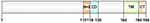Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Role of the HIF-1α/BNIP3 Signaling Pathway in Recurrent Hepatocellular Carcinoma and the Mechanism of Traditional Chinese Medicine
Authors Liu S , Kang L, Song Y, Miao M
Received 9 March 2023
Accepted for publication 1 June 2023
Published 8 June 2023 Volume 2023:10 Pages 893—908
DOI https://doi.org/10.2147/JHC.S409292
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Sizhe Liu, Le Kang, Yagang Song, Mingsan Miao
School of Pharmacy, Henan University of Chinese Medicine, Zhengzhou, 450046, People’s Republic of China
Correspondence: Mingsan Miao, School of Pharmacy, Henan University of Chinese Medicine, Zhengzhou, 450046, People’s Republic of China, Tel/Fax +86-371-65962546, Email [email protected]
Abstract: Recurrence of hepatocellular carcinoma (HCC) negatively affects the quality of life of patients and leads to death. Studies have shown that recurrent hepatocellular carcinoma (RHCC) is closely related to tissue hypoxia and autophagy. It has been shown that hypoxia-inducible factor-1α (HIF-1α) and its downstream factor BCL-2 19 kDa-interacting protein 3 (BNIP3) promote cellular autophagy under hypoxic conditions, resulting in metastasis and RHCC. In this article, the molecular structures of HIF-1α and BNIP3 are described, and the significance of the HIF-1α/BNIP3 signaling pathway in RHCC is explained. Moreover, the role and mechanism of traditional Chinese medicine (TCM) in treating RHCC by modulating the HIF-1α/BNIP3 signaling pathway is discussed. Studies have shown that the HIF-1α/BNIP3 signaling pathway is a potential target of TCM in the treatment of RHCC. The mechanism of the HIF-1α/BNIP3 signaling pathway in RHCC and the progress achieved in TCM research on targeting and regulating this pathway are also reviewed in this article. The objective was to provide a theoretical basis for the prevention and treatment of RHCC, as well as further drug development.
Keywords: recurrent hepatocellular carcinoma, HIF-1α/BNIP3 signaling pathway, traditional Chinese medicine, hypoxia, autophagy
Introduction
Hepatocellular carcinoma (HCC) is the seventh most common type of cancer and the second leading cause of cancer-related mortality worldwide.1 In China, >300,000 individuals expire due to HCC annually, accounting for approximately half of the liver cancer-related deaths globally.2 Hepatitis B infection remains the main cause of HCC globally, while hepatitis C infection and nonalcoholic steatohepatitis associated with a high-fat diet are the major causes of HCC in Western countries.3 Alcohol dependence, smoking, old age, obesity, insulin resistance, and type 2 diabetes are also risk factors for HCC.4 The invasive potential, metastasis, lower surgical resection rate, higher recurrence rate, and insensitivity to chemotherapy are fundamental causes of the higher mortality rate linked to HCC compared with other types of cancer.5 The higher recurrence rate of HCC after surgery is the main reason affecting the long-term survival rate of patients. Patients with HCC are at a 60–70% risk of recurrence after radical liver cancer surgery, and the 5-year survival rate after surgery is <30%.6 Multiple factors influence the development of postsurgical recurrent hepatocellular carcinoma (RHCC). Oxidative stress is an important factor in RHCC.7 Intratumorally hypoxia is a crucial feature in all solid tumors, particularly HCC.8 The tumor hypoxic microenvironment is able to induce cascading metabolic changes in hypoxia-adapted HCC cells through hypoxia-inducible factor (HIF).9 Alpha-functional subunit of HIF (HIF-1α) is a major transcription factor in the hypoxic response and able to activate the transcription of the gene encoding BCL-2 19 kDa-interacting protein 3 (BNIP3), thereby inducing mitochondrial selective autophagy.10 BNIP3 is a stress sensor protein strongly induced by hypoxia. BNIP3 containing hypoxia-responsive element (HRE) is a direct target of HIF-1α and implicated in hypoxia-induced cell death.11,12 In this review, the currently available data on the interaction between HIF-1α and BNIP3 in the development and progression of RHCC are discussed. In addition, the role of the HIF-1α/BNIP3 signaling pathway in RHCC and the progress achieved in traditional Chinese medicine (TCM) research on targeting and regulating this pathway are also reviewed.
Biological Properties of HIF-1α/BNIP3
HIF-1α Structure and Characteristics
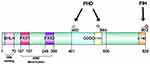
HIF-1 is an oligonucleotide sequence of a protein specifically bound to the erythropoietin gene enhancer. It was found in nuclear extracts of hypoxic HCC cell line Hep3B by Semenza et al in 1992.13 HIF-1 is a member of the basic helix-loop-helix–PER-ARNT-SIM (bHLH–PAS) protein family and composed of α and β subunits.14 The bHLH–PAS is a class of dimeric transcription factors with diverse functions, that are highly conserved in vertebrates and invertebrates.15 These proteins exhibit a relatively conserved structural domain structure, while sharing a common arrangement of structural domains: a bHLH DNA-binding domain; tandemly positioned PAS domains (PAS-A and PAS-B); and a variable trans-activating domain (TAD) or trans-repressive domain (TRD). bHLH domains are usually located at the N terminus of the protein and are responsible for DNA binding and dimerization by two alpha helices.16 This is followed by a PAS structural domain comprising two structurally conserved regions.17
The α-subunit is the functional subunit of HIF-1; it is sensitive to the concentration of oxygen and determines the activity of HIF-1. HIF-1α is a 120-kDa polypeptide subunit containing an 826 amino acid oxygen-regulated polypeptide. The gene is located at 14q21-24 and has a DNA sequence of 3958 bp in length.18,19 HIF-1α contains four structural domains: bHLH; PAS; oxygen-dependent degradation domain (ODDD); and TAD. TAD consists of a transcriptional activation domain with the n-terminal transcriptional activation domain (N-TAD) and a c-terminal transcriptional activation domain (C-TAD), where the N-TAD partially overlaps with the ODDD. The bHLH is a structural domain of HIF-1 that heterodimerizes and binds to DNA, containing arginine and lysine residues. It is a structural domain of HIF-1 that activates gene transcription, particularly for genes involved in angiogenesis and epithelial–mesenchymal transition (EMT), by binding to HRE sequences in the promoters of different genes. PAS is a target gene-specific structural domain that mediates interprotein interactions upon stimulation or binds cofactors in its hydrophobic core to regulate interprotein interactions. The PAS structural domain consists of two very conserved hydrophobic repeats, termed PAS-A and PAS-B, spaced by a less conserved sequence. Under hypoxic conditions, the PAS-B structural domain of HIF-1α heterodimerizes with PAS-B of ARNT. Notably, PAS-B is involved in transcriptional activation via a basic helix-loop-helix. ODDD is mainly associated with the ubiquitin-proteasome degradation pathway, and the region between the two TAD sequences is a repressive structural domain that mainly inhibits the transcriptional activity of TAD. The remaining regulatory regions include the nitrogen- and carbon-terminated nuclear localization signaling domains, proline-serine-threonine-rich protein stabilization domain, and the transcriptional repressor region.20 The structure of HIF-1α is shown in Figure 1.
