Back to Archived Journals » Smart Homecare Technology and TeleHealth » Volume 2
Role of telephone monitoring in patients with chronic heart failure: theory and practical implications
Received 25 September 2013
Accepted for publication 26 November 2013
Published 19 February 2014 Volume 2014:2 Pages 1—12
DOI https://doi.org/10.2147/SHTT.S38820
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Jennifer Farroni Host, Ayesha Hasan
Division of Cardiovascular Medicine, Wexner Medical Center at the Ohio State University, Columbus, OH, USA
Abstract: With the aging of the world's population and the rise of chronic illness such as heart failure (HF), the economic burden, number of hospitalizations, and penalties imposed for failure to meet hospital readmission expectations will continue to rise, thus increasing pressure on clinicians to utilize successful HF monitoring interventions to improve these measures. Telephone monitoring in patients with chronic HF utilizes a proactive approach in the care of such patients, and for this review is grouped into three categories, ie, structured telephone support, telemonitoring, and remote implantable device monitoring. Earlier studies on structured telephone support and telemonitoring suggested a clear benefit on mortality and HF admissions, although several recent large, randomized controlled studies have been neutral. Optimizing medical therapy requires an accurate assessment of volume status by the clinician; therefore, symptom report and weight monitoring alone are often challenging in the identification of true HF decompensation because they are not very sensitive markers. The use of remote monitoring technology for follow-up of patients with implantable devices, including implantable cardiac defibrillators and cardiac resynchronization therapy devices, can aid in identifying HF decompensation. Self-care or self-management is an essential component of a chronic illness such as HF, and it is important for such patients to be engaged in their health care to best utilize the telephone monitoring intervention. System design, adequate staffing, patient satisfaction, and treatment adherence are important for success of the telemonitoring system. Telephone monitoring seems to be an effective approach in the chronic HF population. In the future, large-scale telemonitoring programs may come into place as well as additional remote implantable monitoring devices.
Keywords: heart failure, telemonitoring, remote monitoring, disease management, patient satisfaction, mortality
Introduction
With the aging of the world’s population and the rise of chronic illness such as hypertension, diabetes mellitus, chronic respiratory diseases, and heart failure (HF),1 the economic burden, number of hospitalizations, and penalties imposed for failure to meet hospital readmission expectations will continue to increase, thus placing pressure on clinicians to utilize successful HF monitoring interventions to improve these measures. In the US, the incidence of HF has mostly remained stable over recent decades, with >650,000 new cases diagnosed annually. The incidence of HF increases with age, with approximately 20 per 1,000 individuals aged 65–69 years affected compared with more than 80 per 1,000 among those aged ≥85 years.2
One in five Americans will be >65 years by 2050,3 and since the prevalence of HF is highest in this age group, the number of Americans with HF is projected to rise markedly in the future. The increased prevalence can be expected to increase total annual HF costs significantly from the current annual cost of US$40 billion.2 The Patient Protection and Affordable Care Act was passed in 2010 in the US. Beginning in the fiscal year 2013, certain hospitals with higher than expected 30-day HF readmissions will receive decreased Medicare payments.4
The United Nations reported in 2012 that the number of people >60 years was 11%, which is expected to reach 22% of the world’s population by the year 2050.5 Readmissions are also tracked in some European nations, and to reduce the cost burden of avoidable admissions, several of these countries are utilizing or developing large-scale delivery of telehealth services.6 Thus, a successful transition from hospital to home and the use of patient telephone monitoring has become increasingly important in an effort to reduce 30-day HF readmissions and overall progress in patient care.
Traditionally, ambulatory HF patients were seen in the office and decompensation was addressed reactively, often making it impossible to avoid hospital readmission. Telephone monitoring in patients with chronic HF utilizes a proactive approach in the care of such patients. For the purpose of this review, telephone monitoring is grouped into three categories, ie, structured telephone support (STS), telemonitoring, and remote implantable device monitoring. STS involves contact between the patient and the HF nurse or clinician at regular intervals, whereas telemonitoring involves similar contact but is based on transfer of physiologic data from patients at home that is monitored by their clinician. Remote device monitoring is often included when discussing telemonitoring because it provides more advanced physiologic measurements related to a patient’s HF status and is monitored by the patient’s defibrillator or resynchronization pacemaker. While these methods are distinct, they each involve telephone calls to the patient, with the goals of improving patient symptoms and quality of life, early recognition of clinical decompensation, and reducing emergency room and hospital admissions as well as overall HF expenditure. In this review, we provide an overview of theories employed in telemedicine studies, review comparative studies, and discuss novel approaches to remote and invasive monitoring for the clinical management of this patient population. We also address benefits, disadvantages, patient satisfaction, and adherence with telemonitoring approaches.
Theory in telemedicine
There are few articles referencing the theory behind telephone monitoring in the management of HF,7 which have only been marginally investigated.8 In an overview and analysis of telemedicine-related publications from 1990 to 2005 by Gammon et al, only 5% (83/1,615) referred to a theoretical concept. The main extent of theory discussion was limited, such as “theory X was successfully applied to …”.7 The majority of the theories utilized were imported from other disciplines, such as psychology, sociology, and economics, and applied to study aspects of telemedicine. In a review of mobile technologies, there was a lack of discussion on the health behavior theories or models that provide the basis of the intervention,9 thus emphasizing the limited explanation of theories employed in telemedicine.
