Back to Journals » International Medical Case Reports Journal » Volume 13
Role of Topical Cenegermin in Management of a Cornea Transplant in a Functionally Monocular Patient with Neurotrophic Keratitis and Facial Nerve Palsy: A Case Report
Authors Pocobelli A, Komaiha C , De Carlo L, Pocobelli G, Boni N , Colabelli Gisoldi RAM
Received 21 July 2020
Accepted for publication 28 September 2020
Published 11 November 2020 Volume 2020:13 Pages 617—621
DOI https://doi.org/10.2147/IMCRJ.S273234
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Augusto Pocobelli,1 Chiara Komaiha,1 Luca De Carlo,1 Giulio Pocobelli,2 Nicoletta Boni,1 Rossella Anna Maria Colabelli Gisoldi1
1San Giovanni Addolorata Hospital, UOC Oftalmologia – Banca degli occhi, Rome, Italy; 2Department of Experimental Medicine and Surgery, University of Rome Tor Vergata, Ophthalmology Unit, Rome, Italy
Correspondence: Chiara Komaiha
San Giovanni Addolorata Hospital, UOC Oftalmologia – Banca degli occhi, Via Santo Stefano Rotondo, 6, Rome 00184, Italy
Tel +39-0677052950
Email [email protected]
Background: NK is one of the most challenging ocular conditions to treat and it can represent a devastating complication of acoustic neuroma surgery due to the profound corneal anesthesia and concomitant exposure keratopathy caused by seventh nerve palsy. In such cases, cornea surgery should be considered with extreme caution due to the high risk of devastating complications. The purpose of the study is to report the efficacy of a novel human recombinant nerve growth factor (rhNGF)-based ophthalmic treatment in a functionally monocular patient with a recurrence of severe neurotrophic keratitis (NK) on a corneal graft.
Case Presentation: A 24-year-old woman who underwent acoustic neuroma surgery was referred for the assessment of a lagophthalmos and a paracentral corneal ulcer refractory to medical treatment. The patient presented with a large descemetocele, diagnosed as stage 3 NK that required multilayer amniotic membrane transplantation (AMT) and a following optical penetrating keratoplasty (PK). The recurrence of NK on the graft was successfully treated with a cycle of rhNGF (cenegermin 20 μg/mL) eye drops. Due to the complications of a further NK recurrence after treatment discontinuation, a second AMT and PK approach was chosen. A second cycle of treatment with cenegermin was immediately initiated after PK to prevent further recurrences. No postoperative complications were observed and we report a stable situation at 1 year of follow-up.
Conclusion: The case presented here is, to our knowledge, the first report of a treatment with cenegermin for a NK recurrence after PK and suggests that such early medical approach could be evaluated to prevent postoperative complications.
Keywords: neurotrophic keratitis, nerve growth factor, acoustic neuroma, cornea transplant, cenegermin
Background
Acoustic neuroma surgery can result in a dysfunction of trigeminal and facial nerves leading to the development of severe corneal injury due to combined neurotrophic keratitis (NK) and paralytic lagophthalmos.1
NK is the result of an insult to trigeminal corneal innervation and is characterized by reduced or absent corneal sensitivity associated with persistent epithelial defects or corneal ulcers. If untreated, NK progresses to corneal melting and perforation. A concomitant dysfunction of the facial nerve induces an exposure keratopathy that aggravates the course of NK.2,3
In this report, we describe a complex case successfully treated with a novel rhNGF eye drop formulation (cenegermin 20 µg/mL, Oxervate®, Dompé Farmaceutici Spa, Milan, Italy) to manage and prevent complications of surgical approaches.
Nerve growth factor (NGF), naturally released by corneal epithelium, acts directly on corneal epithelial cells to stimulate their growth and survival, maintains limbal epithelial stem cell potential, binds receptors on lacrimal glands to promote tear production and has been experimentally shown to support corneal re-innervation.4
These mechanisms are essential to overcome the degenerative cycle of NK including impaired corneal innervation, loss of corneal sensitivity, reduced reflex blinking and tear production, and corneal epithelial breakdown. Recent multicenter, randomized controlled trials showed that rhNGF ophthalmic solution was safe and effective in treating patients with moderate-to-severe NK. Up to 74% of patients had complete healing of the neurotrophic corneal lesion on treatment and more than 96% of patients were recurrence free after one year.5–7
Case Presentation
A 24-year-old woman was referred for the assessment of a lagophthalmos and a paracentral corneal ulcer refractory to medical treatment in the left eye.
The patient referred a left acoustic neuroma surgical removal, complicated by the development of hydrocephalus that induced a bilateral optic neuropathy and required shunt surgery. She also developed a left facial nerve palsy as an additional complication of acoustic neuroma surgery. Unfortunately, the right eye was more severely compromised by the optic neuropathy, with a pale optic nerve head and a best-corrected visual acuity (BCVA) of 20/400.
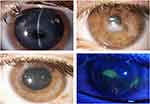
The patient came to our attention with a large paracentral oval descemetocele in the left eye, diagnosed as stage 3 NK,8 that required multilayer amniotic membrane transplantation (AMT) (Figure 1). Three months after surgery, the cornea was still intact but vision was impaired by stromal scarring with a BCVA of 20/400. Due to the optic nerve sub-atrophy in the contralateral eye, after correcting the lagophthalmos with the application of a palpebral gold weight and lateral tarsal strip surgery, it was decided with the patient to proceed with a PK in the left eye.