BNIP3 Structure and Characteristics
BNIP3 is a stress sensor protein strongly induced by hypoxia and associated with autophagy and apoptosis.21 It mediates apoptosis in a cysteine-dependent or cysteine-independent manner and promotes autophagy causing cell death through various mechanisms in different cells. BNIP3 belongs to the BCL-2 family and contains a BCL-2 homology structural domain 3 (BH3). The BNIP3 protein consists of 194 amino acids, has a predicted molecular weight of 21.5 kDa, is located at locus 10q26.3, and comprises six exons of approximately 15 kilobases in size.22 It consists of four major structural domains: proline-glutamic acid-serine-threonine-rich structural domain; BH3 structural domain; conserved structural domain; and transmembrane (TM) structural domain. The BH3 structural domain in BNIP3 is a functional structural domain of BNIP3, which forms a heterodimer with the anti-apoptotic molecules BCL2 and BCL-XL, further enabling the freeing and activation of BCL2 associated X (BAX) and BCL2 antagonist/killer (BAK). BAX and BAK are key regulators of the apoptotic mitochondrial pathway, prompting the release of cytochrome C from mitochondria and activating the caspase 9-dependent apoptosis pathway. The BH3 structural domain plays a dominant role in the caspase-dependent mitochondrial apoptotic pathway mediated by BNIP3. However, it is not required for the induction of cell death. Unlike deletion of the BH3 domain, deletion of the TM domain prevents BNIP3-induced cell death. The TM domain is required for dimerization, mitochondrial targeting, and pro-apoptotic activity, and plays a role in the promotion of cell death by BNIP3. When the cell receives foreign apoptotic signals or introduces exogenous BNIP3, BNIP3 forms dimers through the TM domain and is dispersed on the outer mitochondrial membrane surface. After binding tightly to the outer mitochondrial membrane, it opens the mitochondrial permeability transition pore (MPTP). This leads to mitochondrial depolarization and a decrease in the proton electrochemical gradient, followed by chromosome condensation and DNA breakage. This process can be blocked by MPTP inhibitors; however, caspase inhibitors are unable to inhibit the apoptotic process.23 The structure of BNIP3 is illustrated in Figure 2.
Transduction of the HIF-1α/BNIP3 Signaling Pathway
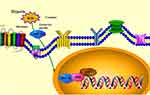
Hypoxia-mediated apoptosis is closely associated with the induction of pro-death BCL-2 family proteins through stimulation of HIF-1.24 BNIP3, which belongs to the BCL-2 family, is a downstream factor of HIF-1α. In vivo and in vitro experiments have shown that it is associated with the initiation of autophagy. BNIP3 is a target molecule of HIF-1α and can be induced by hypoxia or ischemia.25 Under normoxic conditions, HIF-1α is rapidly hydrolyzed by the hypoxia-inducible factor proline hydroxylase (HPH). Following a decrease in oxygen concentration, HPH is inactivated and HIF-1α expression is increased. The bHLH structural domain in HIF-1α is able to bind HRE sequences in different gene promoters. This structure is an important target for the transcriptional regulation of HIF-1.26 The BNIP3 promoter region contains two HREs. HIF-1α binds tightly to HRE2 under hypoxic conditions, thereby stimulating BNIP3 expression.27 Zhang et al28 determined the area of myocardial tissue damage in rats after coronary artery ligation by hematoxylin-eosin staining, the cell viability level of myocardial tissue by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) method, and the expression of HIF-1α and BNIP3 proteins in rat myocardial tissue by Western blotting (WB). It was confirmed that the HIF-1α/BNIP3 pathway could activate mitochondrial autophagy and cause myocardial injury. He et al29 determined the expression levels of HIF-1α and BNIP3 in osteosarcoma cells by co-immunoprecipitation combined with WB. A tibial in situ operating system model was also established in nude mice, and the survival rate was recorded. Moreover, the number of lung lymph nodes in mice was counted by microscopic observation. It was shown that the HIF-1α/BNIP3 pathway inhibits the growth and metastasis of osteosarcoma through the downregulation of β-linked protein. HIF-1α may maintain cell survival by activating the downstream protein BNIP3 and, subsequently, inducing mitochondrial autophagy. It has been shown that HIF-1α activates a functional hypoxia response element (HRE) within the BNIP3 promoter. In hypoxia, the proteasomal degradation of HIF-1α is reduced, leading to the accumulation and translocation of HIF-1α to the nucleus. In the nucleus, HIF-1α binds to the HRE within the BNIP3 promoter to activate the transcription of pro-death target genes.
Zhu et al30 collected serum samples from neonates with hypoxic-ischemic encephalopathy and healthy full-term neonates within 24 h of birth. They measured the levels of BNIP3 in serum using the enzyme-linked reaction adsorption assay. The results showed that the concentration of BNIP3 in serum was significantly higher in neonates with hypoxic-ischemic encephalopathy versus healthy neonates (P<0.001). Under hypoxic conditions (1%), the levels of BNIP3 mRNA and protein were significantly increased in a time-dependent manner. Following pretreatment with a HIF1α inhibitor and hypoxia, BNIP3 expression was significantly lower than that observed in cells cultured under hypoxic conditions only. The number of apoptotic AGE1.HN cells was significantly increased after BNIP3 treatment (P<0.05). After pretreatment with HIF1α or HIF2α inhibitors and hypoxic culture, BNIP3 expression was significantly lower than that noted in cells cultured under hypoxia only. The results of this clinical trial support the ability of HIF-1α to modulate BNIP3 and induce neuronal injury under hypoxic conditions. BNIP3 has limited homology to the BCL-2 family in the BH3 and C-terminal TM structural domains. However, unlike other BH3-containing proteins, deletion of the BH-3 structural domain in BNIP3 does not eliminate its pro-apoptotic activity. The TM structural domain is essential for the translocation of BNIP3 to mitochondria, where it neutralizes its anti-apoptotic function with the pro-survival proteins BCL-2 and BCL-X heterodimerization L. In addition, it has been shown that HIF-1 also responds to various non-hypoxic stimuli, including vasoactive peptides, cytokines, and hormones. These stimuli can initiate the production of reactive oxygen species, which subsequently activate the HIF-1 cascade response. Thus, non-hypoxic stimuli can also activate HIF-1α through redox signaling by activating specific kinases or inactivating phosphatases. BNIP3 acts as a receptor for mitochondrial autophagy and induces autophagy through direct binding to LC3 (microtubule-associated protein one light chain 3)31 Figure 3 depicts the mechanism of HIF-1α/BNIP3 signaling.
Role of the HIF-1α/BNIP3 Signaling Pathway in RHCC
HCC is characterized by a high recurrence rate.32 RHCC is primarily caused by cellular autophagy and hypoxia.33 Tumor cells gain energy through autophagy to enhance their survival and promote outward cell migration34 Autophagy can induce changes in cell adhesion signaling and promote tumor cell invasion and migration. Moreover, tumor cells contain specific inhibitory Focal adhesion kinase (FAK) that activates Src family kinase (SRC), thereby inhibiting autophagy.35 Autophagy plays an important role in RHCC. Furthermore, it acts as an important mediator of HCC cell invasion, inducing changes in cell adhesion signaling and promoting HCC cell migration and glycolytic pathways. In addition, it has been shown that autophagy promotes the expression and invasion of EMT markers in HCC cells. This is an important intermediate stage of cancer metastasis and a major cause of intrahepatic dissemination and metastasis of HCC cells.36 According to the theory of angiogenesis proposed by Judah Folkman, RHCC is also associated with the growth of blood vessels in the tumor. The formation of new blood vessels from endothelial precursors is the prerequisite for the growth and progression of solid malignancies. Access to the host vascular system and the generation of tumor blood supply are rate-limiting steps in tumor growth and progression. Vascular endothelial growth factor (VEGF) refers to a subfamily of growth factors that promote tumor angiogenesis under hypoxic conditions.37 The HIF-1α/BNIP3 signaling pathway is closely related to the above-mentioned tumor cell autophagy, hypoxia, and tumor angiogenic processes.
