Back to Journals » International Journal of General Medicine » Volume 15
Role of Therapeutic Plasmapheresis in SARS-CoV-2 Induced Cytokine Release Syndrome: A Retrospective Cohort Study on COVID-19 Patients
Authors Jamil Z, Khan AA , Yousuf H, Khalid K, Abbasi SM, Waheed Y
Received 22 February 2022
Accepted for publication 22 April 2022
Published 12 May 2022 Volume 2022:15 Pages 4907—4916
DOI https://doi.org/10.2147/IJGM.S362151
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Zubia Jamil,1 Azmat Ali Khan,2 Hamid Yousuf,3 Kashaf Khalid,4 Shahid Mumtaz Abbasi,5 Yasir Waheed4
1Department of Medicine, Foundation University Medical College, Foundation University Islamabad, Islamabad, 44000, Pakistan; 2Pharmaceutical Biotechnology Laboratory, Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia; 3Department of Medicine, Betsi Cadwaladr University Health Board, Wrexham, North Wales, UK; 4Clinical and Biomedical Research Center, Foundation University Medical College, Foundation University Islamabad, Islamabad, 44000, Pakistan; 5Department of Medicine, Fauji Foundation Hospital, Rawalpindi, 46000, Pakistan
Correspondence: Yasir Waheed, Clinical and Biomedical Research Center, Foundation University Medical College, Foundation University Islamabad, Islamabad, 44000, Pakistan, Email [email protected]
Background: Cytokine release syndrome (CRS) significantly contributes to the pathophysiology and progression of COVID-19. It is speculated that therapeutic plasma exchange (TPE) can dampen CRS via elimination of pathogenic cytokines.
Objectives: The study is intended to compare the outcomes of COVID-19 patients with CRS treated with TPE and standard care (SC) to their counterparts receiving SC alone.
Methodology: A retrospective cohort study of severe COVID-19 confirmed patients presenting with CRS and admitted to the medical ICU was conducted between March and August 2021. Using case-control (CC) matching 1:1, 162 patients were selected and divided into two equal groups. The primary outcome was 28-day in-hospital survival analysis in severe COVID-19 patients with CRS. However, secondary outcomes included the effect of plasmapheresis on inflammatory markers, the need for mechanical ventilation, the rate of extubation, and the duration of survival.
Results: After CC matching, the study cohort had a mean age of 55.41 (range 56.41± 11.56 in TP+SC and 54.42± 8.94 in SC alone; p=0.22). There were 25.95% males and 74.05% females in both groups. The mean time from first day of illness to hospitalization was 6.53± 2.18 days. The majority of patients with CRS had comorbid conditions (75.9%). Diabetes mellitus was the most common comorbidity (40.1%), followed by hypertension (25.3%), and chronic kidney disease (21%). Notable reduction in some inflammatory markers (D-dimers, LDH, CRP and serum ferritin) (p< 0.0001) was observed in the group that received TPE+SC. Moreover, the patients in the plasmapheresis plus standard care group required relatively less mechanical ventilation as compared to the group receiving SC alone (46.9% vs 58.1%, respectively; p> 0.05). The rate of extubation in the TP+SC group vs SC alone was 60.5% vs 44.7%, respectively (p> 0.05). Similarly, the mortality percentages in both groups were 19.8% and 24.7%, respectively.
Conclusion: For this particular group of matched patients with COVID-19-induced CRS, TPE+SC was linked with relatively better overall survival, early extubation, and earlier discharge compared to SC alone. As these results were not statistically significant, multi-centered randomized control trials are needed to further elaborate the role of therapeutic plasmapheresis in COVID-19 induced CRS.