Rogers’ diffusion of innovation theory10 was most widely used in the sample reported by Gammon et al7 and referenced in other studies.10,11 This model studies the various phases of diffusion, ie, the process by which an innovation is communicated through channels over time among the members of a social system.7 There are five characteristics associated with adopting an innovation, ie, relative advantage, compatibility, complexity, trialability, and observability.11 This theory has a sociologic focus on social groups and processes and has been used to predict and explain the rate of adoption of telemedicine.7,11
Davis’s technology acceptance model is a theory7 defined as “the users’ perceived usefulness and perceived ease of use of a new technology can explain the intention of users to use or not use the technology”.12 This concept can help to predict and clarify provider attitudes towards telemedicine. The actor-network theory was also referred to, and has the basic idea that technology is socially constructed.12 Actor networks consist of the aligned (or misaligned) interests of individuals or organizations.
Brown et al used the circumplex model of interpersonal traits to help understand the development of trust in collaborative telemedicine.13 This interpersonal behavioral model has 16 personality traits placed in a circle according to their relationship with dominance/control and nurturance/affiliation. Observed trust concerns about telemedicine include loss of patients to other doctors and less presence of the specialist. This theory may help to identify which clinicians may be more compatible and promote sustained use of a telemonitoring system.
Telemedicine (or telemonitoring) encompasses many of these described concepts by targeting patients as a social group with an innovative treatment that is compatible with their lifestyle. The patient is given an interest in his or her own health through transmission of data to the practitioner via a device such as a pacemaker or through communication with the practitioner relaying obtained data such as daily weights and vital signs. One goal is to maintain telemonitoring as a simple procedure for the patient, with the advantage of a more rapid response by practitioners in changing medical management when compared with waiting for a clinic visit to address for such changes. Placing more responsibility on the patient is seen as giving the patient more control over their medical care and ideally improves compliance with treatment. Trialability or success are determined by improved quality of life and reduced hospital admissions for patients.
Comparative studies
Earlier studies on STS and telemonitoring suggested a clear benefit on mortality and HF admissions.14 DIAL (the Randomized Trial of Telephone Intervention in Chronic Heart Failure) is one of the first STS studies and was performed in 51 centers in Argentina, with 1,518 patients randomized to STS by nurses or usual care. A 20% relative risk reduction was seen in the combined endpoint of hospitalization and mortality, although this was largely due to a reduction in hospitalizations.15
Four meta-analyses (by Clark et al [n=4,264], Klersy et al [n=8,612], Inglis et al [n=8,323], and Clarke [n=3,480])16–20 reported similar results, but were generally based on the same set of published studies.21 These meta-analyses showed that telemedical monitoring in chronic HF can reduce total mortality at a follow-up of 6–12 months and can reduce the number and duration of hospital admissions for worsening HF. The Cochrane Collaboration review and meta-analysis18 is the most complete review of telemedical trials in HF and includes 8,323 patients in 25 peer-reviewed studies, with eleven studies evaluating telemonitoring (n=2,710), 16 evaluating STS (n=5,613), and two testing both STS and telemonitoring. The STS review found a nonsignificant 12% reduction in the relative risk of mortality and a significant reduction in HF hospitalizations. Evaluation of the telemonitoring studies alone in the Cochrane review showed a 44% relative risk reduction for mortality. The authors concluded that “Structured telephone support and telemonitoring are effective in reducing the risk of all-cause mortality and chronic HF-related hospitalizations in patients with chronic HF; they improve quality of life, reduce costs, and evidence-based prescribing.”
WHARF (Weight Monitoring in Heart Failure) was an early study of telemonitoring that enrolled 280 patients with severe New York Heart Association (NYHA) class III and IV HF and randomly assigned these patients to weight monitoring using automated scales or to usual care.22 There was a 56% reduction in mortality in the monitored group. These results have not been replicated in larger studies.14
Home telemonitoring and nurse telephone support was compared with usual care in 426 patients from the TEN-HMS (Trans-European Network-Home-Care Management System) study. Usual care was provided by primary care physicians, nurse telephone support consisting of specialist nurses made available by telephone, and home telemonitoring including twice-daily patient self-measurement of weight, blood pressure, heart rate, and rhythm with automated devices linked to a cardiology center. Patients randomized to the usual care group had an alarming 45% one-year mortality rate in contrast with 27% in the nurse telephone support group and 29% in the home telemonitoring arm (P=0.032,23 Figure 1A).
  | Figure 1 Main outcomes of large-scale trials of telemedical treatment in patients with chronic heart failure (A) TEN-HMS trial:23 total mortality in each randomized group. (B) Tele-HF trial:24 Kaplan–Meier time-to-event estimates for the primary endpoint of readmission for any reason or death from any cause. (C) TIM-HF trial:25 Kaplan–Meier cumulative event curves for the primary endpoint of all-cause mortality. (D) CHAMPION:41 hospital admission due to cumulative heart failure during the entire period of randomised single-blind follow-up. Reprinted from The Lancet, 378(9792), Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. The Lancet, 731–739. Copyright © 2011, with permission from Elsevier.21 Copyright © 2011 Elsevier Ltd. All rights reserved. |
The Tele-HF (Telemonitoring to Improve HF Outcomes) study included 1,653 patients with chronic HF who were randomly assigned to a commercial automated interactive telephone voice-response system (n=826) or to usual care (n=827). Patients in the telemonitoring group completed a symptom questionnaire and provided body weight data which were reviewed by a physician every week day, except holidays. The primary endpoint was readmission for any reason or death from any cause within 180 days of enrollment. No significant difference was noted between the groups for rate of the primary outcome, which occurred in 432 patients (52%) in the telemonitoring group and in 426 (51%) patients in the usual care group (hazard ratio [HR] 1.04, 95% confidence interval [CI] 0.91–1.19, P=0.58,24 Figure 1B). However, patient compliance with the system was suboptimal, with 14% of patients in the telemonitoring group never using the system, only 55% of patients using the system at least three times per week by the final week of the study period, and 21% of the study patients not completing the 6-month final telephone interview.