HIF-1α is an upstream factor of BNIP3. Under hypoxic conditions, HIF-1α-driven BNIP3 expression helps to reduce overall cellular oxygen consumption by triggering mitochondrial autophagy, thereby facilitating metabolic adaptation to low oxygen concentrations.38 High BNIP3 and HIF-1α levels are commonly associated with poor prognosis in patients with cancer (eg, melanoma lung, liver). The HIF-1α/BNIP3 pathway promotes cellular autophagy under hypoxic conditions. HCC cells gain energy to migrate outward through autophagy and enhance the degree of cell adhesion, thus contributing to metastasis and RHCC.39 Autophagy is also an important factor in the induction of EMT, which is activated by the HIF-1α/BNIP3 pathway through autophagy in HCC cells. This process produces extracellular matrix, degrades enzymes, and increases the resistance of HCC cells to apoptosis.40 HIF-1α can also promote endothelial cell growth and migration by regulating the hypoxic upregulation of VEGF, thereby inducing liver tumor angiogenesis and causing RHCC.41
By transfecting HepG2 cells with BNIP3 small interfering RNA, followed by treatment with incomplete radiofrequency ablation (IRFA), Xu et al42 performed cell proliferation, migration, and invasion assays, as well as electron microscopy and protein blotting on residual tumor cells. Following in vitro simulated IRFA, the residual HepG2 cells showed increased autophagic vesicles, proliferation, migration, and invasion, as well as increased expression of HIF-1α and BNIP3 proteins. These findings demonstrated that IRFA can induce autophagy through the HIF-1α/BNIP3 signaling pathway and promote RHCC. Méndez-Blanco et al43 developed an in vitro model of acquired resistance to sorafenib in HCC to illustrate the role of HIFs and BNIP3 in HCC resistance to treatment and recurrence. HIF-1α was silenced using siRNA, and its effect on cell viability was tested 24 h after the induction of hypoxia. HIF-1α knockdown significantly reduced cell viability in both HepG2S1 and HepG2S3 sorafenib-resistant cells. The expression of BNIP3 in these drug-resistant cells was also measured under hypoxic conditions. The experiments showed that the protein and mRNA levels of BNIP3 in HepG2 cells tended to decrease following treatment with sorafenib.
The role of autophagy in cancer is bidirectional. Although autophagy is a mechanism involved in cancer cell survival in response to environmental and cellular stresses, it may be associated with cancer cell death in some cases. In a study conducted by Wang et al,44 the induction of autophagy in HCC cells (Huh7, Hep3B, and HepG2) was prevented using sonic hedgehog (Shh) or smoothened agonist (SAG) and violet morpholino. In contrast, autophagy in HCC cells (Huh7, Hep3B and HepG2) was induced using the GLI-selective inhibitor GANT61. Hedgehog inhibition-induced autophagy caused apoptosis in HCC cells (Huh7, Hep3B, and HepG2), as determined by measuring the mouse liver holotype index, monoalkyl cadaverine staining, and transmission electron microscopy. Analyses through WB and quantitative real-time polymerase chain reaction revealed that autophagy was associated with BNIP3. These experiments demonstrated that GANT61 induced autophagy through the upregulation of BNIP3 and that this mechanism contributed to apoptosis, thereby inhibiting the metastasis of HCC. Figure 4 describes in detail the mechanism by which the HIF-1α/BNIP3 signaling pathway causes RHCC through autophagy.
TCM to Combat Recurrent Liver Cancer Through Targeted Regulation of the HIF-1α/BNIP3 Pathway
In TCM, RHCC belongs to the categories of “jaundice”, “meteorism”, and “aggregation”.45 The basic pathogenesis of HCC is liver stagnation, deficiency in the liver and kidneys, deficiency in qi and blood, and dampness-heat. The toxins produced by the above factors stagnate in the liver and develop into HCC; in TCM theory, this process is termed cancer toxicity (Ai-Du).46 Hepatic yin deficiency, syndrome of qi stagnation and blood stasis, hepatospleen transport has no right, and damp heat produces phlegm are factors associated with RHCC and metastatic HCC.47 Patients often exhibit symptoms of yin deficiency, such as low fever, night sweats, dry mouth and throat, and weight loss due to prolonged illness. Due to the rapid progression of the disease, some patients may experience abdominal distension or pain, refusal to press, bulging masses, and a lean body.48 Owing to its multi-targeted intervention, low toxicity, and cost-effectiveness, Chinese medicine offers significant advantages in preventing and treating the recurrence of liver cancer. The HIF-1α/BNIP3 signaling pathway is related to autophagy, which is essential for RHCC. Therefore, it may be a target for intervention to prevent and treat disease recurrence through TCM.
TCM Interferes with the HIF-1α/BNIP3 Pathway Through Its Active Ingredient to Inhibit RHCC
In recent years, several studies demonstrated that the active ingredients contained in some Chinese herbal medicines can effectively inhibit RHCC by interfering with the HIF-1α/BNIP3 pathway. The primary herbal medicines with modulating effects include Shuifeiji, Jiegeng, Wutou, Danshen, and Honghua. Table 1 lists common herbal medicines that interfere with the HIF-1α/BNIP3 pathway and can be used to treat a recurrence of liver cancer recurrence, as well as their active ingredients and intervention mechanisms. Chinese herbal medicines can biphasically regulate the HIF-1α/BNIP3 signaling pathway to inhibit the recurrence of liver cancer. Activation of the HIF-1α/BNIP3 signaling pathway can enhance autophagy of HCC cells, promote apoptosis of tumor cells, and inhibit the recurrence of liver cancer. Xuan et al found that silymarin could induce tumor cell autophagy and apoptosis by activating BNIP3 protein expression in Hep G2 cells. Inhibition of the HIF-1α/BNIP3 signaling pathway could block anaerobic glycolysis in HCC cells, as well as the occurrence of EMT mechanism. Li et al showed that Rehmannia methyl estradiol could inhibit the process of anaerobic glycolysis by downregulating the HIF-1α /BNIP3 signaling pathway to suppress hepatocarcinogenesis. This evidence suggests that the active ingredients of Chinese herbal medicine modulating the HIF-1α /BNIP3 signaling pathway may be effective in inhibiting RHCC.
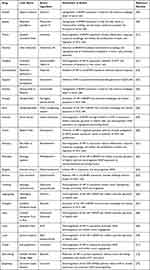 |
Table 1 Common Herbal Medicines That Inhibit Recurrent Hepatocellular Carcinoma by Modulating the HIF-1a/BNIP3 Pathway |
Chinese Herbal Compound Interferes with the HIF-1α/BNIP3 Pathway to Inhibit RHCC
TCM compounds can be combined with other drugs to exert synergistic effects. In TCM theory, this process is known as mutual promotion or mutual assistance. It has been reported that HCC relapses can be inhibited by targeting and modulating the HIF-1α /BNIP3 pathway. We summarize the common groups associated with the HIF-1α/BNIP3 pathway for the treatment of RHCC, as shown in Table 2. The Fuzheng Yiliu Decoction, Jiedu Xiaozheng Decoction, Xuefu Zhuyu Decoction, Huang Qin Decoction, and Yi Qi Hua Yu Jie Du Prescription are discussed below.
 |
Table 2 A Formulas to Inhibit RHCC by Modulating the HIF-1a/BNIP3 Pathway |
Fuzheng Yiliu Decoction
Fuzheng Yiliu Decoction is a classic formula used for the treatment of tumors. It consists of 10 herbs, namely Sanleng (10 g), Ezhu (15 g), Longkui (15 g), Baiying (10 g), Banzhilian (30 g), Baihuasheshecao (30 g), Huangqi (9 g), Danggui (30 g), Fuling (30 g), and Baizhu (10 g). Astragalus polysaccharide in Huangqi activates the HIF-1α/BNIP3 axis, promotes autophagy, and induces apoptosis in HCC cells. The combination of Ezhu and Huangqi can exert a synergistic effect. Moreover, the combination of Curcumol and Astragalus saponin75 reduces the expression of HIF-1α in HCC cells and inhibits aerobic glycolysis and tumor angiogenesis in RHCC.
