G and LN229 cells. T98G and LN229 cells was collected at 48 h after cultivation. Cells were rinsed and suspended with 1 mL binding buffer. T98G and LN229 cells were incubated with Annexin V-FITC and propidium iodide. Results were determined by flow cytometry.
Transwell Assay
The transwell chamber was employed to evaluate the abilities of cell migration and invasion without and with Matrigel (Corning, Corning, New York, USA), respectively. T98G and LN229 cells were cultured in the upper chamber with FBS-free DMEM. DMEM containing 20% FBS was added into the down chamber. The supernatant was discarded after 24 h. Then, cells were stained with crystal violet. The metastasis ability was observed by a microscope at a 100× magnification.
Western Blot Assay
T98G and LN229 cells were lysed by RIPA buffer (Beyotime, Jiangsu, China). The lysate was loaded by 12% sodium dodecyl sulfonate-polyacrylamide gel electrophoresis (SDS-PAGE). After that, bands were transferred onto polyvinylidene fluoride membranes (Membrane Solutions, Shanghai, China). Membranes were blocked in 5% skim milk overnight. The bands were incubated with primary antibodies anti-E-cadherin (1:5000; Abcam), anti-Vimentin (1:1000; Abcam) and anti-β-Actin (1:20,000; Abcam). Following, second antibody labeled with peroxidase was chosen to incubate proteins. The results were observed by enhanced chemiluminescence (KeyGen, Nanjing, China). β-Actin was chosen as a reference.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
RNA was extracted from glioma tissues and cells. cDNA was synthesized using a special kit (Takara, Shiga, Japan). For to quantify circSCAF11/SCAF11 and miR-145-5p, a qRT-PCR detection kit (Takara) was employed. GAPDH and U6 were utilized as controls. The sense and anti-sense primers were circSCAF11 5ʹ-CCTCTCATTTCTTCTGTGTTGCC-3ʹ and 5ʹ-ACTCCAATTTGATCTCTGAGGCT-3ʹ; linear SCAF11 5ʹ-CGACCTCGGTCTGAGGAAAC-3ʹ and 5ʹ-GCATCTGTCAGCCTCACTGT-3ʹ; miR-145-5p 5ʹ-TGCCTCCAACTGACTCCTAC-3ʹ and 5ʹ-ACCTCAAGAACAGTATTTCCAGG-3ʹ; GAPDH 5ʹ-GGAAGGTGAAGGTCGGAGTC-3ʹ and 5ʹ-GACGGTGCCATGGAATTTGC-3ʹ; U6 5ʹ-GCTTCGGCAGCACATATACTAAAAT-3ʹ and 5ʹ-CGCTTCACGAATTTGCGTGTCAT-3ʹ.
Actinomycin D and RNase R Treatment Assay
T98G and LN229 cells were cultivated in a 24-well plate after various treatments. After 24 hrs, cells were incubated with actinomycin D (Thermo Fisher) for 0 hrs, 4 hrs, 8 hrs, 12 hrs and 24 hrs. Results were analyzed by qRT-PCR. For RNase R treatment assay, RNA was extracted and incubated with RNase R (Geneseed, Guangzhou, China) at 37°C. And qRT-PCR was employed to assess results.
Dual-Luciferase Reporter Assay
The target sequence of miR-145-5p in circSCAF11 was predicted via circular RNA Interactome. The wide-type (WT) circSCAF11 containing the binding sequence of miR-145-5p was synthesized and inserted into pmirGLO vector (Promega, Madison, WI, USA), and named as WT-circSCAF11. The mutant (MUT) circSCAF11 was synthesized and sub-cloned into pmirGLO vector, and named as MUT-circSCAF11. The WT-circSCAF11 and MUT-circSCAF11 were transfected into T98G and LN229 cells with miR-145-5p or miR-NC mimic with Lipofectamine 2000 (Thermo Fisher). Luciferase activities were detected by the dual-luciferase reporter system after 48 hrs. Ranilla luciferase activity was chosen as a control. The sense and antisense primers of WT-circSCAF11 were 5ʹ-CCGCTCGAGGAAACTCTTTATACAGTGAAACAGG-3ʹ and 5ʹ-ACGCGTCGACAAATGTTCGAAAGATATGGTACT-3ʹ. The synthesized forward and reverse sequences of MUT-circSCAF11 were 5ʹ-CATTTATAGAAGCCAGTGAAATCAGTGCATTGATTAGGCAGAAGAGACATGAACTGGAATTGTCATGGTTTCCTGATACATTACCTGGAATTGGAAGAATTGGTTTTATT-3ʹ and 5ʹ-CTAGAATAAAACCAATTCTTCCAATTCCAGGTAATGTATCAGGAAACCATGACAATTCCAGTTCATGTCTCTTCTGCCTAATCAATGCACTGATTTCACTGGCTTCTATAAATGAGCT-3ʹ.
