Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 12
Role of stem cells in fertility preservation: current insights
Authors Vermeulen M , Giudice MG, Del Vento F, Wyns C
Received 16 January 2019
Accepted for publication 24 May 2019
Published 5 August 2019 Volume 2019:12 Pages 27—48
DOI https://doi.org/10.2147/SCCAA.S178490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Maxime Vermeulen,1 Maria-Grazia Giudice,1,2 Federico Del Vento,1 Christine Wyns1,2
1Gynecology-Andrology Research Unit, Institut de Recherche Expérimentale et Clinique (IREC), Université Catholique de Louvain, Brussels, 1200, Belgium; 2Department of Gynecology-Andrology, Cliniques Universitaires Saint-Luc, Brussels 1200, Belgium
Abstract: While improvements made in the field of cancer therapy allow high survival rates, gonadotoxicity of chemo- and radiotherapy can lead to infertility in male and female pre- and postpubertal patients. Clinical options to preserve fertility before starting gonadotoxic therapies by cryopreserving sperm or oocytes for future use with assisted reproductive technology (ART) are now applied worldwide. Cryopreservation of pre- and postpubertal ovarian tissue containing primordial follicles, though still considered experimental, has already led to the birth of healthy babies after autotransplantation and is performed in an increasing number of centers. For prepubertal boys who do not produce gametes ready for fertilization, cryopreservation of immature testicular tissue (ITT) containing spermatogonial stem cells may be proposed as an experimental strategy with the aim of restoring fertility. Based on achievements in nonhuman primates, autotransplantation of ITT or testicular cell suspensions appears promising to restore fertility of young cancer survivors. So far, whether in two- or three-dimensional culture systems, in vitro maturation of immature male and female gonadal cells or tissue has not demonstrated a capacity to produce safe gametes for ART. Recently, primordial germ cells have been generated from embryonic and induced pluripotent stem cells, but further investigations regarding efficiency and safety are needed. Transplantation of mesenchymal stem cells to improve the vascularization of gonadal tissue grafts, increase the colonization of transplanted cells, and restore the damaged somatic compartment could overcome the current limitations encountered with transplantation.
Keywords: transplantation, fertility restoration, mesenchymal stem cells, germ-line stem cells, spermatogonial stem cells, in vitro maturation
Introduction
Some years ago, fertility preservation (FP) emerged as a treatment aiming to preserve future reproductive capacity of individuals facing therapies that could potentially affect their gonads, the majority being patients diagnosed with cancer.1 Indeed, chemo- and radiotherapy are associated with gonadotoxicity in both males and females.2 Other health conditions can motivate FP, such as genetic abnormalities or autoimmune diseases.3,4 For adult men or adolescents, cryopreservation of ejaculated or surgically retrieved sperm is routinely proposed before gonadotoxic therapies, while for prepubertal boys, cryopreservation of a testicular biopsy containing spermatogonial stem cells (SSCs) is now ethically accepted as the only way to offer an FP strategy from the perspective of future developments allowing parenthood.5 Several studies have broached the feasibility of cryopreservation of immature testicular tissue (ITT),6–13 and some teams have developed protocols for its clinical implementation.7,10,12,14 Although still at the research stage, autotransplantation and in vitro maturation (IVM) of ITT or SSCs have been considered to restore fertility from cryopreserved ITT. Restoration of the damaged SSC niche with mesenchymal stem cells (MSCs) was also recently proposed to enhance or restore endogenous spermatogenesis.15
For women, cryopreservation of oocytes or embryos is the most common way to preserve fertility.16,17 However, while oocyte cryopreservation may also be proposed to adolescent girls, it cannot be proposed before puberty or to adult women requiring urgent therapy. Cryopreservation of ovarian tissue containing primordial follicles may be proposed with an aim to transplant it back to the patient after cure, a technique that has already proved its efficacy with births of healthy babies.18 However, early postgrafting follicle loss has motivated researchers to improve the procedure, and potential neoplastic tissue contamination (making it unsafe for transplantation) increases the need to find alternative FP methods.