Zhang et al74 prepared a model of HCC in nude mice through the transplantation of Hu-7 cells. They observed the formation of autophagosomes in the model and drug-treated mice by electron microscopy. The expression of BNIP3 protein was measured by WB. The results showed that, compared with the model group, the Fuzheng Yiliu Decoction induced apoptosis and inhibited the growth rate of tumors in the treatment group. WB analysis showed that the expression of autophagy-related proteins beclin-1 (BECN1), BNIP3, and LC3 was upregulated in the Fuzheng Yiliu Decoction group compared with the Fuzheng Yiliu Decoction treatment group. These findings suggested that Fuzheng Yiliu Decoction can inhibit the growth of Hu-7 cells and induce apoptosis of tumor cells in nude mice. Moreover, it can activate the expression of BECN1, BNIP3, and LC3 proteins in liver cancer cells to induce autophagy. Yin et al85 evaluated 80 patients treated after radical surgery for HCC with conventional radiotherapy (n=40) and Fuzheng Yiliu Decoction (n=40). The postoperative survival rate, tumor recurrence rate, and vascular tumor marker levels were compared between the two groups. The results showed that patients in the Fuzheng Yiliu Decoction group had a higher survival rate and lower recurrence rate at 3 years after surgery compared with the conventional radiotherapy group. Moreover, the levels of alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and des-gamma-carboxy prothrombin (DCP) were lower in the former group versus the latter group. This evidence indicates that Fuzheng Yiliu Decoction effectively improves the survival rate and reduces the rate of disease recurrence in patients with HCC. Chen et al86 determined the effects of different concentrations of the ethyl acetate extract of the Fuzheng Yiliu Decoction on the survival rate and HIF-1α protein expression in HepG2 HCC cells under hypoxic conditions. The results showed that the survival rate of HepG2 HCC cells under hypoxic condition was gradually decreased with the increase in the concentration of ethyl acetate extract of Fuzheng Yiliu Decoction. Furthermore, the expression of HIF-1α protein was also inhibited in a dose-dependent manner (P<0.05 or P<0.01). Cao et al87 prepared a nude mouse model of HCC through transplantation of Hu-7 cells and administration of different concentrations of Fuzheng Yiliu Decoction. The results showed that the mean mass of HCC transplanted tumors in the Fuzheng Yiliu Decoction group was lower than that measured in the control group. In addition, the number of bilayer autophagic vesicles was increased and the expression of BNIP3 was increased in the treatment group, as observed through electron microscopy; these effects were dose-dependent (P<0.05). Based on the available evidence, Fuzheng Yiliu Decoction can significantly enhance the autophagic activity of tumor cells and inhibit the proliferation, invasion, and metastasis of HCC cells.
Jiedu Xiaozheng Decoction
Jiedu Xiaozheng Decoction is composed of four herbs, namely, Baihuasheshecao (30 g), Xiakucao (15 g), Shancigu (15 g), and Kushen (15 g); these herbs possess detoxifying and anti-inflammatory properties. The alcoholic extracts of Bupleurum enhance cellular and humoral immunity and inhibit the growth of liver cancer cells.88 The total flavonoids of Xiakucao reduce the expression of HIF-1α in tumor cells and inhibit RHCC by blocking the aerobic glycolytic process in HCC cells. Cao et al89 successfully prepared a liver metastasis tumor model by subcutaneously transplanting HepG2 cells into BALB/c nude mice. This was followed by treatment using ethyl acetate extract of Jiedu Xiaozheng Decoction. The tumor volume and weight were measured, and the apoptotic index and the expression levels of BAX and cytochrome C were determined by immunohistochemistry (IHC). The results showed that the treatment significantly reduced the proliferation of HepG2 cells and significantly increased the apoptosis index was and the expression of intracellular BAX protein. This evidence indicates that the ethyl acetate extract of Jiedu Xiaozheng Decoction can treat HCC by inducing apoptosis and inhibit RHCC. Lu et al75 explored the changes in glucose metabolism after intervention with Jiedu Xiaozheng Decoction in different HCC cell lines by establishing in vitro hypoxic HepG2, Hep3B, and Huh7 HCC cell models. The effects of different concentrations (0, 0.05, 0.1, and 0.2 mg/mL) of Jiedu Xiaozheng Decoction on the survival rate and apoptosis of HCC cells were examined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Hoechst staining. The proliferation of HCC cells and HIF-1α protein expression under hypoxic and normoxic conditions were detected using MTT assay and WB, respectively. The results showed that Jiedu Xiaozheng Decoction promoted the apoptosis of HCC cells, downregulated the expression of HIF-1α and inhibited the glycolytic ability of cells, thus inhibiting the proliferation, invasion, and metastasis of HCC cells.
Xuefu Zhuyu Decoction
Xuefu Zhuyu Decoction is composed of Taoren (12 g), Honghua (9 g), Danggui (9 g), Shudi (9 g), Niuxi (9 g), Chuanxiong (4.5 g), Jiegeng (4.5 g), Baishao (6 g), Zhishi (6 g), Gancao (6 g), and Chaihu (3 g). Hydroxysafflor yellow A, the main component in Honghua, can downregulate the expression of HIF-1α, inhibit the EMT process, and promote the apoptosis of liver tumor cells90 Chaihu saponin can interfere with the glycolytic pathway of liver cancer cells by downregulating HIF-1α expression, thus inhibiting the proliferation of liver cancer cells.88 Platycodon saponin was able to upregulate BNIP3 expression in HCC cells. This caused mitochondrial swelling and decreased membrane potential in tumor cells, thereby triggering autophagy and death of HCC cells.
Meng et al91 investigated 70 patients with advanced HCC; patients in the experimental group received a combination of Xuefu Zhuyu Decoction with XELOX (capecitabine plus oxaliplatin) chemotherapy. The XELOX regimen comprised capecitabine (1000 mg/m2 twice daily, administered orally on days 1–14) and oxaliplatin (135 mg/m2 administered intravenously on day 1). One cycle included 4 weeks of therapy, and three cycles of treatment were performed. The results showed that clinical symptoms (eg, pain in the liver area, bloating, and poor appetite) were improved. In addition, the numbers of CD3+ and CD4+ cells were decreased, whereas those of CD8+ and CD4+/CD8+ cells were increased. The serum levels of HIF-1α were decreased by the combination treatment, indicating that XELOX chemotherapy with Xuefu Zhuyu Decoction improves immunity and serum HIF-1α expression, as well as prognosis in patients with advanced HCC.