RIP Assay
T98G and LN229 cells were collected and lysed using RIP lysis buffer (Abcam). Cells were incubated with magnetic beads coated with anti-Ago2 (Abcam) or anti-IgG (Abcam). The beads were washed after 24 hrs and RNA were purified. The results were assessed by qRT-PCR.
In vivo Tumor Formation Assay
Charles River (Beijing, China) provided male nude mice (N=20, 6-week old), and nude mice were averagely divided into 4 groups. Three of these groups were administrated with ropivacaine (40 μmol/kg) via tail vein injection once a day from the day of tumor formation to the end of the experiment. 5×106 cells transfected with pcDNA-circSCAF11 or pcDNA-NC were injected into the right axilla of mice treated with ropivacaine. Tumor volume was measured every 7 days. All mice were euthanized after 28 days. And tumor weight was calculated. The effects between ropivacaine and circSCAF11 overexpression on circSCAF11 and miR-145-5p expression were detected by qRT-PCR. The Animal Care and Use Ethics Committee of Danyang People’s Hospital of Jiangsu agreed with this study. This study complied with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Statistical Analysis
Results were derived from three experiments. GraphPad Prism version 5.0 was chosen to assess data and make figures. Data were showed as means ± standard deviations. Significant differences were compared by two-tailed Student’s t-tests and one-way analysis of variance. Spearman correlation analysis was employed to assess the relationship between circSCAF11 and miR-145-5p. P < 0.05 was considered statistically significant.
Results
Ropivacaine Inhibits Cell Proliferation, Migration and Invasion, Whereas Induces Oxidative Stress and Cell Apoptosis in Glioma
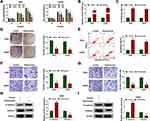
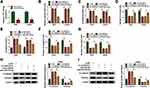
In order to explore the effects of ropivacaine in glioma progression, T98G and LN229 cells treated with 10 μM, 100 μM and 1 mM of ropivacaine were cultured for 24 hrs, 48 hrs and 72 hrs, respectively. CCK-8 assay showed that 10 μM, 100 μM and 1 mM ropivacaine repressed the viability of T98G and LN229 cells at 72 h after ropivacaine treatment and 1 mM ropivacaine inhibited the viability of T98G and LN229 cells at 48 hrs after ropivacaine treatment (Figure 1A); therefore, in subsequent ropivacaine-related study, cells were treated with 1 mM ropivacaine for 48 hrs. Subsequently, ROS measurement assay revealed that 1 mM ropivacaine obviously upregulated ROS level (Figure 1B). 8-OHdG determination assay explained that 1 mM ropivacaine promoted 8-OHdG production (Figure 1C). In addition, cell colony formation assay investigated that the proliferation of T98G and LN229 cells was obviously inhibited by 1 mM ropivacaine (Figure 1D). Flow cytometry analysis showed 1 mM ropivacaine-induced cell apoptosis in T98G and LN229 cells (Figure 1E). Transwell assay illustrated that 1 mM ropivacaine suppressed cell migration and invasion abilities (Figure 1F and G). Furthermore, Western blot was employed to detect the effects of ropivacaine on the expression of epithelial–mesenchymal transition (EMT)-related proteins E-cadherin and Vimentin. Results showed that 1 mM ropivacaine upregulated E-cadherin protein level and downregulated Vimentin protein expression in T98G and LN229 cells (Figure 1H and I). These data demonstrated that ropivacaine repressed glioma progression.