While SSCs, originating from differentiation of gonocytes after birth, continuously divide asymmetrically to give rise to new SSCs and differentiating germ cells,19 embryonic oogonia enter a resting stage (prophase of meiosis I) and undergo final maturation only at the onset of puberty, thus constituting a fixed ovarian reserve that decreases during a lifetime.20 This classical scheme was questioned during the last decade with the discovery of potential female germ-line stem cells (FGSCs) in the ovary, opening a debate that is not over yet.21
In this review, we present current FP approaches for male and female patients facing gonadotoxic therapies and methods that could be applied to improve their impaired fertility using cryostored gonadal material and other sources of stem cells (SCs) that may enhance in vitro and vivo germ-cell differentiation or develop into gametes.
Materials and methods
Methods
A search was performed on PubMed using the following combination of terms without time limitation: ([fertility] AND [restoration OR preservation]) AND (stem cell OR germline stem cell OR oogonia OR spermatogonial stem cell OR spermatogonia). Articles in languages other than English, guidelines, reviews, and scientific video protocols were excluded.
Results
Literature search
Figure 1 shows a flowchart describing the selection of papers. From the 458 results, 60 focusing on the main topic were selected and 136 added for their relevance to understanding and discussion.
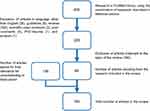 |
Figure 1 Flowchart of paper selection. |
SSCs to restore fertility in the male
SSCs are known as a subpopulation of spermatogonia localized at the basement membrane of seminiferous tubules (STs) and estimated to represent 0.03% of germ cells in the adult mouse.22 These diploid SCs are able both to self-renew and give rise to differentiated haploid cells at the end of the spermatogenic process.19
Due to the smallness of testicular biopsies taken for cryopreservation in prepubertal boys, the scarcity of SSCs in the testes,23 the low efficiency of the transplantation process observed in mice and nonhuman primates,24,25 and the low proportion of human haploid germ cells generated with IVM,26 amplification of SSCs is an essential step for fertility restoration.
SSC propagation
The development of SSC propagation–culture systems has mainly been achieved through studies in rodents. In 2003, Kanatsu-Shinohara et al reported the first long-term amplification of murine SSCs for >5 months in a specific medium containing glial cell line-derived neurotrophic factor (GDNF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and leukemia inhibitory factor (LIF), which were considered as essential for SSC culture.27 Indeed, both in vivo and vitro studies brought evidence that GDNF plays a pivotal role in SSC self-renewal.28,29 Moreover, bFGF was shown to potentiate the effect of GDNF, as addition of bFGF to culture media containing GDNF increased the number of SSC colonies compared to culture without bFGF,28 while LIF and EGF were shown to act on SSC colony formation30 and diameter,31 respectively. Subsequently, several teams attempted to find a culture system of dissociated testicular cell suspensions (TCSs) able to propagate human SSCs in vitro (Table 1).32–51 Sadri-Ardekani et al adapted the protocol developed by Kanatsu-Shinohara et al for human testicular cells (TCs). Briefly, this culture system relies on the capacity of somatic cells to adhere to the plate while the germ-cell fraction stays in suspension, allowing enrichment of SSCs after differential plating.32,33 This technique led to an 18,450-fold enrichment of adult SSCs after 64 days and to a 9.6-fold enrichment of prepubertal SSCs after 11 days of culture using xenotransplantation as the gold standard to identify SSCs able to migrate along the basement membrane of the STs, colonize their niches, and generate germ-cell colonies. Among researchers who have xenotransplanted long-term cultured human SSCs,32,33,39,40,42,43,51 only Sadri-Ardekani et al and Nickkholgh et al quantified SSCs in STs after transplantation and demonstrated SSC enrichment.32,33,43 However, several other teams using the same protocol could not reproduce such results due to the complexity and skills needed to distinguish between SSCs and human embryonic stem cell-like (hESC-like) cells,34 because of low germ-cell survival and overgrowth of remaining somatic cells.35,47 Indeed, the importance of the germ- versus somatic-cell ratio in culture was demonstrated, showing an impact on SSC proliferation.52,53 The influence of the medium was also pinpointed when Gat et al observed more germ-cell