Keywords: coronavirus, therapeutic plasma, cytokines
Introduction
The prevailing COVID-19 pandemic, otherwise known as SARS-CoV-2, has been confirmed in more than 494.6 M people and caused 6.2 M deaths globally.1 Despite the severe negative impact of the disease on the healthcare system, there are still no effective therapeutic treatments.2 Researchers have reported the efficacy of Remdesivir, an antiviral drug previously used against the Ebola virus;3 other studies have shown ineffective results when previously known antiviral drugs were used against severe COVID-19 diseases.4,5
While the cause of severe SARS-CoV-2 syndrome is not fully understood, an excessive inflammatory response typically plays the major role.6 The overly reactive immune response, referred to as cytokine release syndrome (CRS), is an umbrella term that typifies numerous life-threatening clinical manifestations engendered by severe and acute COVID-19 infections, where the immune system starts producing excessive inflammatory signals, thus causing deleterious health effects on the affected host.7 Three key indicators to help identify CRS among affected patients have been reported by Fajgenbaum, et al., namely, pronounced levels of cytokines, acute systemic inflammation, and secondary organ dysfunction.8 Therefore, the severity of COVID-19 infection increases the risk of developing fatal conditions such as acute respiratory distress syndrome, multiple organ failure, and sepsis.
It has been suggested that plasmapheresis, also known as therapeutic plasma exchange (TPE), is a rational treatment modality for patients with severe CRS.9 The TPE method allows substances with large molecular weights to be removed from the body by way of extracorporeal blood purification, thus reversing pathological processes associated with their presence.10 Previously, TPE has been used successfully for the treatment of patients suffering from multiorgan failure and septic shock.11,12 The use of TPE in infectious diseases dates back to the pre-antibiotic era in 1892, when it was successful in treating diphtheria. There have been recent reports showing the therapeutic effects of TPE during SARS-CoV-1 and MERS outbreaks, which resulted in a drop in overall viral loads in a safe manner.13 In light of the available background information, studies are being implemented to investigate the therapeutic effects on COVID-19 patients; the first report about its use in COVID-19 cases was published in March 202014. A plethora of case reports advocating the positive effects of TPE among fulminant COVID-19 infections have been published.15–17 With this study, we seek to assess the efficacy of therapeutic plasmapheresis on 28-day in-hospital mortality among severe COVID-19 cases presenting with CRS.
Materials and Methods
This study was designed primarily to evaluate the role played by therapeutic plasmapheresis on 28-day in-hospital mortality among patients with severe COVID-19 infections and CRS. The effect of plasmapheresis on inflammatory markers, the need for mechanical ventilation, the rate of extubation and survival duration were studied as secondary outcomes.
A retrospective cohort study involved all COVID-19 confirmed patients admitted to the medical ICU of Foundation University Hospital, Islamabad between March and August, 2021 due to severe COVID-19 infection resulting in hypoxemia and increased need for oxygen.
Indication for Plasmapheresis
In accordance with the treatment and diagnosis guidelines of COVID-19 (Trial Version 7) proposed by the NHC of China18 and the guidelines for clinical treatment of the infection suggested by the Ministry of National Health Services, Regulations, and Co-ordinations (NHSRC) of Pakistan,19 TPE was undertaken only in selective COVID-19 patients who had consented and were carefully monitored for safety. The study comprised the following patients:
Patients with COVID-19 infection, as determined by RT-PCR, and who were admitted to the medical ICU for hypoxemia and need for supplemental oxygen. This severe/critical COVID-19 was complicated by CRS.
Among the exclusion criteria were: Patients for whom plasmapheresis was impossible due to absolute or relative contraindications, patients who could not tolerate the central line placement, patients with hemodynamic instability (septic shock or hypovolemic shock), patients with documented Heparin and fresh frozen plasma (FFP) allergies, patients who died within 24 hours of admission, and patients with severe congestive cardiac failure [ejection fraction (EF)<20%].
Study Methods
Foundation University Hospital is a large tertiary care hospital in which a medical ICU was established in March 2020 to respond to the COVID-19 outbreak. Aside from mechanical ventilators, non-invasive ventilators, and oxygen ports, it offers new therapies (convalescent plasma therapy and plasma exchange), as well as pharmacological agents (remdesivir, anticoagulants, steroids, and tocilizumab, etc.). The COVID-19 patients who were hypoxemic and needed oxygen therapy were admitted to the medical intensive care unit. At the time of admission to ICU, each patient was given an admission registration (AR) number. Data about each patient was stored in the hospital’s MEDIX system and could be traced by the AR number.