The impact of remote telemedical management on mortality and hospitalization rates in ambulatory patients with HF was investigated in the Tim-HF (Telemedical Interventional Monitoring in Heart Failure) trial. Seven hundred and ten patients with NYHA class II or III HF were randomly assigned to either telemonitoring (n=354) or usual care (n=356). A self-assessed health questionnaire, blood pressure, electrocardiogram, and weight were transmitted via a personal digital assistant using Bluetooth wireless transmission and a mobile phone network to a central server. No mortality or HF hospitalization benefit was seen. Compared with usual care, telemonitoring had no significant effect on all-cause mortality (HR 0.97, 95% CI 0.67–1.41, P=0.87,25 Figure 1C). This study had a longer follow-up than most other telemonitoring studies. The latter two individual telemonitoring trial findings24,25 do not support the results of the previously mentioned four large meta-analyses. It is key to note the method and parameter being monitored in each study. Checking vital signs, weight, and an electrocardiogram focuses on individual patient issues and is not comparable with a telephone-based voice response system. The patient’s acceptance of the system and compliance play a role as well.14 The severity of HF, number of patients, patient clinical status, and usual care are also contributing factors because they differ across these studies. It is also unknown when interpreting these studies how much telemonitoring information each site had a responsibility to review, which group of staff members reviewed the information, how much of the data was acted upon, and what intervention took place. This could have a significant impact on the final outcomes.
Practical implications
Structured telephone support
STS, the first platform of telemedicine, involves contact between the patient and the HF nurse or clinician at regular intervals. Information is gathered about the patient’s condition through a structured telephone conversation at 24–72 hours post- discharge, in the 30-day transition period after discharge, and between clinic visits. Patients are given appropriate medical recommendations and directed to follow-up with their health care provider if evidence of deterioration is noted.
The hospital discharge phone call should be initiated within 2 business days according to the Center for Medicare and Medicaid Services guidelines for transition care.4 Not only does the Center for Medicare and Medicaid Services recommend that an interaction such as the discharge phone call be completed within 2 days, but this is also referred to in the most recent 2013 American College of Cardiology Foundation/American Heart Association HF guidelines as part of an effective system of care transition.2 This interaction between the provider and patient allows for follow-up regarding patient symptoms, weight, and pharmacotherapy. In addition, patients can receive continual education and encouragement to make the appropriate lifestyle changes needed to manage HF. As mentioned in the 2010 Heart Failure Society of America HF guidelines, education initiated during hospitalization should be supplemented and reinforced within 1–2 weeks after discharge.26
Telemonitoring
STS is a mode of telemonitoring, although telemonitoring is largely considered to involve technology and equipment to monitor data and transmit it to the health care provider. Telemonitoring generally should be initiated by the patient and can include physiologic parameters such as blood pressure, heart rate, weight, electrocardiography, or symptoms.14 With the rapid development and use of technology, the traditional STS has developed into telemonitoring platforms used to obtain and transfer patient data, such as personal digital assistants (smart phones) and the wireless communication infrastructure.21
Bui and Fonarow have commented that extensive use of mobile phone-based monitoring systems with application-based support of disease management and HF patient education can be a cost-efficient and convenient tool to improve home management of HF.27 Mobile phones are widely accessible, portable, less expensive than traditional telemonitoring,28 and can provide monitoring where mobile phone reception is available.27 The US Agency for Healthcare Research and Quality has recently provided substantial funding to support research on the use of wireless and telephone care management to reduce hospital readmissions for HF patients.29
At our institution, we have structured telemonitoring similar to that shown in Figure 2, ie, the Circle of Home Management of Heart Failure developed by Chaudhry et al in the Tele-HF trial.24 Our program utilizes STS and telemonitoring of data transmitted from patients. The remote HF diagnostic report is accessed by the office associate and reviewed by a registered clinical nurse in our HF program. The registered nurse reviews the impedance and parameters collected to identify any abnormalities, particularly an increased Optivol Fluid Index™, decreased impedance, an abnormal HF symptom questionnaire, or blood pressure, heart rate, or weight log specific to the Latitude™ remote monitoring system. Such abnormalities prompt a telephone call to the patient with standard HF questions designed by our clinic that focus on symptoms, weight, compliance with sodium/fluid restriction, and changes in medications. The reports are then sent to the nurse practitioner for review and recommendations, with final review by a physician prior to inclusion in the medical record. Remote data for each patient are reviewed on a monthly basis because this is what our staffing allows with the additional review of patient alerts.