After surgery the patient received topical treatment with netilmicin 0.3% preservative free eye drop 4 times a day for four weeks and dexamethasone 0.1% preservative free eye drops 6 times a day (gradually reduced over the next 12 months) and lubricants (hyaluronic acid 0.2% 6 times a day). One week post-surgery the patient presented with a slightly dystrophic epithelium, a transparent graft and no inflammatory reaction. However, two weeks post-surgery a central epithelial defect developed, that progressed rapidly to a frank central ulcer despite aggressive lubrication and the application of a therapeutic contact lens. The subsequent onset of peripheral melting required to remove the running suture and replace it with an interrupted suture (Figure 1). Since in the meantime cenegermin 20 µg/mL eye drops, the first topical treatment proven effective in NK, became available we decided to begin topical treatment with this novel drug, which was administered as per protocol 6 times a day for 8 weeks.
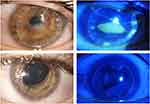
The patient responded well to Oxervate treatment, with a progressive corneal healing and full closure of the corneal lesions at week 5 (Figure 2).
The corneal epithelium remained intact until the end of treatment with cenegermin eye drops, despite a severe evaporative dry eye probably induced by the altered blinking. However, one week after the end of treatment, a recurrence of NK was observed (Figure 2), which was first managed by AMT surgery and later by a tectonic PK due to the persistence of stromal melting..
Due to the previous NK diagnosis and profound corneal anesthesia (0mm by Cochet-Bonnet) measured post-surgery, and following the positive outcome of the previous Oxervate treatment, we decided to prescribe immediately after the surgery a second cycle of this novel medication (starting from the first postoperative day) in presence of a mild epitheliopathy. This earlier postoperative treatment approach was decided to prevent the development of an ulcer and subsequent scarring that would have impaired the patient’s vision again.
The 8-week treatment course was successful, with a fully preserved corneal epithelium integrity. The patient was managed with preservative-free artificial tears in the follow-up (hyaluronic acid 0.2% 6 times a day) and presented at the last visit (12 months after the end of Oxervate treatment) with a stable ocular surface and a BCVA of 20/40 (Figure 2).
Discussion and Conclusions
The impairment of corneal sensory and facial innervation caused by acoustic neuroma surgery induces a reduction of protective reflexes such as blinking and tearing, together with a decrease of trophic support to the avascular cornea. As a result, severe NK can arise as a postoperative complication that is difficult and challenging to treat. Before the advent of rhNGF eye drops, management of NK was based on clinical severity, and the aim of the therapy was to prevent or slow down the progression of corneal damage.2,9,10
Oxervate, the first rhNGF-based treatment approved for NK, has clearly demonstrated its efficacy in masked randomized clinical trials on moderate-to-severe NK patients.5–7
To our knowledge, this is the first case report of a patient with facial nerve palsy and NK on a corneal graft, successfully treated with Oxervate eye drops.
Clinical trial data suggest that one cycle of rhNGF eye drop treatment is sufficient to maintain long-term efficacy (up to 1 year) in the vast majority of patients.7 In this exceptionally severe and complicated case of a functionally monocular patient in whom optic PK was deemed necessary, a recurrence of NK on the graft required a second PK and a second cycle of rhNGF treatment. We speculate that the early use of rhNGF post-surgery allowed to prevent further complications and the patient’s cornea is still healed after 12 months from the end of treatment with good vision and simple medical care with ocular lubricants. These results suggest that the advent of rhNGF eye drops may change the natural history of NK and may represent the first medical treatment to successfully manage severe NK cases complicated by facial nerve palsy and multiple ocular surgery procedures.4
The experience made in cases like the one presented here will allow to establish the most appropriate treatment protocol for these patients, who are difficult to study in controlled settings due to the rarity of their condition.
Abbreviations
rhNGF, recombinant human nerve growth factor; NK, neurotrophic keratitis; AMT, amniotic membrane transplantation; PK, penetrating keratoplasty.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Published research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The institutional approval was not required to publish the case details.
Consent for Publication
The subject gave her written informed consent to publish the case.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding or grant support was received.
Disclosure
AP, RAMCG, LDC, GP, and NB declare that they have no competing interests. CK was a consultant for Dompé Farmaceutici Spa, Milan, Italy until May 2020, reports personal fees from dompè, outside the submitted work, and report no other potential conflicts of interest for this work.
References
1. Portelinha J, Passarinho MP, Costa JM. Neuro-ophthalmological approach to facial nerve palsy. Saudi J Ophthalmol. 2015;29(1):39–47. doi:10.1016/j.sjopt.2014.09.009
2. Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–579.
3. Sohrab M, Abugo U, Grant M, Merbs S. Management of the eye in facial paralysis. Facial Plast Surg. 2015;31(2):140–144. doi:10.1055/s-0035-1549292
4. Sacchetti M, Bruscolini A, Lambiase A. Cenegermin for the treatment of neurotrophic keratitis. Drugs Today (Barc). 2017;53(11):585–595. doi:10.1358/dot.2017.53.11.2722395
5. Ferrari MP, Mantelli F, Sacchetti M, et al. Safety and pharmacokinetics of escalating doses of human recombinant nerve growth factor eye drops in a double-masked, randomized clinical trial. BioDrugs. 2014;28(3):275–283. doi:10.1007/s40259-013-0079-5
6. Bonini S, Lambiase A, Rama P, et al.; REPARO Study Group. Phase I trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1468–1471. doi:10.1016/j.ophtha.2018.03.004.
7. Bonini S, Lambiase A, Rama P, et al.; REPARO Study Group. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1332–1343. doi:10.1016/j.ophtha.2018.02.022.
8. Mackie I. Neuroparalytic keratitis. In: Roy F, Mitchell P, editors. Current Ocular Therapy. Philadelphia, PA: WB Saunders; 1995:452–454.
9. Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves. J Cell Physiol. 2017;232:717–724. doi:10.1002/jcp.25623
10. Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107–131. doi:10.1016/j.preteyeres.2018.04.003
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.