As various studies have strongly suggested that therapeutic plasmapheresis may be beneficial for COVID-19-induced CRS and our hospital also offers plasmapheresis for selective severe COVID-19 patients with CRS, we decided to conduct a retrospective analysis of the efficacy of TPE on clinical outcomes of severe COVID-19 patients with CRS. Following approval by the hospital’s ethical committee (Letter No. 506/RC/FFH/RWP 20 January 2021), all the medical history data of severe COVID-19 patients admitted to the medical ICU was analyzed. Patients in whom therapeutic plasmapheresis was performed due to CRS (n=91) were placed in a group called ‘therapeutic plasmapheresis with standard care’ (TPE+SC) and those in whom CRS was diagnosed but the patient did not consent to TPE (n=101) were placed in a group called ‘standard care alone’ (SC). Each patient was informed at the time of admission that data collected from them might be used for retrospective or prospective research to contribute to the development of guidelines for the treatment of COVID-19, and that any personal information collected would be protected. Therefore, written informed consent was taken from every patient or their relative at admission to the medical ICU.
Several important terms that are used in the manuscript have been discussed below.
Severe COVID-19 infection is a term that refers to a patient who has symptomatic pneumonia; fever/cough with rapid respiratory rate at 30 bpm or higher, SpO2 ≤ 90% with infiltrates covering more than half of lung fields on a chest X-ray. In contrast, a Critical COVID-19 infection involves SARS-CoV-2 cases with acute respiratory distress syndrome (ARDS) or multi-organ involvement. The Berlin definition of ARDS was used to define acute respiratory distress syndrome (ARDS).20 According to this definition, ARDS refers to the phenomenon where an individual has become infected with COVID-19 and has developed new respiratory symptoms or worsened their existing respiratory symptoms within one week of infection. A chest X-ray reveals bilateral infiltrates and cardiogenic pulmonary edema should be ruled out by echocardiogarphy. ARDS is further divided into three stages; severe; PaO2/FiO2 ≤ 100 mmHg; moderate; paO2/FiO2 100–200 mmHg; mild; paO2/FiO2 200–300 mmHg20 where PaO2/FiO2 was calculated by Horowitz index MDCalc calculator. .
The (Cytokine release syndrome) CRS was defined using national guidelines.19
Patients with severe or critical COVID-19 infection situations with presence of ANY of the following: Ferritin >1000 mcg/L and rising in the last 24 hours.
Ferritin >2000 mcg/L in patients requiring high-flow oxygen or ventilation.
Lymphopenia *800 cells/ml or lymphocyte percentages of 20%
AND any TWO of the following:
CRP >70 mg/L with a rising tendency in the last 24 hours in the absence of bacterial infection.
LDH > 300 U/L and rising over the last 24 hours.
Ferritin >700 ng/ml with a tendency to rise in the last 24 hours.
D-dimers >1000 ng/ml (or >1 mcg/ml) with a tendency to rise in the last 24 hours.
As defined by the hospital protocol, standard care includes the administration of all standard pharmacological agents (anticoagulants, vitamin D, steroids, antivirals, zinc, and vitamin C) to all severe COVID-19 patients admitted to the medical ICU, as well as placement of the patients in a prone position while awake and oxygen administration to maintain SpO2 ≥ 92%.19
TPE was performed under supervision of the apheresis team of the hospital. All patients with CRS were informed about the investigational role of TPE and those who were willing to participate, underwent 5 sessions of therapeutic plasmapheresis on 5 consecutive days after giving written informed consent. The number of sessions was decided by the NHS guidelines21 on plasma exchange, which states that the first exchange removes 50–60% of pathological factors, the second exchange removes an additional 20–30% of pathological factors, the third exchange removes 10% of the remaining factors, the fourth exchange and fifth exchange remove accumulated pathological factors.