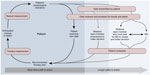  | Figure 2 The Circle of Home Management of Heart Failure. |
Remote implantable device monitoring
Optimizing medical therapy requires an accurate assessment of volume status by the clinician; therefore, symptom report and weight monitoring alone are often challenging to identify true HF decompensation given that they are not very sensitive markers. The use of remote monitoring technology for follow-up of patients with implantable devices, including implantable cardiac defibrillators and cardiac resynchronization therapy devices, is considered standard practice if indicated and can aid in identifying HF decompensation. The term “telemonitoring” is preferred when referring to devices designed solely to monitor patient status as opposed to routine remote monitoring of implantable cardiac defibrillators and cardiac resynchronization therapy devices to assess device performance and patient status indicators.30
Remote implantable device monitoring is available from five different medical technology companies. Medtronic Carelink™ (Minneapolis, MN, USA), Biotronik Home Monitoring™ (Lake Oswego, OR, USA), Boston Scientific Latitude (Natick, MA, USA), and St Jude Medical Merlin.net™ (St Paul, MN, USA) are accessible in the USA, and in addition to these, the Sorin Smartview™ (Milan, Italy) is available in Europe. Most are wireless and do not require the patient to manually download their data. Medtronic CareLink is the only one to still utilize a system where patients must place a wand over the device to transmit data.30
Parameters routinely monitored by devices include percentage of ventricular pacing, presence of arrhythmia, activity levels, heart rate variability, and mean heart rate at rest. Early clinician notification of atrial fibrillation through remote monitoring allows for appropriate anticoagulation, cardioversion, and other management decisions.30 This occurred a median of 148 days prior to the next scheduled in-office follow-up in 2009 in an Italian study of 166 patients using the home monitoring system.31 Most cardiac resynchronization therapy devices can estimate heart rate variability, a measure of autonomic nervous tone, and measure continuous heart rate variability as the standard deviation of 5-minute median atrial-to-atrial intervals (SDAAM). The results from a study by Adamson et al32 show that SDAAM <50 msec when averaged over 4 weeks were associated with an increased mortality risk (HR 3.20, P=0.02) and SDAAM were persistently lower over the entire follow-up period in patients who required hospitalization or died. SDAAM decreased at a median of 16 days before hospitalization and returned to baseline after treatment. Automated detection of decreases in SDAAM was 70% sensitive in detecting cardiovascular hospitalization.
Remote implantable device monitoring relies on the theory that routine monitoring of selected physiologic indicators will facilitate early detection of clinical deterioration and direct timely interventions to prevent adverse events and avoid hospitalization. Studies have shown that hemodynamic decompensation often precedes clinical decompensation, pointing to the need for more intense monitoring of ambulatory HF patients beyond standard management33 (ie, clinic follow-up several times a year, laboratory tests, standard cardiac testing such as echocardiography and electrocardiography, and patient instructions to monitor their weight and symptoms).
The four medical technology companies in the USA have device-based heart failure diagnostics. The Medtronic implantable cardiac defibrillator and cardiac resynchronization therapy devices measure thoracic impedance, which should decrease if significant pulmonary congestion is present and may provide an indication of the patient’s clinical status or impending cardiac decompensation. Trended daily impedance data have been shown to correlate well with pulmonary capillary wedge pressure.34 The OptiVol™ fluid index (Medtronic Inc) is also reported, which is the accumulation of the difference between the daily impedance and the reference impedance, adjusted for individual patient variation. The SENSE-HF (Sensitivity and Positive Predictive Value of Implantable Intrathoracic Impedance Monitoring as a Predictor of Heart Failure Hospitalizations) trial studied the OptiVol feature for monitoring of thoracic impedance to predict HF events.35 An intrathoracic impedance-derived fluid index had low sensitivity and a positive predictive value in the early period after implantation of a device in patients with chronic HF, but sensitivity improved within the first 6 months after implantation. Additionally, the thoracic impedance is not specific to HF alone because it can be affected by other conditions, such as pleural effusion, pneumonia, and pocket hematoma. The results of the PARTNERS-HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure) study demonstrated that patients with positive combined HF device diagnostics had a 5.5-fold increased risk of HF hospitalization with pulmonary signs or symptoms within the next month, indicating that they should be followed more closely.36 A diagnostic HF result was defined as a fluid index >100 Ω or any two of the following: a prolonged episode of atrial fibrillation, a rapid ventricular rate during atrial fibrillation, a high fluid index (≥60 Ω), low patient activity, low heart rate variability, high night heart rates, low percentage of cardiac resynchronization therapy pacing, or implantable cardiac defibrillator shocks. Biotronik (Berlin, Germany) and St Jude Medical remote implantable devices also have thoracic impedance data in their reports.
The Latitude report includes blood pressure and weight monitoring, an HF symptom questionnaire, and device diagnostics. DECODE (the Decompensation Detection Study) using the Latitude system data did not improve prediction of HF decompensation events.37 A 48% sensitivity for early detection of HF decompensation events was demonstrated with two false-positive events per patient per year.