Huang Qin Decoction
Huang Qin decoction is composed of Huangqin (9 g), Gancao (6 g), Shaoyao (6 g), and 12 Dazao. Han baicalin in Huangqin activates the HIF-1α/BNIP3 axis and promotes autophagy and apoptosis of liver cancer cells. Paeoniflorin (PF) contained in Paeonia lactiflora exerts a wide range of anti-inflammatory and immunomodulatory effects.92 Studies have reported that paeoniflorin downregulates HIF-1α expression in vivo, affects the expression of downstream factors BNIP3 and VEGF, and inhibits autophagy and angiogenesis in tumor cells.93 Hu et al94 investigated the effect of Huang Qin Decoction on the expression of HIF-1α in human hepatoma HepG2 cells and the potential mechanism involved in this process. The results of WB analysis showed that, after 24 h of treatment with 5-fluorouracil and baicalin soup, the HIF-1α protein expression in HepG2 cells was decreased in all intervention groups. Notably, the lowest levels of HIF-1α were detected in the group treated with high-dose baicalin. This evidence indicates that Huang Qin Decoction dose-dependently decreased HIF-1α protein expression in human HCC HepG2 cells, inhibited the autophagy-activated EMT pathway and the production of extracellular matrix degrading enzymes in HCC cells, and induced apoptosis. Li et al67 assigned 112 patients with primary liver cancer into control and observation groups (n=56 per group). Patients in both groups were treated with conventional treatment, such as hepatic artery chemoembolization. In the observation group, patients were also treated with Huang Qin Decoction for 4 months. Treatment effectiveness was significantly higher in the observation group versus the control group (67.86% vs 48.21%, respectively; P<0.01). Furthermore, the levels of HIF-1α and AFP and tumor volume were significantly lower in the observation group compared with the control group (P<0.01). These results showed that treatment with Huang Qin Decoction in combination with hepatic artery chemoembolization could inhibit the growth and spread of HCC cells by reducing HIF-1α and AFP expression in patients with HCC. Wang et al95 examined 120 patients with HCC and classified them into observation and control groups. There were 60 patients in each of the two groups. Patients in the control group were treated with sequential hepatic artery chemoembolization, while those in the observation group were treated with Huang Qin Decoction for 8 weeks. The results showed that the levels of aspartate transaminase, aspartate transaminase, total bilirubin, AFP, and HIF-1α were decreased in the observation group compared with the control group after treatment (P<0.05). This evidence demonstrates that Huang Qin Decoction combined with sequential hepatic artery chemoembolization can reduce the serum levels of HIF-1α and effectively improve liver function in patients with HCC.
Yiqi Huayu Jiedu Prescription
The formula of Yiqi Huayu Jiedu is composed of Baishen (10 g), Huangqi (30 g), Ezhu (15 g), Chonglou (15 g), Hanzhilian (30 g), and Gancao (5 g), and improves qi and blood stasis. This formula is rich in ginsenosides, Astragalus polysaccharides, curcuminoids, curcuminoids, shigella saponins, glycoproteins, and other anti-tumor active components. Curcumin can inhibit the HIF-1α/BNIP3 axis and block the EMT of HCC, thus exerting anti-metastatic and anti-invasive effects. Modern pharmacological analysis shows that Yiqi Huayu Jiedu Prescription can improve immunity, promote phagocytosis, and enhance the function of the adrenal cortex. These effects can stabilize tumors, prevent recurrence and metastasis, and improve the quality of life of patients.
Zeng et al78 evaluated the effect of Yiqi Huayu Jiedu Prescription on human liver tumors and HIF-1α expression by establishing a liver cancer transplantation tumor model in nude rats. IHC was performed on tumor tissues after 21 days of intervention with Yiqi Huayu Jiedu Prescription. The results showed that the tumor size was significantly reduced and HIF-1α expression was decreased in the treatment group versus the control group. Wang et al96 established a transplantation tumor model of human HCC sorafenib-resistant cells in nude mice. The purpose was to evaluate the effects of the combination of Yiqi Huayu Jiedu Prescription with sorafenib on the size of the transplantation tumors and HIF-1α expression in tumor tissues. Mice in the experimental group were treated with Yiqi Huayu Jiedu Prescription (7.5 g/kg) combined with sorafenib (15 mg/kg) through gavage. The tumor volume was measured after 21 days of drug administration, and the expression of HIF-1α was detected by IHC. The results showed that the treatment decreased the tumor volume and expression of HIF-1α in experimental mice. Hence, this formula can improve the prognosis of HCC by downregulating the expression of HIF-1α, improving the hypoxic microenvironment of tumor tissues, and preventing the development of resistance to sorafenib.
Conclusion and Future Perspective
This article summarizes the biological characteristics of HIF-1α and BNIP3, and discusses the role of the HIF-1α/BNIP3 signaling pathway in RHCC. The hypoxic or ischemic state of the body activates HIF-1α and the downstream factor BNIP3, which mediates cellular autophagy. Autophagy promotes HCC cell migration, glycolysis, and EMT process, but can also directly kill tumor cells. The article also outlines currently available information on potential herbs/extracts/combinations for treating RHCC. These agents inhibit disease recurrence through biphasic modulation of the HIF-1α/BNIP3 signaling pathway. The main components of these formulations include saponins, flavonoids, and polysaccharides. These components enhance autophagy and promote apoptosis in HCC cells by activating the HIF-1α/BNIP3 signaling pathway, or inhibiting anaerobic glycolysis and the EMT mechanism in tumor cells by downregulating the expression of the HIF-1α/BNIP3 signaling pathway.
TCM is widely used in China as an essential complement or alternative to pharmacological therapy for improving the clinical outcomes of patients with recurrent liver cancer. TCM formulations are effective in modulating the HIF-1α/BNIP3 signaling pathway to prevent the recurrence of liver cancer. However, as most clinical trials are conducted in China with a limited sample size, additional detailed animal and clinical studies are warranted to confirm their efficacy and safety. It is also essential to elucidate the molecular mechanism underlying the effects of the above herbs/extracts/combinations in the treatment of recurrent liver cancer by targeting HIF-1α/BNIP3 signaling. Of note, there are few studies on the toxicity of the above herbs/extracts/combinations and their target organs. Therefore, our future research studies will investigate the side effects and toxicity of TCM in RHCC.
Overall, this article provides the latest information on natural agents used in the treatment of recurrent liver cancer through modulation of the HIF-1α/BNIP3 signaling pathway. This review may provide new directions for the development of natural agents targeting RHCC.
Abbreviations
HCC, Hepatocellular carcinoma; RHCC, recurrent hepatocellular carcinoma; HIF-1α, Hypoxia-inducible factor-1α; BNIP3, BCL-2 19 kDa interacting protein 3; TCM, traditional Chinese medicine; NASH, nonalcoholic steatohepatitis; bHLH - PAS, basic helix - loop - helix - PER - ARNT - SIM; ODDD, oxygen-dependent degradation; TAD, trans-activating domain; TRD, trans-repressive domain; N-TAD, N-terminal transcriptional activation domain; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; HRE, hypoxia response element; C-TAD, C-terminal transcriptional activation domain; NLS-N, nuclear localization signaling domains-nitrogen; NLS-C, carbon-terminated nuclear localization signaling domains; PSTD, proline-serine-threonine-rich protein stabilization domain; BH3, Bcl-2 homology 3; CD, conserved structural domain; TM, transmembrane; MPTP, Mitochondrial Permeability Transition Pore; HPH, hypoxia-inducible factor proline hydroxylase; WB, Western Blot; LC3, microtubule-associated protein one light chain 3; ROS, reactive oxygen species; FAK, Focal adhesion kinase; specific inhibitory adhesion kinase; SRC, Src family kinase; EMT, Epithelial-mesenchymal transition; VEGF, Vascular endothelial growth; IRFA, incomplete radiofrequency ablation; Shh, Sonic hedgehog; SAG, Smoothened Agonist.