An ultrasound-guided double lumen catheter was passed through the femoral vein observing complete aseptic measures. Before each session of plasmapheresis, a complete blood profile, serum calcium levels and coagulation profile were assessed. The plasma volume was calculated by the formula 30–40 mL/Kg. COBE Spectra apheresis machine version 7 (TERUMO BCT, Lakewood co-operation, USA) was used for plasmapheresis. The extracorporeal circuit was primed with 1 L of heparinized saline in order to prevent the filter and circuit from blood clotting. The total calculated plasma volume was removed during each session and, depending on the hemodynamic condition of patients, the flow rate was adjusted from 20 to 30 mL/minutes usually taking 2–4 hours per session. At the end of each session, 75% of plasma volume was replaced by FFP and 25% was replaced by normal saline. Intravenous calcium gluconate was given over 10–15 minutes in patients who were hypo calcemic.21
Statistical Analysis
The statistical analysis was conducted using SPSS (version 26) and MedCalc Statistical Software 19.6.4 (MedCalc software, Ostend, Belgium). Mean, standard deviation and ranges were used for quantitative variables whereas percentages were used to express qualitative variables. Patients with severe COVID-19 infection complicated by CRS were grouped into two categories according to therapeutic plasmapheresis: therapeutic plasmapheresis + SC vs SC alone. Both groups had severe disease with CRS but further case-control matching in a ratio of 1:1 was performed using MedCalC for four variables; age, gender, presence of comorbid conditions and disease severity. After matching, 81 patients were selected in each group who were statistically identical to each other in terms of age, gender distribution, comorbidities and biochemical parameters in order to minimize any confounders affecting the results. Student t-tests and chi-square tests were used for analyzing quantitative and qualitative variables, respectively. For analysis of 28-day in-hospital mortality and median survival duration (from time of onset of illness to death), Kaplan Meier and log rank analysis were employed. The comparison of inflammatory markers pre- and post-plasmapheresis and clinical outcome in terms of rate of mechanical ventilation, rate of extubation and mortality percentages were also calculated. At the end, independent predictors of outcome were determined by Cox-regression analysis.
Results
We conducted this study primarily for the purpose of evaluating the role of therapeutic plasmapheresis on 28-day in-hospital mortality among severe COVID-19 patients with CRS. The effect of plasmapheresis on inflammatory markers, need for mechanical ventilation, rate of extubation and survival duration were also studied as secondary outcomes of this study.
Study Cohort Characteristics
From March to August 2021, 234 COVID-19 patients were admitted to the medical ICU with documented CRS. Among them, 47.4% (n=91) patients received therapeutic plasmapheresis along with standard care and 52.6% (n=101) patients received only standard care. Therefore, a total of 192 patients were included in the study cohort. Case control matching 1:1 was performed and 81 matched patients in both groups were selected (total n=162) as shown in Figure 1.
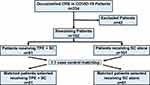 |
Figure 1 Patient selection and matching flowchart. Abbreviations: TPE, therapeutic plasma exchange; CRS, cytokine release syndrome; SC, standard care. |
Therapeutic Plasmapheresis
The mean time from the day of onset of illness to hospitalization was 6.53±2.18 days. In all patients, therapeutic plasmapheresis was initiated within 12 days of illness, with the exception of 1 patient who presented at the 11th day of illness and therapeutic plasmapheresis was initiated on the 13th day. All except 3 patients underwent 5 sessions of plasmapheresis; 2 patients showed notable clinical and biochemical improvement after 3 sessions, 1 patient died after two sessions. No major complications were observed after therapeutic plasmapheresis. Only 2 patients developed a femoral artery puncture that was managed at the bedside by applying pressure and 1 patient developed thrombophlebitis of the femoral vein in whom the subclavian vein was used for plasmapheresis afterwards.
Clinical and Laboratory Parameters for Cases and Controls
Comparison of clinical and biochemical parameters after cross-matching showed that both groups (plasmapheresis plus standard care vs standard care alone) were not statistically different from each other in terms of age, gender, presence of comorbid conditions, biochemical markers and inflammatory markers (p>0.05).
The majority of patients with CRS had co-morbid conditions (75.9%; 123/162). Diabetes mellitus was the most common (40.1%) followed by hypertension (25.3%) and chronic kidney disease (21%). This shows that patients with comorbidities are more likely to develop complications of COVID-19 infection. Table 1 compares the clinical and laboratory profiles of the 2 groups.