Remote implantable device monitoring changes the pattern from the usual intermittent scheduled downloads to live transmitted data which can be helpful for addressing HF clinical status or decompensation. These remote diagnostic data allow for close monitoring of patients, but are dependent on availability and quantity of nursing or clinical staff to make patient telephone calls. Some offices or clinics may be able to view daily patient alerts and contact patients accordingly, which is ideal. However, others may find it feasible to review data only once a month. Standard HF questions can be directed to patients with evidence of HF decompensation and acted upon immediately, or the information can be given to the clinician for review. These diagnostic reports are another clinical tool used in the management of patients with chronic HF and are particularly useful for diuretic adjustments, but they should not be the only parameter reviewed when making medication changes.
Remote monitoring approaches rely on the principle that routine surveillance of select physiologic parameters will allow early detection of clinical decompensation and allow timely intervention to prevent negative outcomes. An effective home monitoring strategy, whether it is telemonitoring or remote device monitoring, must contain all the necessary elements to complete the circle of HF management38 (Figure 2).
The first step is to reliably measure physiologic indicators that reflect early decompensation, and the information must then be transmitted efficiently in a form that allows a timely response. Qualified clinicians must receive the information and recommend an intervention. The patient must receive the recommendation and correctly implement it. The final step, which does not always occur, is reassessment to determine if the issue is resolved or warrants further intervention.38
Invasive approaches
Data from clinical trials of implantable hemodynamic monitors suggest that weight gain may be inadequate to recognize decompensation in enough time to intervene and prevent hospitalization.39 The heart failure sensor developed by CardioMEMS Inc (Atlanta, GA, USA) is a 15 mm long and 3 mm wide device which is delivered through a catheter-based system from the femoral vein (Figure 3). The heart failure sensor has a three-dimensional housing of silicone and consists of a pressure-sensitive capacitor that is tethered into location by two nitinol loops to avoid migration.40 The pulmonary artery diameter must be 7–10 mm and placement is confirmed by selective angiography. The heart failure sensor is powered externally by an antenna placed on the back or side of the patient in the approximate location of the sensor. Detected frequency shifts are converted to real-time pressure waveform, after calibrating for atmospheric pressure. Pulmonary artery target pressures were a systolic of 15–35 mmHg, a diastolic of 8–20 mmHg, and a mean of 10–25 mmHg with the use of neurohormonal, diuretic, and/or vasodilator therapy.40 Patients remain on warfarin if indicated for other reasons or are placed on an aspirin/clopidogrel combination for 30 days.
The CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients) study is a multicenter, prospective, randomized, controlled, single-blind clinical trial that evaluated the safety and efficacy of the heart failure sensor used to measure pulmonary artery pressures and the effectiveness of pressure-guided therapy in reducing HF-related hospitalizations in NYHA Class III patients with acute decompensation. The study met its primary efficacy endpoint, with a 30% reduction in HF hospitalization rates at 6 months (HR 0.64, 95% CI 0.55–0.75, P<0.001) and 38% in annualized rates in HF patients whose treatment was guided by pulmonary artery pressures obtained through this small permanent wireless implant41 (Figure 1D). Improvement was also seen in mean pulmonary artery pressures, quality of life, and days alive out of hospital. Advantages to this system compared with others are implantation through right heart catheterization, lack of battery change out, and the wireless feature of the sensor.40 This device is currently under review by the US Food and Drug Administration.
Other invasive devices include the left atrial pressure sensor with the HeartPOD® device (St Jude Medical).42 Patients can be engaged in their pharmacologic management through provider-programmed medication adjustments (usually diuretics and vasodilators) based on the value of measured left-sided filling pressures.
Clinical perspectives: benefits and disadvantages
HF disease management programs provide frequent monitoring, either telephone-based or telemonitoring-based, which is designed to detect changes in fluid and/or hemodynamic status and implement short-term interventions to restore clinical stability and prevent hospitalizations.43 Elements of HF programs may be grouped into the following categories: monitoring of salt and fluid management, sustained therapeutic modifications, and patient education.
A systematic review of randomized trials showed that multidisciplinary management strategies for HF patients achieved a 27% reduction in HF hospitalization rates and a 43% reduction in total number of HF hospitalizations.44 The recent American College of Cardiology Foundation/American Heart Association and European Society of Cardiology HF guidelines recommend that HF patients be followed in an HF disease management program,2,45 particularly those at high risk for hospital readmission.2 HF disease management programs attempt to shift care to self-management, promote self-care,2,27 and recommend remote monitoring of symptoms.45 The multidisciplinary HF team consists of physicians, nurse practitioners, registered nurses, a social worker, a dietician, the pharmacy, and the device clinic. Psychology, sleep medicine, and palliative care may also work closely with the multidisciplinary team.
Self-care or self-management is an essential component of a chronic illness such as HF, and it is important for such patients to be engaged in their health care to best utilize the telephone monitoring intervention. Self-care is defined as a process whereby individuals and/or their caregivers perform the daily activities that serve to maintain or restore health and well-being, prevent illness, and manage chronic illness. Self-care, when done correctly, is associated with prevention and early detection of health problems or decompensation, better overall health, and quality of life, and in HF, improved clinical outcomes and reduced health care costs.46 It has been shown that up to 64% of HF hospitalizations could be prevented by adherence to the prescribed medication and diet, but up to 90% of HF patients do not fully adhere to their recommended regimens.47
Self-efficacy is defined as the confidence one has in performing a specific behavior and is essential to making sustained behavioral changes. Weaker self-efficacy beliefs are associated with nonadherence to self-care recommendations for HF.47 The positive effect of such an intervention was seen in two studies: one study tested a sliding scale diuretic titration protocol in HF patients where patients using this self-management strategy had increases in their exercise tolerance, quality of life, and fewer emergency department visits;48 and the other study admitted HF patients randomized to an HF management group and taught self-management skills compared with usual care.49 Unfortunately, the majority of patients with HF are not followed in an HF disease management program but by primary care physicians. Many live in rural areas where there is limited access to health care, thus making the challenge of extending telemedicine to a greater population and teaching self-care to these patients a primary goal. Our program focuses on self-efficacy for patients who demonstrate an ability to follow a sliding scale diuretic titration protocol based on their daily weights, eg, a patient on a maintenance furosemide dose of 40 mg twice a day is instructed to take an extra 40 mg tablet if his or her weight has increased by 2 lb in a 24-hour period or by 5 lb over one week.