circSCAF11 Expression is Upregulated in Glioma Tissues and Cells
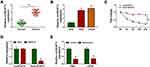
In order to reveal the role of circSCAF11 in glioma, the expression level of circSCAF11 in glioma tissues was firstly detected. QRT-PCR results showed that circSCAF11 expression was obviously upregulated in glioma tissues compared with normal tissues (Figure 2A). Similarly, circSCAF11 expression level was significantly upregulated in T98G and LN229 cells relative to NHA cells (Figure 2B). Actinomycin D treatment assay showed that circSCAF11 level had no obvious change, whereas linear SCAF11 was greatly decreased after actinomycin D treatment (Figure 2C). RNase R treatment assay showed that circSCAF11 expression level was not obviously changed, but linear SCAF11 expression was dramatically decreased after RNase R incubation, which meant that circSCAF11 was more stable than linear SCAF11 (Figure 2D). In addition, the impact of ropivacaine treatment on circSCAF11 expression was detected in T98G and LN229 cells. QRT-PCR results showed that ropivacaine exposure obviously downregulated circSCAF11 expression (Figure 2E). These results showed that ropivacaine might inhibit glioma progression by regulating circSCAF11.
circSCAF11 Overexpression Partially Attenuates the Inhibition Effects of Ropivacaine on Glioma Progression
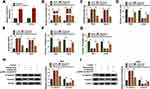
To investigate the functional effects between ropivacaine and circSCAF11 on glioma progression, the overexpression vector of circSCAF11 was built. QRT-PCR results showed that circSCAF11 expression was greatly upregulated after pcDNA-circSCAF11 transfection in T98G and LN229 cells (Figure 3A). Subsequently, ROS measurement assay showed that cellular ROS level was upregulated by ropivacaine treatment, whereas this effect was partially blocked by circSCAF11 overexpression in T98G and LN229 cells (Figure 3B). 8-OHdG determination assay revealed that ropivacaine treatment induced 8-OHdG production and circSCAF11 overexpression hindered this phenomenon in T98G and LN229 cells (Figure 3C). Cell colony formation assay explained that ropivacaine exposure repressed cell proliferation, whereas which was partially attenuated by circSCAF11 overexpression in T98G and LN229 cells (Figure 3D). Furthermore, flow cytometry assay showed that ropivacaine treatment promoted cell apoptosis; however, this effect was decreased by circSCAF11 overexpression (Figure 3E). Transwell assay revealed that the migration and invasion abilities of T98G and LN229 cells were repressed by ropivacaine exposure, whereas circSCAF11 overexpression hindered these effects (Figure 3F and G). Western blot also showed that ropivacaine treatment upregulated E-cadherin protein expression and downregulated Vimentin protein expression, whereas these impacts were impeded by circSCAF11 overexpression in T98G and LN229 cells (Figure 3H and I). These results suggested that circSCAF11 overexpression could decrease the effects of ropivacaine on glioma progression.
circSCAF11 Functions as a Sponge of miR-145-5p
In order to explore the regulatory mechanism of circSCAF11 on glioma progression, the target gene of circSCAF11 was predicted by circular RNA Interactome. Results showed that circSCAF11 contained the binding sites of miR-145-5p (Figure 4A). Subsequently, dual-luciferase reporter assay revealed that the luciferase activity was obviously decreased in WT-circSCAF11+miR-145-5p mimic group in T98G and LN229 cells, whereas there was no significant change in MUT-circSCAF11+miR-145-5p mimic group (Figure 4B). RIP assay showed that circSCAF11 was obviously enriched by anti-Ago2 in T98G and LN229 cells, whereas circSCAF11 was no change in anti-IgG group (Figure 4C). In addition, the effects of circSCAF11 overexpression or circSCAF11 knockdown on miR-145-5p expression were detected. The interfering efficiency of si-circSCAF11 was firstly detected and the result showed that circSCAF11 expression was greatly downregulated by si-circSCAF11 (Figure 4D). QRT-PCR analysis explained that circSCAF11 overexpression inhibited miR-145-5p expression level and circSCAF11 knockdown upregulated miR-145-5p expression (Figure 4E). Then, miR-145-5p expression was detected in glioma tissues and cells. QRT-PCR results showed that miR-145-5p expression was downregulated in glioma tissues and cells compared with control groups (Figure 4F and G). Spearman correlation analysis revealed that circSCAF11 was negatively related to miR-145-5p (Figure 4H). All evidences illustrated that circSCAF11 was associated with miR-145-5p in T98G and LN229 cells.