Pre- and Post-Plasmapheresis Inflammatory Markers
The inflammatory markers pre and post-therapeutic plasmapheresis were also compared and this showed a reduction in some inflammatory markers (D-Dimers, LDH, CRP and serum ferritin (p<0.0001). PaO2/FiO2 ratio also improved post-plasmapheresis (p<0.0001). The group that received standard care alone showed an improvement in lymphocyte count and PaO2/FiO2 and a reduction in D-dimers level (p<0.05); however, no improvement in other inflammatory markers (p>0.05). This indicates that therapeutic plasmapheresis may remove inflammatory cytokines from the body, hence helping the patients with COVID-19 and CRS.
The comparison of lymphocytes, inflammatory markers and PaO2/FiO2 pre- and post-plasmapheresis in the plasmapheresis plus standard care group vs parameters at the time of admission to the medical ICU and during admission in the standard care alone group is described in Table 2.
Clinical Outcome
Clinical outcome was studied in terms of rate of mechanical ventilation, rate of extubation and mortality percentages in both groups. The patients in the plasmapheresis plus standard care group required less mechanical ventilation as compared to the group on standard care alone (46.9% vs 58.1%, respectively; RR=0.81, 95% CI=0.60–1.09, p>0.05). However, statistics did not show a significant difference between the 2 groups. The rate of extubation in the plasmapheresis plus standard care group vs the standard care alone group was 60.5% vs 44.7%, respectively (RR=0.71, 95% CI=0.45–1.14, p>0.05). Similarly, the mortality percentages in both groups were 19.8% and 24.7%, respectively (RR=0.80, 95% CI=0.45–1.43, p>0.05). Thus, we observed relatively lower rates of mechanical ventilation and mortality with higher rates of extubation and survival in the group receiving therapeutic plasmapheresis plus standard care compared to the group receiving standard care alone in this study although these results are not statistically significant (p>0.05). The comparison of clinical outcome in terms of different clinical parameters (invasive ventilation, rate of extubation, mortality and survival) among the 2 groups plasmapheresis + SC vs SC alone is depicted in Table 3.
Survival Analysis
Overall, the mortality rate among 162 patients admitted with CRS was 22.2% (36/162) while 77.8% (126/162) patients survived. The 28-day mortality was 19.8% (16 out of 81 patients) for cases receiving plasmapheresis plus standard care and 24.7% (20 out of 81 patients) for patients receiving standard care alone (HR=0.80, 95% CI=0.45-0.43, p=0.45). The overall survival duration (from onset of illness to death) was 24 days for the plasmapheresis plus standard care group (HR=0.70, 95% CI=0.36–1.37); for the standard care alone group, the median survival duration was 18 days (HR=1.42, 95% CI=0.73–2.76). Although the median survival time was greater in the plasmapheresis plus standard care group, this difference was not statistically significant (Log rank x2=1.06, p=0.30). The Kaplan–Meier analysis curve exhibiting the general survival likelihood among the two groups of the study cohort (plasmapheresis + SC vs SC alone) is denoted in Figure 2.
Regression Analysis
To categorize predictors of outcomes among COVID-19 patients with CRS, regression analysis was applied. The linear regression analysis begins with identification of statistically important predictors. Advancing age, low platelet count, high Troponin T levels and presence of multiple comorbid conditions were found to be predictors of survival in these patients. Afterward, Cox-regression was used to analyze the predictors. This model covered 81.5% of classified cases and 16% to 24% variation of variables (Cox and Snell pseudoR2 and Nagelkerke pseudoR2 respectively). This model was fitted statistically to analyze the predictors (Chi-square test=34.83, p=0.00) according to Omnibus tests. Upon analyzing the plasmapheresis in a regression analysis, we did not find therapeutic plasmapheresis to be a positive predictor of survival of COVID-19 patients with CRS (p>0.05). The various variables affecting the survival of COVID-19 patients presenting with CRS are denoted in Table 4.