aggregate formation when using DMEM/F12 instead of StemPro-34.54 Others also examined the efficiency of differential plating to select germ cells from TCSs, but did not find a difference in germ-cell numbers recovered from whole TCSs and differentially plated cells after 14 days of culture.55 To improve SSC propagation, cell sorting prior to culture was further applied. Coculture of SSCs sorted by fluorescence-activated cell sorting based on their HLA–/EPCAM+ phenotype onto inactivated somatic feeder cells resulted in putative SSCs coexpressing DDX4 and UTF1, although their proliferation rate was poor and no survival was found after 4 weeks.55 Other phenotypic markers, ie, GFRα1, GPR125, SSEA-4, KIT–/ITGβ1–, CD9, ITGα6, THY1, and FGFR3, have been used to select monkey or human SSCs,38,43,44,48–50,56–65 but among 16 studies, only 5 cultured the sorted SSCs.38,48–50,59 Lim et al succeeded in long-term culture of CD9-sorted spermatogonia onto laminin-coated plates, but reported a low proliferation rate (20,000–80,000 cells in 130 days).49 However, when GPR125 was used to select spermatogonia from testicular tissue (TT) of patients diagnosed with obstructive azoospermia, a five fold enrichment was achieved in the first month when cultured onto hydrogel without a feeder layer.38 While the authors claimed an advantage of their system over differential plating, as it avoided overgrowth of somatic cells, the SC potential was not evaluated. Human SSC sorting based on their SSEA-4 expression was performed by two teams with contradictory results, since one reported successful SSC amplification for 21 weeks onto Matrigel,48 while the second achieved amplification only onto γ-irradiated feeder cells and observed an inability of SSCs to attach to Matrigel.59 Coculture of ITGα6+ SSCs onto collagen-coated plates with Sertoli cells allowed a five fold increase in colony numbers.50 Culturing unsorted cells prior to cell selection has also been attempted, showing that 50 days in the same culture conditions followed by isolation of ITGα6+ cells resulted in a seven fold enrichment of SSCs.43
 |
Table 1 Studies including long-term culture (>1 month) of human SSCs |
 |
Table 1 (Continued). |
 |
Table 1 (Continued). |
 |
Table 1 (Continued). |
Together, these results point to the need to identify the best method to propagate SSCs most efficiently. Recently, Bhang et al discovered that human endothelial TCs secreted GDNF, bFGF, stromal cell-derived factor-1 (SDF-1), macrophage inflammatory protein 2, and insulin-like growth factor-binding protein 2 and could support SSC growth for at least 150 days.51 It also appeared that cells with MSC characteristics were able to support spermatogonia in vitro. Indeed, Smith et al showed that a THY1+ fraction isolated from TCSs was of mesenchymal origin and could support SSEA-4+ SSC growth, while mouse embryonic fibroblasts and human placental and fetal testicular stromal cells could not.59 Interestingly, human umbilical perivascular cells (HUPVCs), which are also of mesenchymal origin and share common properties with somatic TCs (LIF, bFGF, and BMP4 secretion as well as expression of testicular extracellular matrix markers) also supported germ-cell proliferation and survival.66
SSC transplantation
Spermatogenesis restoration can be achieved both by injection of isolated SSCs into germ-cell-depleted testes and transplantation of an ITT piece where SSCs remain within their intact niche or original microenvironment.67
Transplantation of isolated SSCs
The first success using SSC transplantation to restore fertility was achieved in mice by Brinster and Avarbock who reported complete spermatogenesis and offspring after SSC injection into STs of busulfan-sterilized mice.68 In order to evaluate the capacity of transplanted SSCs to colonize their niche, recipient mice were injected intraperitoneally with busulfan inducing germ-cell depletion and improving donor SSC colonization (Figure 2). Recently, a higher proportion of donor-derived offspring generation was reported when busulfan was injected directly into testes.69 The power of the technique for FP was further demonstrated with offspring in several species, including rats, goats, chickens, and sheep, and embryo development in nonhuman primates.70–74 The spermatogenic process has also been completed in bovines, pigs, and dogs, but sperm functionality was not evaluated.75–77 In addition, cryopreservation of mouse, rat, rabbit, and baboon SSCs did not affect their viability neither their ability to colonize mouse STs,78 and culture of thawed mouse78-80 and rat78 SSCs resulted in spermatogenesis after transplantation. The safety of the procedure was studied in mice, and although differences in histone acetylation of germ cells were observed,81 no modifications in the genomes of offspring were found.82 In addition, propagation of moue SSCs before transplantation did not increase the incidence of cancer or decrease the survival of mice that had undergone SSC transplantation.83