circSCAF11 Knockdown Represses Cell Proliferation, Migration and Invasion, Whereas Induces Cell Oxidative Stress and Apoptosis by Sponging miR-145-5p
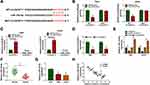
In order to study the effects between circSCAF11 knockdown and miR-145-5p inhibitor on glioma progression, the interfering efficiency of anti-miR-145-5p was firstly detected. QRT-PCR analysis showed that miR-145-5p expression was obviously downregulated after anti-miR-145-5p transfection (Figure 5A). Subsequently, ROS measurement assay revealed that circSCAF11 knockdown upregulated cellular ROS level in T98G and LN229 cells, whereas this effect was decreased by an miR-145-5p inhibitor (Figure 5B). 8-OHdG determination assay showed that circSCAF11 silencing promoted 8-OHdG production, whereas miR-145-5p deletion hindered this promotion effect (Figure 5C). Cell colony formation assay explained that circSCAF11 deletion inhibited the colony-forming ability of T98G and LN229 cells and miR-145-5p inhibitor partially attenuated this effect (Figure 5D). In addition, flow cytometry analysis investigated that circSCAF11 knockdown induced cell apoptosis in T98G and LN229 cells; however, this phenomenon was decreased by an miR-145-5p inhibitor (Figure 5E). Transwell assay demonstrated that circSCAF11 silencing repressed cell migration and invasion abilities, whereas these effects were partially restored by anti-miR-145-5p (Figure 5F and G). Furthermore, Western blot results showed that circSCAF11 deletion promoted the protein expression level of E-cadherin and inhibited Vimentin expression, whereas these effects were hindered by anti-miR-145-5p (Figure 5H and I). Collectively, these data showed that circSCAF11 knockdown repressed glioma progression by associating with miR-145-5p.
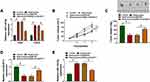
Ropivacaine Represses Tumor Growth in vivo by Downregulating circSCAF11 Expression and Upregulating miR-145-5p Expression
In vivo tumor formation assay was carried out to determine the effects between ropivacaine and circSCAF11 on tumorigenesis. The impacts between ropivacaine and circSCAF11 overexpression on miR-145-5p expression were firstly detected in T98G and LN229 cells. QRT-PCR showed that miR-145-5p expression level was upregulated by ropivacaine treatment and circSCAF11 overexpression partially abolished this effect (Figure 6A). Subsequently, ropivacaine exposure was found to inhibit tumor volume and circSCAF11 overexpression hindered this impact (Figure 6B). QRT-PCR results revealed that ropivacaine treatment decreased tumor weight, whereas circSCAF11 overexpression partially relieved this inhibition effect (Figure 6C). Furthermore, the effects between ropivacaine and circSCAF11 overexpression on circSCAF11 and miR-145-5p expression were revealed in vivo. QRT-PCR analysis showed that ropivacaine treatment downregulated circSCAF11 expression, whereas this impact was hindered by circSCAF11 overexpression (Figure 6D). Ropivacaine was revealed to upregulate miR-145-5p expression; whereas this promotion effect was decreased by circSCAF11 overexpression (Figure 6E). All the above data showed that ropivacaine inhibited glioma growth in vivo by regulating circSCAF11 and miR-145-5p.
Discussion
Ropivacaine, a regional anesthetic, is effective in cancer therapy. Glioma is an epidemic malignant tumor and mainly occurs in people over the age of 18.20 The metastasis and recurrence of glioma pose a burden to people’s life. Herein, we mainly explore the effects of ropivacaine on glioma progression.
Ropivacaine is related to cancer progression. Zhang et al revealed that ropivacaine suppressed cell migration in esophageal cancer.8 Gong et al indicated that ropivacaine hindered cell proliferation and promoted cell apoptotic rate in breast cancer.21 In our view, ropivacaine was explained to repress cell proliferation, metastasis and induced cell apoptosis in glioma. These findings were consistent with previous studies. In addition, ropivacaine also was revealed to promote the production of ROS and 8-OHdG.