 |
Table 4 Cox Regression Analysis Showing Variables in Predicting Survival of Patients with COVID-19 Complicated by CRS |
Discussion
Therapeutic plasma exchange treatment, when used in conjunction with standard care (including steroids) among COVID-19 patients with CRS might have survival benefits. Only a few treatments have been demonstrated to reduce the morbidity and mortality caused by COVID-19. To reduce the rate of fulminant COVID-19 infections, it is necessary to rigorously evaluate the existing treatment methods, among which TPE has been postulated to be a viable solution. Treatment with TPE shortens hospital stays and decreases the severity of CRS. An overlapping picture of usage of TPE in COVID-19 infections complicated by CRS has been reported by Kamran et al.22 The study outcome demonstrated that TPE reduced inflammatory markers, improved oxygenation and ameliorated the clinical course of COVID-19. We used case control matching analysis to match TPE and SC treatment groups more specifically to neutralize the influence of treatment choice biases. Several variables given in Table 1 had to be matched in both arms prior to investigation, which strengthens the conclusion. Statistical analysis showed that both groups (TPE+SC and SC alone) did not differ significantly with regards to age, gender, presence of comorbid conditions, biochemical markers and inflammatory markers (p>0.05).
It was found out that patients with comorbidities were prone to developing CRS. A study by Zhou et al. reported that 48% of patients with COVID-19 had a comorbidity, with hypertension as the leading health problem (30%), followed by diabetes (19%) and coronary heart disease (8%).23 A meta-analysis of the global position has also reported the role of comorbid conditions (such as hypertension and diabetes) in inducing the cytokine storm and thus increasing the fatality rate among COVID-19 patients.24 While using TPE as a treatment option for COVID-19 patients, there were no apparent ill-effects such as coagulopathy, worsening renal or cardiac function, and allergies which could be related to TPE’s immunosuppressive properties. In general, this effect of TPE is reported to be a result of the body being deprived of circulating immune factors, thus significantly suppressing immunity.21 Therefore, in our study, plasmapheresis resulted in a notable increase in lymphocyte count and decrease in some inflammatory biomarkers. The standard care alone group also exhibited a reduction in D-dimer level. The possible explanation is that D-dimer is not only an inflammatory marker but is also a prime marker for thromboembolism. Anti-coagulants have demonstrated their role in the COVID-19-induced hypercoagulable state by reducing D-dimer levels.25 A similar increase in the PaO2/FiO2 ratio occurred in the standard care alone group as different modes of oxygen delivery improved hypoxemia in these patients.26
Furthermore, clinical outcome was studied in terms of rate of mechanical ventilation, rate of extubation and mortality percentages in both groups. Although statistically insignificant, we observed lower rates of mechanical ventilation and mortality along with the higher rates of extubation and survival in the group receiving therapeutic plasmapheresis plus standard care compared to the group receiving standard care alone in this study. Our results are much more comparable to the study by Kamran, et al.22 They conducted a study to evaluate the role of plasma exchange among severe COVID-19 patients with CRS. Despite a small sample size (n=45+45), the study was unique in that it presented data on patients with CRS who underwent plasma exchange. In their study, they found that nearly 48% patients with CRS required mechanical ventilation. Whereas in our study, 46.9% patients in the TP + SC group needed invasive ventilation compared to the SC group in which higher rates were observed (58.1%). The survival rate in the TP + SC group is 80.2% in our study, which was higher compared to the SC group alone in which 75.3% patients survived. Comparing these results to Kamran, et al.,22 the overall survival rate was around 83% in patients with therapeutic plasmapheresis compared to only 46% in patients who had not received plasmapheresis. Although a difference in the sample size could be affecting the results, the overall survival rate is around 80% in both studies. Our mortality analysis revealed that the 28-day mortality was 19.8% for patients with TP + SC and 24.7% for patients with SC alone (p>0.05).