In view of these encouraging results, SSC transplantation is considered a potential fertility-restoration method for future clinical application (Figure 3). Using cadaver testes, ultrasound-guided injection in the rete testis has been determined as the best technique for cell transplantation in larger testes.84,85
So far, only one report has described autotransplantation of cryopreserved human TCSs in patients cured of non-Hodgkin’s lymphoma, but no follow-up was published.86 An important clinical concern is the risk of cancer-cell contamination of the TCSs to be transplanted, since transplantation of only 20 leukemic cells in rats has resulted in cancer relapse.87 To address this issue, several teams searched for extracellular markers allowing separation of human SSCs from cancer cells but completely safe purification is not yet possible using cell-sorting techniques.56,58,88–90 However, the culture protocol developed by Sadri-Ardekani et al allowed elimination of malignant cells added to the cell suspension, and may represent a good alternative to sorting approaches.46
Furthermore, long-term culture of human SSCs did not show increased chromosomal abnormalities in another study, but methylation assays demonstrated demethylation of three paternally imprinted genes and increased methylation of two maternally imprinted genes after 50 days.44 The impact of such modifications on offspring are not known and difficult to predict. While it is possible that once transplanted, SSCs and generated spermatozoa could retrieve a normal methylation pattern, it was also hypothesized that cultured and transplanted human SSCs might be unable to enter meiosis or lead to embryos that will degenerate because of their inability to pass cellular checkpoints.44
Transplantation of ITT (SSCs within their niche)
The main aim of tissue transplantation rather than cell transplantation is that cellular interactions within the SC niche are preserved, which is important for germ-cell proliferation and maturation.91 However, as grafting of thawed ITT contaminated by leukemic cells has resulted in development of generalized leukemia in rats,92 this technique must be restricted to nonhematological or nonmetastasizing cancers and to benign disorders requiring gonadotoxic therapies.
Xenotransplantation of mouse, rabbit, porcine, Japanese quail, and cynomolgus monkey ITT to nude mice leads to offspring generated with sperm retrieved from the in vivo matured grafts.93–96 With regard to human ITT, experiments have shown a blockade of differentiation at the pachytene spermatocyte stage, probably due to the phylogenetic distance between the mice and humans.11,97 Different grafting sites have been put forward. Intratesticular grafting was proposed as a grafting site, assuming that it could be advantageous to transplant the tissue into its natural environment with high testosterone levels and that breeches created in the parenchyma to insert the graft favor donor SSC colonization, although human germ-cell differentiation was still arrested at the spermatocyte stage.98,99 For obvious microbiological reasons, xenotransplantation cannot be considered for clinical purposes. Autologous transplantation of ITT, however, suppresses such animal contamination risks (Figure 3). Initially, ectopic transplantation in monkeys showed meiotic arrest.100,101 Importantly, Jahnukainen et al reported sperm maturation after autologous grafting of cryopreserved ITT into the scrota of busulfan-treated monkeys, suggesting that the technique could be translated to the clinic.13 Very recently, this potential was further supported by successful production of sperm and generation of a healthy baby following autologous transplantation of rhesus macaque ITT. Interestingly, offspring were obtained with sperm recovered from a scrotal graft, but the authors did not detect any differences in the percentage of STs displaying complete spermatogenesis between grafting sites (back skin and scrotum).102
In vitro maturation of SSCs
The aim of IVM is to promote in vitro differentiation of SSCs into spermatozoa able to fertilize an oocyte during an assisted reproductive technology (ART) procedure (Figure 3). This strategy presents an advantage over transplantation to avoid the risk of disease relapse in cases of tissue contamination with neoplastic cells.