CircSCAF11 was found to promote glioma progression. Meng et al investigated that circSCAF11 expression was upregulated in glioma tissues or cells, and that its knockdown-repressed cell proliferation and invasion.14 Similarly, in our studies, circSCAF11 was revealed to be overexpressed in glioma tissues and cells and its silencing repressed cell proliferation and metastasis. Additionally, we also found that circSCAF11 inhibited cell apoptosis and the production of ROS and 8-OHdG, and that circSCAF11 overexpression decreased the inhibition effect of ropivacaine on glioma progression. Furthermore, we also found that circSCAF11 sponged miR-145-5p.
MiR-145-5p was investigated to repress cell proliferation and migration in bladder cancer.22 Chen et al reported miR-145-5p was downregulated and its overexpression promoted cell apoptosis in prostate cancer.23 Wang et al revealed that ectopic miR-145-5p expression hindered the proliferation and migration of hepatocellular carcinoma cells.24 In agreement with their studies, miR-145-5p was indicated to be downregulated in glioma tissues or cells in this study, and its knockdown hindered the inhibition effects of circSCAF11 silencing on cell proliferation and metastasis and the promotion effect of circSCAF11 silencing on cell apoptosis in glioma. These data indicated that miR-145-5p inhibited cell proliferation and metastasis, whereas promoted glioma cell apoptosis. Additionally, miR-145-5p knockdown was revealed to repress ROS and 8-OHdG production.
Collectively, ropivacaine was revealed to inhibit cell proliferation and metastasis, but induced cell apoptotic rate and oxidative stress in glioma. CircSCAF11 overexpression decreased the inhibition effects of ropivacaine on glioma progression in vitro and in vivo. Furthermore, circSCAF11 was investigated to regulate glioma development by associating with miR-145-5p. These data provided a new sight in glioma therapy.
Abbreviations
EMT, epithelial–mesenchymal transition; ROS, reactive oxygen species; 8-OHdG, 8-hydroxy-2-deoxyguanosine; CCK-8, cell counting kit-8; RIP, RNA immunoprecipitation; SDS-PAGE, sodium dodecyl sulfonate-polyacrylamide gel electrophoresis; WT, wide-type; MUT, mutant.
Ethics Approval and Consent to Participate
Written informed consent was obtained from patients with approval by the Institutional Review Board in Danyang People’s Hospital of Jiangsu.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no financial or non-financial conflicts of interest for this work.
References
1. Wang M, Yang C, Liu X, et al. An upstream open reading frame regulates vasculogenic mimicry of glioma via ZNRD1-AS1/miR-499a-5p/ELF1/EMI1 pathway. J Cell Mol Med. 2020. doi:10.1111/jcmm.15217
2. Li J, Zhao J, Tan T, et al. Nanoparticle drug delivery system for glioma and its efficacy improvement strategies: a comprehensive review. Int J Nanomedicine. 2020;15:2563–2582. doi:10.2147/IJN.S243223
3. Xu X, Ban Y, Zhao Z, Pan Q, Zou J. MicroRNA-1298-3p inhibits proliferation and invasion of glioma cells by downregulating Nidogen-1. Aging (Albany NY). 2020;12. doi:10.18632/aging.103087.
4. Xuan C, Jin M, Wang L, et al. PART1 and hsa-miR-429-mediated SHCBP1 expression is an independent predictor of poor prognosis in glioma patients. Biomed Res Int. 2020;2020:1767056. doi:10.1155/2020/1767056
5. Zou Y, He X, Peng Q-Y, Guo Q-L. Inhibition of CD38/cyclic ADP-ribose pathway protects rats against ropivacaine-induced convulsion. Chin Med J (Engl). 2017;130(19):2354–2360. doi:10.4103/0366-6999.215333
6. Siekmann W, Tina E, Von Sydow AK, Gupta A. Effect of lidocaine and ropivacaine on primary (SW480) and metastatic (SW620) colon cancer cell lines. Oncol Lett. 2019;18(1):395–401. doi:10.3892/ol.2019.10332