Financial implications become an important part of the decisions regarding what specific therapies can be offered to HF patients once they have been discharged from hospital. In the US, the Center for Medicare and Medicaid Services has recently established guidelines to bill for transitional care beginning on the date of discharge from the inpatient hospital setting and continuing for the next 29 days.4 The following components are included in the transitional care management services: interactive contact within 2 business days following discharge via telephone, email, or face-to-face, followed by a face-to-face visit within 7–14 days depending on the medical complexity of the patient. This interactive contact can be successfully completed via the discharge phone call and by licensed clinical staff under the direction of a physician or nurse practitioner.
Currently there is no reimbursement available for telephone nursing support but reimbursement occurs for remote implantable device monitoring analysis. Current procedural terminology (CPT) code 93297 may be utilized by a physician or nurse practitioner when reviewing, analyzing, and reporting one or more recorded physiologic cardiovascular data elements from an implantable cardiovascular monitor system obtained from a remote setting. CPT code 93290 is used in the clinic setting, whereas CPT code 93299 deals with the technical aspects of receiving the remote data.50 Cost-effectiveness should increase by targeting patients such as recent hospital discharges who have the highest chance of adverse events and the greatest opportunity for improvement in diet and medication adherence.
Several studies have shown that the elderly can utilize telephone monitoring51,52 and mobile phone applications53 with favorable outcomes. The three-lead tele-electrocardiogram seems to be as reliable as the 12-lead device in elderly patients,54 and these data can be very helpful to the clinician when reviewing patient symptoms. Many patients do not check their weights as directed, and more possibly would if they were followed in a telemonitoring program.27 Remote implantable device monitoring, in most cases, eliminates the patient compliance component because data are automatically available to the clinician. Patients feel at ease when someone is “looking out” for them, and telemedicine increases the number of patient encounters. Frequent follow-up of patients may provide early warning signs if a patient is deteriorating.
Some research demonstrates that shifting the burden of self-care management to patients has not been effective due to the complexity of therapy, the advancing age of HF patients, and marginal or inadequate health literacy.55 Scherr et al showed that the elderly had some difficulties using a telemonitoring system with mobile phones.56
Disadvantages with telemedicine include availability and commitment to review the transmitted patient data. Most centers using telemedical approaches do not operate 24 hours a day 7 days a week, thus leaving some concern over legal implications for abnormal data not viewed instantly.21 Alerts may be lost or not answered in a timely manner due to limited staff or time. The remote data can be reviewed rapidly, but telephone contact with the patient to provide medical recommendations, education, and scheduling an appointment if needed can be time-consuming. One nurse should care for 50 patients in telephone support structures, and current remote management systems employ one staff member per 200–300 patients during the daytime.21 Employing additional staff to carry out these measures is expensive. Additionally, the newer investigational remote monitoring devices, such as the heart failure sensor monitor and HeartPOD devices, are costly, given their invasive nature and the risk of associated device complications.
Long-term impact on patient satisfaction and treatment adherence
Patient adherence and satisfaction with remote monitoring overall seems to be high.30 A meta-analysis of remote monitoring of HF patients demonstrated high patient acceptance of STS, with improvement in quality of life and medication adherence.17 A study by Clark et al showed that the elderly found telephone monitoring to be acceptable as part of their health care routine, adapted quickly, and adhered well for 12 months.52 Patients seem to be more satisfied and are likely to be more adherent if the telemonitoring platform is user-friendly and not intrusive or time-consuming. To increase the success of the telemedicine intervention, the patient must have a reasonable cognitive level, want to play an active role in their care, and be interested in the technology.57
Patient satisfaction as an outcome has been poorly reported and presented in telemedicine studies. Kraai et al58 reviewed 14 papers, and found that poorly constructed instruments and inadequately designed measurements were used in the majority of the research reported. In review, the overall consensus of patients was above average satisfaction with their telemedicine experience. This increased satisfaction was more directly correlated with noninvasive telemonitoring or STS. Although these results were deemed positive, the underexposure of patient satisfaction as an outcome within telemedicine leaves room for further exploration.
Conclusion
Chronic HF management is a medical and economic challenge in both developed countries and rural areas where specialized care is scarce. Despite advances in HF treatment, morbidity and mortality remain high, with 20%–30% of patients being readmitted after 30 days and 50% within 6 months. While the effectiveness of telemedicine in HF is not consistent across studies, many have demonstrated reduction in all-cause mortality, reduction of HF-related hospitalization and costs, and improvement in quality of life.