IVM of dissociated TCs
In mammals, in vitro differentiation of germ cells seems to require a 3D rather than 2D environment considering promising results obtained in monkeys103 and humans104 using soft-agar and methylcellulose-culture systems. With regard to human SSCs, postmeiotic cells in 2 of 6 immature TCSs cultured in a methylcellulose system and spermatozoon-like cells (based on mitochondria localization) in 1 out of 6 cultured TCSs were obtained in one study.104 In another, spermatozoon-like cells were also generated using chitosan cylinders to culture dissociated STs from adult transsexual patients after hormonal therapy.105 However, whether differentiated germ cells originate from SSCs or spermatogonia already committed to differentiation remains unknown. Recently, the fertilization capacity of round spermatids obtained after IVM of human GPR125+ spermatogonia was demonstrated using mouse oocytes with subsequent 8-cell stage embryo development.106
IVM of intact ITT (SSCs within their niche)
Organotypic culture of ITT allows preservation of cell interactions inside the niche and leads to germ-cell differentiation up to the haploid stage in rodents, with generation of offspring in mice.107,108 Recently, a long-term organotypic culture of human ITT able to preserve ST integrity and Leydig cell functionality and achieve Sertoli cell maturation with partial establishment of the blood–testicular barrier109,110 eventually led to the generation of haploid germ cells.26 As a decrease in spermatogonial numbers and only a few postmeiotic germ cells were observed, the next hurdles to overcome before clinical translation are enhancing the efficiency of the technique and demonstrating the fertilizing capacity and genetic integrity of in vitro matured cells. Recently, Ogawa developed a microfluidic culture system allowing growth of mice ITT for up to 6 months and resulting in higher spermatogenesis efficiency compared to standard organotypic culture, which could eventually address issues that have been encountered with human tissue.111 In this well-designed system, a porous polydimethylsiloxane (PDMS) membrane separated mouse ITT from flowing medium, allowing physiological exchanges between the chamber and the media as secreted molecules were maintained for a longer period in the chamber compared to free diffusion occurring in the classical organotypic culture system. Moreover, diffusion of oxygen through the PDMS membrane resulted in a reduction in oxygen toxicity compared to direct exposure. Later, the same group modified their culture system by suppressing the need for a pump, making its use simpler.112,113
Using other SCs to restore male fertility
In vitro spermatogenesis from embryonic and induced pluripotent SCs
Different SC sources have been considered to generate haploid germ cells in vitro. In mice, while the first generation of spermatids derived from ESCs led to abnormal offspring,114 viable offspring with normal karyotype and methylation status were achieved a decade later.115 Differentiation of hESCs into germ cell-like cells was first reported in 2004.116 However, ESCs are genetically unrelated to patients, and their procurement is complicated by ethical issues on embryo destruction. Researchers thus focused on human-induced pluripotent stem cells (hiPSCs) derived from skin and cord-blood cells that were also differentiated in haploid germ cells, though with incomplete imprinting reestablishment (Figure 3).117 Other teams derived male germ cells from hESCs or hiPSCs, but most of the differentiated cells remained at early stages, suggesting low efficiency of the process.118–124 Lower efficiency has been observed for differentiation of skin-derived iPSCs into haploid cells for patients with azoospermic factor C deletion.125 One group suggested the existence of another source of SCs they called “very small embryonic stem cells (VSELs)” residing in the testes, where they undergo asymmetric divisions, giving rise to A (dark) spermatogonia that proliferate and differentiate into A (pale) and B spermatogonia.126 In humans, the potential of these cells to differentiate in vitro has never been investigated, although based on the nuclear expression of OCT4 and cytoplasmic expression of SSEA-4 and STELLA, their presence was suggested in testes of childhood cancer survivors aged 23 to 35 years.127 However, a large part of the scientific community is not convinced about the existence of VSELs, and researches refuted their SC properties.128,129 While researchers are currently actively working on these approaches, it is important to note that besides a high degree of uncertainty regarding functionality and safety, the fertilizing capacity of human in vitro differentiated ESCs and iPSCs has not been evaluated.