7. Kao H-W, Lin -Y-Y, Gwathney WJ, Hong K. Formulation and evaluation of multilamellar vesicles ropivacaine in pain management. Int J Nanomedicine. 2019;14:7891–7901. doi:10.2147/IJN.S215952
8. Zhang Y, Peng X, Zheng Q. Ropivacaine inhibits the migration of esophageal cancer cells via sodium-channel-independent but prenylation-dependent inhibition of Rac1/JNK/paxillin/FAK. Biochem Biophys Res Commun. 2018;501(4):1074–1079. doi:10.1016/j.bbrc.2018.05.110
9. Yang W, Cai J, Zhang H, Wang G, Jiang W. Effects of lidocaine and ropivacaine on gastric cancer cells through down-regulation of ERK1/2 phosphorylation in vitro. Anticancer Res. 2018;38(12):6729–6735. doi:10.21873/anticanres.13042
10. Wang W, Zhu M, Xu Z, et al. Ropivacaine promotes apoptosis of hepatocellular carcinoma cells through damaging mitochondria and activating caspase-3 activity. Biol Res. 2019;52(1):36. doi:10.1186/s40659-019-0242-7
11. Zhao W, Dong M, Pan J, et al. Circular RNAs: a novel target among non‑coding RNAs with potential roles in malignant tumors (Review). Mol Med Rep. 2019;20(4):3463–3474. doi:10.3892/mmr.2019.10637
12. Shi F, Shi Z, Zhao Y, Tian J. CircRNA hsa-circ-0014359 promotes glioma progression by regulating miR-153/PI3K signaling. Biochem Biophys Res Commun. 2019;510(4):614–620. doi:10.1016/j.bbrc.2019.02.019
13. Jin P, Huang Y, Zhu P, Zou Y, Shao T, Wang O. CircRNA circHIPK3 serves as a prognostic marker to promote glioma progression by regulating miR-654/IGF2BP3 signaling. Biochem Biophys Res Commun. 2018;503(3):1570–1574. doi:10.1016/j.bbrc.2018.07.081
14. Meng Q, Li S, Liu Y, et al. Circular RNA circSCAF11 accelerates the glioma tumorigenesis through the miR-421/SP1/VEGFA axis. Mol Ther Nucleic Acids. 2019;17:669–677. doi:10.1016/j.omtn.2019.06.022
15. Si W, Shen J, Zheng H, Fan W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin Epigenetics. 2019;11(1):25. doi:10.1186/s13148-018-0587-8
16. Sanjay S, Girish C. Role of miRNA and its potential as a novel diagnostic biomarker in drug-induced liver injury. Eur J Clin Pharmacol. 2017;73(4):399–407. doi:10.1007/s00228-016-2183-1
17. Ma Y. The challenge of microRNA as a biomarker of epilepsy. Curr Neuropharmacol. 2018;16(1):37–42. doi:10.2174/1570159x15666170703102410
18. Chen J, Chen T, Zhu Y, et al. circPTN sponges miR-145-5p/miR-330-5p to promote proliferation and stemness in glioma. J Exp Clin Cancer Res. 2019;38(1):398. doi:10.1186/s13046-019-1376-8
19. Donzelli S, Mori F, Bellissimo T, et al. Epigenetic silencing of miR-145-5p contributes to brain metastasis. Oncotarget. 2015;6(34):35183–35201. doi:10.18632/oncotarget.5930
20. Li J, Li Q, Lin L, et al. Targeting the Notch1 oncogene by miR-139-5p inhibits glioma metastasis and epithelial-mesenchymal transition (EMT). BMC Neurol. 2018;18(1):133. doi:10.1186/s12883-018-1139-8
21. Gong X, Dan J, Li F, Wang L. Suppression of mitochondrial respiration with local anesthetic ropivacaine targets breast cancer cells. J Thorac Dis. 2018;10(5):2804–2812. doi:10.21037/jtd.2018.05.21
22. Zhang H, Jiang M, Liu Q, Han Z, Zhao Y, Ji S. miR-145-5p inhibits the proliferation and migration of bladder cancer cells by targeting TAGLN2. Oncol Lett. 2018;16(5):6355–6360. doi:10.3892/ol.2018.9436
23. Chen Z, Zhen M, Zhou J. LncRNA BRE-AS1 interacts with miR-145-5p to regulate cancer cell proliferation and apoptosis in prostate carcinoma and has early diagnostic values. Biosci Rep. 2019;39(3):BSR20182097. doi:10.1042/BSR20182097
24. Wang B, Dong W, Li X. miR-145-5p acts as a novel tumor suppressor in hepatocellular carcinoma through targeting RAB18. Technol Cancer Res Treat. 2019;18:1533033819850189. doi:10.1177/1533033819850189
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.