Funding
This work was supported by the QI Huang Scholars (National Chinese Medicine Human Education Letter 2022-6); the Major Public Welfare Special Project of Henan Province (201300310100); and the Joint Open Project of the State Administration of Traditional Chinese Medicine (GZY-KJS-2022-040-1).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi:10.1002/hep.31288
3. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
4. Cernea S, Onișor D. Screening and interventions to prevent nonalcoholic fatty liver disease/nonalcoholic steatohepatitis-associated hepatocellular carcinoma. World J Gastroenterol. 2023;29(2):286–309. doi:10.3748/wjg.v29.i2.286
5. Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20(1):37–49. doi:10.1038/s41575-022-00688-6
6. Allaire M, Bruix J, Korenjak M, et al. What to do about hepatocellular carcinoma: recommendations for health authorities from the International Liver Cancer Association. JHEP Rep. 2022;4(12):100578. doi:10.1016/j.jhepr.2022.100578
7. Gadallah EA, Elkomos BE, Khalil A, Fawzy FS, Abdelaal A. Central hepatectomy versus major hepatectomy for patients with centrally located hepatocellular carcinoma: a systematic review and meta-analysis. BMC Surg. 2023;23(1):2. doi:10.1186/s12893-022-01891-7
8. Facciorusso A, Villani R, Bellanti F, Mitarotonda D, Vendemiale G, Serviddio G. Mitochondrial signaling and hepatocellular carcinoma: molecular mechanisms and therapeutic implications. Curr Pharm Des. 2016;22(18):2689–2696. doi:10.2174/1381612822666160209153624
9. Xiong XX, Qiu XY, Hu DX, Chen XQ. Advances in hypoxia-mediated mechanisms in hepatocellular carcinoma. Mol Pharmacol. 2017;92(3):246–255. doi:10.1124/mol.116.107706
10. Mellor HR, Harris AL. The role of the hypoxia-inducible BH3-only proteins BNIP3 and BNIP3L in cancer. Cancer Metastasis Rev. 2007;26(3–4):553–566. doi:10.1007/s10555-007-9080-0
11. Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57(10):1009–1014. doi:10.1136/jcp.2003.015032
12. Gorbunova AS, Yapryntseva MA, Denisenko TV, Zhivotovsky B. BNIP3 in lung cancer: to kill or rescue? Cancers. 2020;12(11):3390. doi:10.3390/cancers12113390
13. Semenza GL. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2017;36(3):252–259. doi:10.15252/embj.201695204
14. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510–5514. doi:10.1073/pnas.92.12.5510
15. Edwards HE, Gorelick DA. The evolution and structure/function of bHLH-PAS transcription factor family. Biochem Soc Trans. 2022;50(3):1227–1243. doi:10.1042/BST20211225
16. Lee DG, Kim YK, Baek KH. The bHLH transcription factors in neural development and therapeutic applications for neurodegenerative diseases. Int J Mol Sci. 2022;23(22):13936. doi:10.3390/ijms232213936
17. Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004;36(2):189–204. doi:10.1016/S1357-2725(03)00211-5
18. Yang W, Ma J, Zhou W, et al. Reciprocal regulations between miRNAs and HIF-1α in human cancers. Cell Mol Life Sci. 2019;76(3):453–471. doi:10.1007/s00018-018-2941-6
19. Pezzuto A, Carico E. Role of HIF-1 in cancer progression: novel insights. a review. Curr Mol Med. 2018;18(6):343–351. doi:10.2174/1566524018666181109121849
20. Huang X, Zhao L, Peng R. Hypoxia-inducible factor 1 and mitochondria: an intimate connection. Biomolecules. 2022;13(1):50. doi:10.3390/biom13010050
21. Guo K, Searfoss G, Krolikowski D, et al. Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell Death Differ. 2001;8(4):367–376. doi:10.1038/sj.cdd.4400810
22. Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson ÅB. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem. 2012;287(23):19094–19104. doi:10.1074/jbc.M111.322933
23. Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–368. doi:10.1126/science.2876518
24. Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A. 2002;99:12825–12830. doi:10.1073/pnas.202474099
25. Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91:226–231. doi:10.1161/01.RES.0000029232.42227.16
26. Li J, Gong SH, He YL, et al. Autophagy is essential for neural stem cell proliferation promoted by hypoxia. Stem Cells. 2023;41(1):77–92. doi:10.1093/stmcls/sxac076
27. Xu K, Lu C, Ren X, Wang J, Xu P, Zhang Y. Overexpression of HIF-1α enhances the protective effect of mitophagy on steroid-induced osteocytes apoptosis. Environ Toxicol. 2021;36(11):2123–2137. doi:10.1002/tox.23327
28. Zhang Y, Liu D, Hu H, Zhang P, Xie R, Cui W. HIF-1α/BNIP3 signaling pathway-induced-autophagy plays protective role during myocardial ischemia-reperfusion injury. Biomed Pharmacother. 2019;120:109464. doi:10.1016/j.biopha.2019.109464
29. He G, Nie JJ, Liu X, et al. Zinc oxide nanoparticles inhibit osteosarcoma metastasis by downregulating β-catenin via HIF-1α/BNIP3/LC3B-mediated mitophagy pathway. Bioact Mater. 2022;19:690–702. doi:10.1016/j.bioactmat.2022.05.006
30. Zhu L, Qi B, Hou D. Roles of HIF1α- and HIF2α-regulated BNIP3 in hypoxia-induced injury of neurons. Pathol Res Pract. 2019;215(4):822–827. doi:10.1016/j.prp.2019.01.022
31. Cyran AM, Zhitkovich A. HIF1, HSF1, and NRF2: oxidant-responsive trio raising cellular defenses and engaging immune system. Chem Res Toxicol. 2022;35(10):1690–1700. doi:10.1021/acs.chemrestox.2c00131
32. Huang F, Wang BR, Wang YG. Role of autophagy in tumorigenesis, metastasis, targeted therapy and drug resistance of hepatocellular carcinoma. World J Gastroenterol. 2018;24(41):4643–4651. doi:10.3748/wjg.v24.i41.4643
33. Wu XZ, Xie GR, Chen D. Hypoxia and hepatocellular carcinoma: the therapeutic target for hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22(8):1178–1182. doi:10.1111/j.1440-1746.2007.04997.x
34. Hashemi M, Nadafzadeh N, Imani MH, et al. Targeting and regulation of autophagy in hepatocellular carcinoma: revisiting the molecular interactions and mechanisms for new therapy approaches. Cell Commun Signal. 2023;21(1):32. doi:10.1186/s12964-023-01053-z
35. Zandieh MA, Farahani MH, Rajabi R, et al. Epigenetic regulation of autophagy by non-coding RNAs in gastrointestinal tumors: biological functions and therapeutic perspectives. Pharmacol Res. 2023;187:106582. doi:10.1016/j.phrs.2022.106582
36. You L, Wu W, Wang X, et al. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev. 2021;41(3):1622–1643. doi:10.1002/med.21771
37. Haddad JJ. Science review: redox and oxygen-sensitive transcription factors in the regulation of oxidant-mediated lung injury: role for hypoxia-inducible factor-1α. Crit Care. 2002;7(1):1–8. doi:10.1186/cc1840
38. Burton TR, Gibson SB. The role of Bcl-2 family member BNIP3 in cell death and disease-NIPping at the heels of cell death. Cell Death Differ. 2009;16(4):515–523. doi:10.1038/cdd.2008.185
39. Aryapour E, Kietzmann T. Mitochondria, mitophagy, and the role of deubiquitinases as novel therapeutic targets in liver pathology. J Cell Biochem. 2022;123(10):1634–1646. doi:10.1002/jcb.30312
40. Zhang J, Hu Z, Horta CA, Yang J. Regulation of epithelial-mesenchymal transition by tumor microenvironmental signals and its implication in cancer therapeutics. Semin Cancer Biol. 2023;88:46–66. doi:10.1016/j.semcancer.2022.12.002
41. Olejarz W, Kubiak-Tomaszewska G, Chrzanowska A, Lorenc T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. Int J Mol Sci. 2020;21(16):5840. doi:10.3390/ijms21165840
42. Xu WL, Wang SH, Sun WB, et al. Insufficient radiofrequency ablation-induced autophagy contributes to the rapid progression of residual hepatocellular carcinoma through the HIF-1α/BNIP3 signaling pathway. BMB Rep. 2019;52(4):277–282. doi:10.5483/BMBRep.2019.52.4.263
43. Méndez-Blanco C, Fondevila F, Fernández-Palanca P, et al. Stabilization of hypoxia-inducible factors and BNIP3 promoter methylation contribute to acquired sorafenib resistance in human hepatocarcinoma cells. Cancers. 2019;11(12):1984. doi:10.3390/cancers11121984
44. Wang Y, Han C, Lu L, Magliato S, Wu T. Hedgehog signaling pathway regulates autophagy in human hepatocellular carcinoma cells. Hepatology. 2013;58(3):995–1010. doi:10.1002/hep.26394
45. Liu L, Cheng Q, Bai C. Advances of traditional Chinese medicine in prevention and treatment of recurrence and metastasis of liver cancer. J Clin Hepatol. 2021;26(05):1216–1220. Chinese.