The overall survival duration (from the onset of illness to death) was only 18 days for the SC-alone group, yet it was 24 days for the TP plus SC group. This finding indicates that therapeutic plasmapheresis has a relatively better impact on survival outcomes (18 vs 24 days) for COVID-19 patients with severe CRS but findings are statistically insignificant. Linear regression did not indicate the plasmapheresis to be an important predictor of survival. We should consider some important limitations of the study that might improve these results further. Firstly, the retrospective nature of the study; secondly, a need for more multi-centered randomized controlled trials with a greater number of patients; thirdly, use of continuous hemofiltration instead of centrifugal therapeutic plasmapheresis, which is used in our study;27 fourthly, early initiation of plasmapheresis within 48 hours of onset of cytokine release syndrome. In a nutshell, therapeutic plasmapheresis may be a beneficial modality if used early in the course of disease in severe COVID-19 patients presenting with cytokine release syndrome by decreasing the need for ventilation and enhancing overall survival outcome, along with increasing the extubation rate.
Conclusion
For the particular group of patients with COVID-19-induced CRS, Therapeutic plasmapheresis + Standard care may be linked with relatively better overall survival, reduced levels of some inflammatory cytokines, early extubation, and earlier discharge compared to Standard care alone. As these results were statistically insignificant in this study, further multi-centered randomized control trials are needed to elaborate the role of therapeutic plasmapheresis in COVID-19 induced CRS before incorporating this modality as possible choice of management in COVID-19 induced CRS.
Ethics Statement
Ethical approval was obtained from the ethical review committee of Fauji Foundation Hospital, Rawalpindi, Pakistan (letter No. 524/RC/FFH/RWP, 4th January 2021). The study was conducted in compliance with the Declaration of the Helsinki.
Informed Consent Statement
All participants gave informed consent to take part in the study.
Acknowledgments
AAK's work is funded by the Researchers Supporting Project (No. RSP-2021/339), King Saud University, Riyadh, Saudi Arabia. All patients in the study were panel patients of Fauji Foundation Hospital, Rawalpindi, who received treatment, consultations, labs, etc., funded by FFH. We acknowledge all the hospital faculty, trainees and staff who performed duties in the HDU during the COVID-19 pandemic. The authors are thankful to MedCalc software limited, Ostend, Belgium, for providing free MedCalc software that significantly helped in statistical analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. WHO coronavirus (COVID-19) dashboard | WHO coronavirus (COVID-19) dashboard with vaccination data.
2. Basiri A, Pazhouhnia Z, Beheshtizadeh N, Hoseinpour M, Saghazadeh A, Rezaei N. Regenerative medicine in COVID-19 treatment: real opportunities and range of promises. Stem Cell Rev Reports. 2021;17(1):163–175. doi:10.1007/S12015-020-09994-5/TABLES/1
3. Jiang Y, Chen D, Cai D, Yi Y, Jiang S. Effectiveness of remdesivir for the treatment of hospitalized COVID-19 persons: a network meta-analysis. J Med Virol. 2021;93(2):1171–1174. doi:10.1002/JMV.26443
4. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 — final report. N Engl J Med. 2020;383(19):1813–1826. doi:10.1056/NEJMOA2007764
5. Young B, Tan TT, Leo YS. The place for remdesivir in COVID-19 treatment. Lancet Infect Dis. 2021;21(1):20–21. doi:10.1016/S1473-3099(20)30911-7
6. Merad M, Subramanian A, Wang TT. An aberrant inflammatory response in severe COVID-19. Cell Host Microbe. 2021;29(7):1043–1047. doi:10.1016/J.CHOM.2021.06.018
7. Li X, Shao M, Zeng X, Qian P, Huang H. Signaling pathways in the regulation of cytokine release syndrome in human diseases and intervention therapy. Signal Transduct Target Ther. 2021;6(1):1–16. doi:doi:10.1038/s41392-021-00764-4
8. Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383(23):2255–2273. doi:doi:10.1056/NEJMRA2026131
9. Memish ZA, Faqihi F, Alharthy A, Alqahtani SA, Karakitsos D. Plasma exchange in the treatment of complex COVID-19-related critical illness: controversies and perspectives. Int J Antimicrob Agents. 2021;57(2):106273. doi:doi:10.1016/J.IJANTIMICAG.2020.106273
10. Kaplan AA. Therapeutic plasma exchange: a technical and operational review. J Clin Apher. 2013;28(1):3–10. doi:doi:10.1002/JCA.21257
11. Keith PD, Wells AH, Hodges J, Fast SH, Adams A, Scott LK. The therapeutic efficacy of adjunct therapeutic plasma exchange for septic shock with multiple organ failure: a single-center experience. Crit Care. 2020;24(1):1. doi:doi:10.1186/S13054-020-03241-6
12. Knaup H, Stahl K, Schmidt BMW, et al. Early therapeutic plasma exchange in septic shock: a prospective open-label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers. Crit Care. 2018;22:1. doi:doi:10.1186/S13054-018-2220-9
13. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. doi:doi:10.1172/JCI138003
14. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Available from: https://jamanetwork.com/journals/jama/article-abstract/2763983.