Using SCs to rescue damaged SSC niches
From the perspective of future clinical application, the question of whether SSC transplantation in a chemotherapy/radiotherapy-damaged niche may restore fertility arises, as Sertoli and Leydig cell defects have both been reported after gonadotoxic therapy.130,131 As healthy Sertoli cells present in the TCSs were shown to enhance SSC engraftment and bring adequate signals to surviving endogenous SSCs,132,133 the use of SCs as supporting cells was considered to improve SSC-transplantation outcomes (Figure 3). In this regard, MSCs can be considered deal candidates, since several studies have suggested that male fertility can be improved, thanks to their paracrine secretions (Table 2). Indeed, umbilical cord-derived MSCs secrete factors known to play an important role in spermatogenesis such as granulocyte-colony stimulating factor, vascular endothelial growth factor, and GDNF,134 as well as enhanced expression of meiotic genes, when injected into busulfan-sterilized mice.135 Also, SDF-1 is another MSC-secreted factor136 involved in SSC migration and homing, as deletion of the CXCR4 in mouse germ cells reduces SSC homing, but not their proliferation or survival.137 It can thus be hypothesized that cotransplantation of MSCs with SSCs could improve colonization efficiency, previously reported as low.24 Moreover, in one study HUPVCs shared molecular properties with adult somatic TCs, notably secretion of LIF, bFGF, and BMP4, known as regulators of spermatogenesis, and their transplantation promoted ST regeneration after exposure to mono-2-ethylhexyl phthalate, while all STs were damaged in controls.66 The authors assumed that the mesenchymal origin shared by Sertoli cells and HUPVCs explained the common properties of the two cell types and their ability to support SSCs. In the same way, adipose-derived stem cell (ASC) transplantation in efferent ducts of busulfan-sterilized hamsters allowed resumption of spermatogenesis.138 Furthermore, in a rat model of testicular torsion, injection of MSCs from human fat orbital tissue into the testes of animals not only resulted in rescue of germ cells from apoptosis but also in higher levels of testosterone, suggesting that MSCs may also support Leydig cells.139
 |
Table 2 Studies that attempted to restore male fertility using stem cells of mesenchymal origin |
Moreover, pure MSCs (CD45–Sca1+Lin–) isolated from bone marrow of GFP+ mice injected into testes of busulfan-treated GFP– mice resulted in more STs presenting spermatogenesis (70%) compared to injection of hematopoietic SCs (CD45+Sca1+Lin–) (18%) or DMEM (19%).15 Pretreatment of MSCs before transplantation was also evaluated with the objective of improving SSC-transplantation efficiency. Interestingly, while cotransplantation of SSCs with or without TGFβ1-treated MSCs in sterilized mice testes resulted in an equivalent resumption of endogenous spermatogenesis, a higher proportion of STs containing donor-derived spermatogenesis was observed when TGFβ1-treated MSCs were cotransplanted with SSCs. This observation could be explained by the lower expression of genes involved in inflammation and cell migration in TGFβ1-treated MSCs, resulting in reduced lymphatic migration toward other organs.140
SCs to restore fertility in the female
Current evidence of SCs in the ovary
The conventional view that mammalian ovaries do not produce oocytes after birth has been challenged in recent decades with the discovery of FGSCs in ovaries of juvenile and adult mice.21 Mathematical calculations demonstrated that the rate of follicular atresia did not coincide with the age at which mice exhausted their follicular reserve, suggesting that neo-oogenesis occurred in ovarian tissue to reestablish the follicle pool and ensure reproductive potential during adulthood.21 Indeed, it was demonstrated that FGSCs isolated from mice ovaries maintained proliferative activity in vitro and led to offspring after transplantation to sterile mice.21,141–145 Their presence was also demonstrated in prepubertal rat146 and pig147 and adult pig148 and human149 ovaries. Indeed, when FGSCs isolated from adult minipig ovaries were infected by an EGFP lentivirus and injected into human ovarian cortex pieces, EGFP+ oocytes were observed after 3 weeks in ovarian cortical xenografts.148 In addition, FGSCs isolated from human cortical tissue (based on DDX4 expression) and transduced with a GFP-expression vector were shown to reform structures resembling follicles in culture with dispersed adult ovarian cells and to differentiate into oocytes when injected into human cortical tissue before xenotransplantation to nude mice.149 In that study, the authors attributed FGSCs not being detected earlier by other teams to their smallness size (5–8 μm) and proportion (0.014±0.002%) of total ovarian cells. Ding et al also reported oocyte differentiation of FGSCs obtained from small cortical tissue fragments present in IVF patients’ follicular aspirates.150 However, the existence of FGSCs is not accepted universally. Even more controversy on the subject arose when Johnson et al published a study suggesting an extragonadal source from bone marrow and peripheral blood.151 Eventually, with transplantation and parabiotic mouse models, the hypothesis that circulatory bone-marrow