46. Wang W, Gao Z, Yin C. A methodological study of traditional Chinese medicine treatment of primary liver cancer. J Clin Hepatol. 2021;37(09):2009–2015. Chinese.
47. Huang Q, Wei A. Discussion on the treatment of metastasis and recurrence of hepatocellular carcinoma from the method of promoting blood circulation and removing blood stasis. Lishizhen Med Materia Medica Res. 2018;29(08):1944–1945. Chinese.
48. Xiao Z, Chen H, Lin L. LIN Li-zhu’s experience on the phased treatment strategy of hepatocellular carcinoma. China J Trad Chin Med Pharma. 2019;34(06):2526–2528. Chinese.
49. Xuan L, Jin X, Meng G. Effect of silymarin on HepG2 protein expression in human hepatocellular carcinoma cell lines in vitro and its clinical significance. Zhejiang Pract Med. 2014;19(06):400–402. Chinese.
50. Xu J, Li X, Lai P. Study on the killing effect and mechanism of platycodin D in vitro on human hepatocellular carcinoma cell HepG2. Chin J Health Lab Technol. 2015;25(16):2754–2757, 2760. Chinese.
51. Peng F, Zhang N, Wang C, et al. Aconitine induces cardiomyocyte damage by mitigating BNIP3-dependent mitophagy and the TNFα-NLRP3 signalling axis. Cell Prolif. 2020;53(1):e12701. doi:10.1111/cpr.12701
52. Cui S, Chen T, Wang M, et al. Tanshinone I inhibits metastasis of cervical cancer cells by inducing BNIP3/NIX-mediated mitophagy and reprogramming mitochondrial metabolism. Phytomedicine. 2022;98:153958. doi:10.1016/j.phymed.2022.153958
53. Zhang Y, Liu Y, Cui Q, et al. Hydroxysafflor yellow A alleviates ischemic stroke in rats via HIF-1[Formula: see text], BNIP3, and Notch1-mediated inhibition of autophagy. Am J Chin Med. 2022;50(3):799–815. doi:10.1142/S0192415X22500331
54. Zhu F, Gao J, Zeng F, et al. Hyperoside protects against cyclophosphamide induced ovarian damage and reduced fertility by suppressing HIF-1α/BNIP3-mediated autophagy. Biomed Pharmacother. 2022;156:113743. doi:10.1016/j.biopha.2022.113743
55. Xie W, Zhu T, Zhang S, Sun X. Protective effects of Gypenoside XVII against cerebral ischemia/reperfusion injury via SIRT1-FOXO3A- and Hif1a-BNIP3-mediated mitochondrial autophagy. J Transl Med. 2022;20(1):622. doi:10.1186/s12967-022-03830-9
56. Sun X, Hong Y, Shu Y, et al. The involvement of Parkin-dependent mitophagy in the anti-cancer activity of Ginsenoside. J Ginseng Res. 2022;46(2):266–274. doi:10.1016/j.jgr.2021.06.009
57. Zhang SY, Wang F, Zeng XJ, Huang Z, Dong KF. Astragalus polysaccharide ameliorates steroid-induced osteonecrosis of femoral head through miR-206/HIF-1α/BNIP3 axis. Kaohsiung J Med Sci. 2021;37(12):1089–1100. doi:10.1002/kjm2.12426
58. Liu XW, Lu MK, Zhong HT, Wang LH, Fu YP. Panax Notoginseng saponins attenuate myocardial ischemia-reperfusion injury through the HIF-1α/BNIP3 pathway of autophagy. J Cardiovasc Pharmacol. 2019;73(2):92–99. doi:10.1097/FJC.0000000000000640
59. Xu B, Zhu L, Chu J, et al. Esculetin improves cognitive impairments induced by transient cerebral ischaemia and reperfusion in mice via regulation of mitochondrial fragmentation and mitophagy. Behav Brain Res. 2019;372:112007. doi:10.1016/j.bbr.2019.112007
60. You M, Zhang H, He G. Inhibitory effect of saikosaponin b2 on diethyl nitrosamine-induced primary liver cancer of mice by regulating SIRT6-mediated glucose metabolism pathway. Chin J Pharmacol Toxicol. 2022;36(10):739–745. Chinese.
61. Dong W, Yang A, Li X. Effects of Rhei Radix et Rhizoma on mTOR/HIF-1α/VEGF signal pathway in cell RAW264.7 inflammation model induced by LPS. Chin J Inform Trad Chin Med. 2020;27(02):38–42. Chinese.
62. Wei Y, Lyu B, Jin L. Effects of plumbagin on proliferation, apoptosis, invasion and expression of HIF-1α in hepatocellular HepG2 cells under hypoxia condition. Chin J Mod Appl Pharma. 2022;39(14):1789–1795. Chinese.
63. Liu Y, Liu F, Li X. Effects of dihydroartemisinin on VEGF in hepatoma HepG2 cells and angiogenesis in HUVECs. J Shandong Univ Trad Chin Med. 2022;46(04):524–532, 548. Chinese.
64. Li Y, Wang H, Deng X. Effect and mechanism of cantharidin on PD-1/PD-L1 expression in mice with liver cancer. J Med Res. 2022;51(12):52–57, 181. Chinese.
65. Cheng Z, Cheng W, Xing D. Asparagus polysaccharide combined HIF1α RNAi to inhibit angiogenesis of liver cancer in the absence of oxygen. Lishizhen Med Materia Medica Res. 2020;31(12):2817–2820. Chinese.
66. Li T, Jin M, Song S. Triptolide inhibits human hepatocarcinoma SMMC-7721 cells by regulating glycolysis. World J Integr Trad West Med. 2020;15(06):981–985, 990. Chinese.
67. Li Y, Guo P, Tian Y. Effects of Huangqin Decoction combined with sequential hepatic arterial chemoembolization on the expression of NF-κB and HIF-1α in patients with primary liver cancer. China J Trad Chin Med Pharma. 2019;34(08):3870–3873. Chinese.
68. Wang L, Xie R, Wu Y. Growth inhibition of SMMC7721 by Artemisia quinoa flavonoids and its relationship with HIF-1α expression. Chin J Gerontol. 2016;36(22):5521-–5523. Chinese.
69. Jin A, Gao F, Xu H. Effects of Rhaponticum uniflorum on angiogenesis and apoptosis of H22 transplanted tumor tissue in mice. Chin Pharma J. 2016;51(04):280–283. Chinese.