Telephone monitoring appears to be a feasible intervention for reducing admission rates and bridging the gap between the patient and clinician, but future research should focus on identifying the target population most likely to respond to the telemedicine intervention. It is essential that patients are taught to master self-care and self-management skills and they should be offered the opportunity to become actively involved in their health care, particularly diuretic management. Telemedical home assessment units to measure biomarkers, such as brain natriuretic peptide monitoring, may soon be on the horizon and are currently being studied in clinical trials.59
System design, adequate staffing, patient satisfaction, and treatment adherence are important for success of the telemonitoring system. With the persistent worldwide economic burden of HF, home monitoring of HF patients should progress from clinic and home visits with promotion of self-care and self-efficacy to the utilization of telemedicine and remote monitoring of implantable devices. Telephone monitoring seems to be an effective approach in the chronic HF population. In the future, large-scale telemonitoring programs may come into place as well as additional remote implantable monitoring devices to transmit physiologic data obtained from individual patients. Expanding theoretical models could also benefit research in telemedicine. Having a consistent foundation in this field could improve the communication process between patients and practitioners, provide patients with an active role and greater satisfaction in their management, and advance telemedicine in terms of efficient response times and outcome benefit.
Disclosure
The authors report no conflicts of interest in this work.
References
World Health Organization. Chronic diseases and health promotion. Available from: http://www.who.int/chp/en/. Accessed September 4, 2013. | |
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–1852. | |
United Nations Department of Economics and Social Affairs. World population prospects: the 2012 Revision. Available from: http://esa.un.org/wpp/. Accessed August 8, 2013. | |
CMS.gov [homepage on the Internet]. c2013. Available from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed September 4, 2013. | |
United Nations Department of Economics and Social Affairs. Available from: http://www.un.org/en/development/desa/population/publications/pdf/popfacts/popfacts_2012-4.pdf. Accessed September 4, 2013. | |
Burrillreport.com. Hospital Readmissions in Europe. Available from: http://www.burrillreport.com/content/Hospital-Readmissions-Europe_HiRes.pdf. Accessed September 4, 2013. | |
Gammon D, Johannessen LK, Sørensen T, Wynn R, Whitten P. An overview and analysis of theories employed in telemedicine studies: a field in search of an identity. Methods Inf Med. 2008;47:260–269. | |
Lehoux P, Sicotte C, Denis JL, Berg M, Lacroix A. The theory of use behind telemedicine: how compatible with physicians’ clinical routines? Soc Sci Med. 2002;54:899–904. | |
Riley WT, Rivera DE, Atienza AA, Nilsen W, Allison SM, Mermelstein R. Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med. 2011;1:53–71. | |
Zanaboni P, Wootton R. Adoption of telemedicine: from pilot stage to routine delivery. BMC Med Inform Decis Mak. 2012;12:1. | |
Spaulding RJ, Russo T, Cook DJ, Doolittle GC. Diffusion theory and telemedicine adoption by Kansas health-care providers: critical factors in telemedicine adoption for improved patient access. J Telemed Telecare. 2005;11 Suppl 1:107–109. | |
Mackert M. Expanding the theoretical foundations of telemedicine. J Telemed Telecare. 2006;12:49–52. | |
Brown HG, Poole MS, Rodgers TL, et al. Trust, trait theory, and collaboration in telemedicine: a circumplex perspective. In: Proceedings of the 36th Hawaii International Conference on System Sciences. 2003:1–10. | |
Stoyanov N, Paul V. Clinical use of telemonitoring in chronic heart failure: keeping up with the times or misuse of time? Curr Heart Fail Rep. 2012;9:75–80. | |
GESICA Investigators. Randomised trial of telephone intervention in chronic heart failure: DIAL trial. BMJ. 2005;331:425–427. | |
Clark RA, Inglis SC, McAlister FA, Cleland JG, Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ. 2007;334:942. | |
Klersy C, De Silvestri A, Gabutti G, Regoli F, Auricchio A. A meta-analysis of remote monitoring of heart failure patients. J Am Coll Cardiol. 2009;54:1683–1694. | |
Inglis SJ, Clark RA, McAlister FA, et al. Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane Database Syst Rev. 2010;8:CD007228. | |
Inglis SC, Clark RA, McAlister FA, Stewart S, Cleland JGF. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structures telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane Review. Eur J Heart Fail. 2011;13:1028–1040. | |
Clarke M, Shah A, Sharma U. Systematic review of studies on telemonitoring of patients with congestive heart failure: a meta-analysis. J Telemed Telecare. 2011;17:7–14. | |
Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. Lancet. 2011;378:731–739. | |
Goldberg LR, Piette JD, Walsh MN, et al. A daily electronic home monitoring system in patients with advanced heart failure improves survival: the WHARF (weight monitoring in heart failure) trial. Am Heart J. 2003;146:705–712. | |
Cleland JGF, Louis AA, Rigby AS, Janssens U, Balk AH; TEN-HMS Investigators. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) Study. J Am Coll Cardiol. 2005;45:1654–1664. | |
Chaudhry SI, Mattera JA, Curtis JP. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. | |