15. Alharthy A, Faqihi F, Balhamar A, Memish ZA, Karakitsos D. Life-threatening COVID-19 presenting as stroke with antiphospholipid antibodies and low ADAMTS-13 activity, and the role of therapeutic plasma exchange: a case series. SAGE Open Med Case Reports. 2020;8:2050313X20964089. doi:doi:10.1177/2050313X20964089
16. Faqihi F, Alharthy A, Memish ZA, Kutsogiannis DJ, Brindley PG, Karakitsos D. Peripheral neuropathy in severe COVID-19 resolved with therapeutic plasma exchange. Clin Case Reports. 2020;8(12):3233–3238. doi:doi:10.1002/CCR3.3397
17. Dogan L, Kaya D, Sarikaya T, et al. Plasmapheresis treatment in COVID-19–related autoimmune meningoencephalitis: case series. Brain Behav Immun. 2020;87:155–158. doi:doi:10.1016/J.BBI.2020.05.022
18. National Health Commission & National Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J. 2020;133(9):1087. doi:doi:10.1097/CM9.0000000000000819
19. Clinical Management Protocol Covid-19. Guidelines Clinical Management Guidelines for COVID-19 Infections; 2020. Available from: https://covid.gov.pk/new_guidelines/5.June.2020_Clinical_Management_Guidelines_for_COVID-19_infection_v3_cleaned_1-6-20_for_submission.pdf.
20. Fanelli V, Vlachou A, Ghannadian S, Simonetti U, Slutsky AS, Zhang H. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis. 2013;5(3):326. doi:10.3978/J.ISSN.2072-1439.2013.04.05
21. Department Of Critical Care Clinical Guideline. Guidelines for therapeutic plasma exchange in critical care; 2016. Available from: https://www.bsuh.nhs.uk/library/wp-content/uploads/sites/8/2020/09/TPE.pdf.
22. Kamran SM, Mirza ZE, Naseem A H, et al. Therapeutic plasma exchange for coronavirus disease-2019 triggered cytokine release syndrome; a retrospective propensity matched control study. PLoS One. 2021;16(1):e0244853. doi:10.1371/JOURNAL.PONE.0244853
23. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
24. Ng WH, Tipih T, Makoah NA, et al. Comorbidities in SARS-CoV-2 patients: a systematic review and meta-analysis. MBio. 2021;12(1):1–12. doi:10.1128/mBio.03647-20
25. Jamil Z, Khan AA, Khalid S, Asghar M, Muhammad K, Waheed Y. Beneficial effects of anticoagulants on the clinical outcomes of COVID-19 patients. Antibiot. 2021;10:1394. doi:10.3390/ANTIBIOTICS10111394
26. Jamil Z, Khalid S, Abbasi SM, Waheed Y, Ahmed J. Clinical outcomes of moderate to severe COVID-19 patients receiving invasive vs. non-invasive ventilation. Asian Pac J Trop Med. 2021;14(4):176. doi:10.4103/1995-7645.312518
27. Lee PA, Weger GW, Pryor RW, Matson JR. Effects of filter pore size on efficacy of continuous arteriovenous hemofiltration therapy for Staphylococcus aureus-induced septicemia in immature swine. Crit Care Med. 1998;26(4):730–737. doi:10.1097/00003246-199804000-00024
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