70. Feng B, Zhu Y, He S. Effect of basil polysaccharide on the expression of histone demethylase LSD1, JMJD2B and JARID1B in hepatoma cells under hypoxic conditions. Lishizhen Med Materia Medica Res. 2015;26(08):1835–1840. Chinese.
71. Shen J, Tan S. Transcatheter arterial embolization with cinobufacini on terminal stage of hepatocellular carcinoma. Jilin J Trad Chin Med. 2015;35(07):678–680. Chinese.
72. Luo Y, Chen L. Regulation of saponins from Pulsatilla chinensis on energy metabolism of Bel-7402 xenograft in nude mice. Chin Trad Herbal Drugs. 2014;45(07):973–977. Chinese.
73. Li S, Sun J. Effects of curcumin on the expression of cyclooxygenase-2 in human hepatocellular carcinoma BEL-7402 cells. J China Med Univ. 2014;43(03):222–225, 230. Chinese.
74. Zhang W, Cheng W, Bao Y. Fuzheng Yiliu Decoction on apoptosis and autophagy in human hepatoma HepG2 cells. Pharmacol Clin Chin Materia Medica. 2013;29(05):102––106. Chinese.
75. Lu Q, Guan J, Zeng J. Study on the biological mechanism of Jiedu Xiaozheng Yin in regulating glucose energy metabolism and inhibiting hepatocellular carcinoma cell proliferation through HIF-1/miR-210. Fujian J Trad Chin Med. 2022;53(11):34–41. Chinese.
76. Shi X, Zhu H, Zhang Y, Zhou M, Tang D, Zhang H. XuefuZhuyu decoction protected cardiomyocytes against hypoxia/reoxygenation injury by inhibiting autophagy. BMC Complement Altern Med. 2017;17(1):325. doi:10.1186/s12906-017-1822-0
77. Lam W, Jiang Z, Guan F, et al. PHY906(KD018), an adjuvant based on a 1800-year-old Chinese medicine, enhanced the anti-tumor activity of Sorafenib by changing the tumor microenvironment. Sci Rep. 2015;5:9384. doi:10.1038/srep09384
78. Zeng P, Gao W, Pan M. Effect of Yiqi Huayu Jiedu formula and its separates on nude mice transplantation tumor of HepG2 cells and expression of HIF1a and E-cad. Liaoning J Trad Chin Med. 2014;41(09):2004–2006. Chinese.
79. Huang Q, Zhang Y, Liang L, et al. Effect of Aitongxiao Recipe on angiogenesis mimicry of liver cancer in hypoxic microenvironment. Guangxi J Trad Chin Med. 2021;44(05):77–80. Chinese.
80. Deng Z, Ou Y, Hu Y, et al. Shuyu pills improves mitochondrial damage by regulating the expression of HIF-1α and p53 to prevent hepatocellular carcinoma. J Hunan Univ Chin Med. 2021;41(07):1010–1016. Chinese.
81. Wu X, Pan J, Yu JJ, et al. DiDang decoction improves mitochondrial function and lipid metabolism via the HIF-1 signaling pathway to treat atherosclerosis and hyperlipidemia. J Ethnopharmacol. 2023;308:116289. doi:10.1016/j.jep.2023.116289
82. Chen J, Zheng X, Xu G, et al. Sini decoction inhibits tumor progression and enhances the anti-tumor immune response in a murine model of colon cancer. Comb Chem High Throughput Screen. 2023. doi:10.2174/1386207326666230320103437
83. Tang L, Wang F, Xiao L, et al. Yi-Qi-Jian-Pi formula modulates the PI3K/AKT signaling pathway to attenuate acute-on-chronic liver failure by suppressing hypoxic injury and apoptosis in vivo and in vitro. J Ethnopharmacol. 2021;280:114411. doi:10.1016/j.jep.2021.114411
84. Hou F, Li W, Shi Q, et al. Yi Ai Fang, a traditional Chinese herbal formula, impacts the vasculogenic mimicry formation of human colorectal cancer through HIF-1α and epithelial mesenchymal transition. BMC Complement Altern Med. 2016;16(1):428. doi:10.1186/s12906-016-1419-z
85. Yin Q, Wang Y, Xu L. Effect of Fuzheng Yiliu Decoction on postoperative recurrence and vascular tumor markers in patients with primary hepatocellular carcinoma undergoing radical resection. Liaoning J Trad Chin Med. 2022;49(08):133–135. Chinese.
86. Chen X, Lin W, Zheng L, et al. Jiedu Xiaozheng Decoction regulates the expression of HIF-1 in hepatocellular carcinoma cells to inhibit the mechanism of hepatocellular carcinoma angiogenesis. Jiangxi J Trad Chin Med. 2019;50(12):55–58. Chinese.
87. Cao S, Fan L, Wang N, et al. Effect of Fuzheng Yiliu Decoction on nude mice model of human hepatoma cell Hu-7 xenograft. Guiding J Trad Chin Med. 2018;24(24):66–68, 71. Chinese.
88. He S, Lu G, Hou H, et al. Saikosaponin-d suppresses the expression of cyclooxygenase-2 through the phospho-signal transducer and activator of transcription 3/hypoxia-inducible factor-1α pathway in hepatocellular carcinoma cells. Mol Med Rep. 2014;10(5):2556–2562. doi:10.3892/mmr.2014.2574
89. Cao Z, Chen X, Lin W, et al. Jiedu Xiaozheng Yin decoction inhibits hepatoma cell proliferation by inducing apoptosis via the mitochondrial-mediated pathway. Mol Med Rep. 2015;12(2):2800–2806. doi:10.3892/mmr.2015.3696
90. Ji DB, Zhu MC, Zhu B, et al. Hydroxysafflor yellow A enhances survival of vascular endothelial cells under hypoxia via upregulation of the HIF-1 alpha-VEGF pathway and regulation of Bcl-2/Bax. J Cardiovasc Pharmacol. 2008;52(2):191–202. doi:10.1097/FJC.0b013e318181fb02
91. Meng T, Zhang T, Deng Q. Effect of Xuefu Zhuyu decoction assisted XELOX chemotherapy on the expression of serum HIF-1α and TSP-1 in patients with advanced liver cancer. Chin J Integr Trad West Med Liver Dis. 2022;32(07):606–609. Chinese.
92. Yu W, Ilyas I, Hu X, Xu S, Yu H. Therapeutic potential of paeoniflorin in atherosclerosis: a cellular action and mechanism-based perspective. Front Immunol. 2022;13:1072007. doi:10.3389/fimmu.2022.1072007
93. Li J, Gao L, Xue Y. Effects of paeoniflorin on lung function and mTOR/HIF-1α/VEGF pathway in rats with Klebsiella pneumonia. J Medical Pest Control. 2023;39(02):186–191, 206. Chinese.
94. Hu J, Liu W, Zhao Y. Effect and mechanism of Huangqin decoction on expression of PTEN, HIF-1α and VEGF in human hepatoma cells. Mod Intervent Diagn Treat Gastroenterol. 2022;27(07):859–862. Chinese.
95. Wang X, Zhu X, Li D. Effects of Huangqin Decoction combined with sequential hepatic arterial chemoembolization on the serum levels of NF-κB, HIF-1α and AFP and the liver Function in patients with primary hepatic carcinoma. J Changchun Univ Chin Med. 2022;38(02):175–178. Chinese.
96. Wang Y, Zeng P, Gao W. Effects of Yiqi Huayu Jiedu prescription combined with sorafenib on the growth and expression of hypoxia inducible factor-1α and vascular mimicry formation of human hepatocellular carcinoma sorafenib resistant cells in nude mice transplanted tumor mode. Shaanxi J Trad Chin Med. 2020;41(02):143–146. Chinese.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.