Koehler F, Winkler S, Schieber M. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123:1873–1880. | |
Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:475–539. | |
Bui AL, Fonarow GC. Home monitoring for heart failure management. J Am Coll Cardiol. 2012;59:97–104. | |
Seto E, Leonard KJ, Masino C, Cafazzo JA, Barnsley J, Ross HJ. Attitudes of heart failure patients and health care providers towards mobile phone-based remote monitoring. J Med Internet Res. 2010;12:e55. | |
Agency for Healthcare Research and Quality. Evidence generation awards. Available from: http://archive.ahrq.gov/funding/arra/awards/awevgen.html. Accessed September 4, 2013. | |
Cronin EM, Varma N. Remote monitoring of cardiovascular implanted electronic devices: a paradigm shift for the 21st century. Expert Rev Med Devices. 2012;9:367–376. | |
Ricci RP, Morichelli L, Santini M. Remote control of implanted devices through home monitoring technology improves detection and clinical management of atrial fibrillation. Europace. 2009;11:54–61. | |
Adamson PB, Smith AL, Abraham WT, et al; InSync III Model 8042 and Attain OTW Lead Model 4193 Clinical Trial Investigators. Continuous autonomic assessment in patients with symptomatic heart failure; prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110:2389–2394. | |
Adamson PB, Magalski A, Braunschweig F, et al. Ongoing right ventricular hemodynamics in heart failure. Clinical value of measures derived from an implantable monitoring system. J Am Coll Cardiol. 2003;41:565–571. | |
Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–848. | |
Conraads VM, Tavazzi L, Santini M. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE-HF trial. Eur Heart J. 2011;32:2266–2273. | |
Whellan DJ, Ousdigian KT, Al-Khatib SM, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure) study. J Am Coll Cardiol. 2010;55:1811–1813. | |
Gilliam FR III, Ewald GA, Sweeney RJ. Automated HF decompensation detection: results from the DECOmpensation DEtection study (DECODE). Available from: http://www.innovationsincrm.com/cardiac-rhythm-management/2012/april/258-automated-heart-failure-decompensation-detection | |
Desai AS, Stevenson LW. Connecting the circle from home to heart-failure disease management. N Engl J Med. 2010;363:2364–2367. | |
Zile MR, Bennett TD, St John Sutton M. Transition from chronic compensated to acute decompensated heart failure: Pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. | |
Hasan A, Paul V. Telemonitoring in chronic heart failure. Eur Heart J. 2011;32:1457–1464. | |
Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. | |
Walton AS, Krum H. The Heartpod implantable heart failure therapy system. Heart Lung Circ. 2005;14S:S31–S33. | |
Konstam MA, Konstam V. Heart failure disease management: a sustainable energy source for the health care engine. J Am Coll Cardiol. 2010;56:379–381. | |
McAlister FA, Stewart S, Ferrua S, McMurray J. Multidisciplinary strategies for the management of heart failure patients at high risk for admission. J Am Coll Cardiol. 2004;44:810–819. | |
McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. | |
Moser DK. Conceptualizing self-care in heart failure: A life course model of patient characteristics. J Cardiovasc Nurs. 2008;23:205–218. | |
Ciere Y, Cartwright M, Newman SP. A systematic review of the mediating role of knowledge, self-efficacy, and heart failure behaviour in telehealth patients with heart failure. J Telemed Telecare. 2012;18:384–391. | |
Prasun MA, Kocheril AG, Klass PH, Dunlap SH, Piano MR. The effects of a sliding scale diuretic titration protocol in patients with heart failure. J Cardiovasc Nurs. 2005;20:62–70. | |
Wright SP, Walsh H, Ingley KM, et al. Uptake of self-management strategies in a heart failure management programme. Eur J Heart Fail. 2003;5:371–380. | |
Centers for Medicare and Medicaid Services. Physician fee schedule look-up tool. Available from: http://www.cms.hhs.gov/PfsLookup/. Accessed September 18, 2013. | |
Antonicelli R, Testarmata P, Spazzafumo L, et al. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare. 2008;14:300–305. | |
Clark RA, Yallop JL, Piterman L, et al; CHAT Study Team. Adherence, adaptation and acceptance of elderly chronic heart failure patients to receive healthcare via telephone-monitoring. Eur J Heart Fail. 2007;9:1104–1111. | |
Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. J Med Internet Res. 2012;14:e31. | |
Antonicelli R, Ripa C, Abbatecola AM, Capparuccia CA, Ferrara L, Spazzafumo L. Validation of the 3-lead tele-ECG versus the 12-lead tele-ECG and the conventional 12-lead ECG method in older people. J Telemed Telecare. 2012;18:104–108. | |
Bhimaraj A. Remote monitoring of heart failure patients. Methodist DeBakey Cardiovasc J. 2013;9:26–31. | |
Scherr D, Kastner P, Kollman A, et al. Effect of home-based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: randomized controlled trial. J Med Internet Res. 2009;11:e34. | |
Paré G, Moqadem K, Pineau G, St-Hilaire C. Clinical effects of home telemonitoring in the context of diabetes, asthma, heart failure and hypertension: a systematic review. J Med Internet Res. 2010;12:e21. | |
Kraii IH, Luttik MLA, De Jong RM, Jaarsma T, Hillege HL. Heart failure patients monitored with telemedicine: patient satisfaction, a review of the literature. J Card Fail. 2011;17:684–690. | |
Maisel A, Barnard D, Jaski B, et al. Primary results of the HABIT trial (heart failure assessment with BNP in the home). J Am Coll Cardiol. 2013;61:1726–1735. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.