cells can generate ovulated oocytes both in the steady state and after induced damage was discredited by several teams.152,153 Later, Lei and Spradling concluded that FGSCs could be dedifferentiated cells able to become germ cells under specific conditions as they did not detect these cells in mouse ovaries using a cell lineage–labeling system and demonstrated that the pool of primordial follicles generated during fetal life is sufficient to sustain adult oogenesis without a source of renewal.154 Subsequently, other studies corroborated this hypothesis, as different teams were not able to detect FGSCs in mouse and human ovarian tissue using DDX4 lineage tracing, RT-PCR, or immunohistochemistry.155–157 Reizel et al carried out an interesting study in which somatic mutations accumulated in microsatellites were used to reconstruct cell-lineage trees, which gave information on lineage relationships among different cell types. Reconstructed cell trees showed that oocytes formed clusters distinct from bone-marrow cells in both young and adult mice, suggesting that the two cell types belong to separate lineages. A second interesting observation was that oocyte depth increased with mouse age. In other words, oocytes of older mice had undergone more mitotic divisions than those of younger mice, which could be explained by either depth-guided selection of oocytes for ovulation or postnatal renewal.158
Use of SCs to treat ovarian reproductive failure
MSCs have been shown to act on the somatic compartment of the ovary, leading to reactivation and differentiation of “dormant” SCs (Figure 4). Notably, transplanted menstrual blood-derived endometrial MSCs (MenSCs) are able to migrate to the ovarian cortex and differentiate to granulosa cells, which improves FGSC renewal and restores fertility of sterilized mice.159,160 Other studies have demonstrated fertility restoration of sterilized mice or rats using SCs isolated from bone marrow,161–166 adipose tissue,167,168 amniotic fluid,169 amnion,170 and chorion (Table 3).171 Moreover, repeated bone marrow–derived MSCs (BMSCs) infusions through the tail vein not only postpone age-related ovarian failure in mice but improve the survival rate of offspring, suggesting a potential effect on egg quality.172 With regard to humans, one team investigated transplantation of BMSCs into ovaries of 10 women diagnosed with premature ovarian failure and reported recovery of menstruation in two cases and one pregnancy with delivery of a healthy baby.173 Even if promising, these results should be further confirmed and viewed with caution, since risks of transformation and tumorigenicity in MSC-based therapies are still debatable.174
 |
Table 3 Studies that attempted to improve female fertility using stem cells of mesenchymal origin |
Use of SCs to improve ovarian transplantation outcomes
Orthotopic autotransplantation of freeze–thawed pre- and postpubertal ovarian tissue already proved its efficacy, with more than 100 live births reported thus far18,175–178 and a cumulative success rate of 57% (Figure 4).179
Although these results are encouraging, an important loss of primordial follicles has been reported after transplantation.180 To overcome this issue, several types of SCs have been used to improve graft oxygenation and follicle survival (Figure 4). Aware that MSCs play an important role in angiogenesis and stabilization of the blood-vessel network, Xia et al cotransplanted MSCs and ovarian tissue, both encapsulated in Matrigel and demonstrated that MSCs promoted neoangiogenesis and prevented loss of primordial follicles in grafts.181 Angiogenin, which plays a role in angiogenesis and endothelial cell proliferation, has been further identified as a key MSC-secreted factor involved in follicle survival and revascularization of xenografted human ovarian tissue.182 ASCs as another source of MSCs, with the advantage of easier access compared to BMSCs, have also been evaluated. After encapsulation of human ovarian tissue using a mix of ASCs and fibrin, higher graft oxygenation and vascular density with improved survival of primordial follicles was achieved compared to tissue transplantation only.183 These results highlight the potency of MSCs in promoting graft revascularization.
Use of stem cells to improve follicle IVM
As autotransplantation has the potential risk of reintroducing cancer cells, succeeding in IVM of primordial follicles recovered from cryopreserved ovarian tissue is of paramount importance.184 IVM of preantral and antral follicles isolated from thawed human ovarian tissue until a competent oocyte stage has been achieved,185–187 although with lower efficiency for prepubertal tissue,188 which could be explained by the higher proportion of abnormal follicles before puberty.189 In an attempt to improve follicle IVM, MSCs have been exploited (Figure 4). Experiments conducted in vitro demonstrated that conditioned medium from human umbilical cord MSCs increased microvessel density and decreased apoptosis of in vitro cultured cortical tissue compared to serum-free culture.190 Human menstrual blood–derived endometrial MSCs increased follicular growth and IVM rates when cocultured with mouse alginate-encapsulated preantral follicles.191 In the same way, coculture of BMSCs with human alginate-encapsulated follicles improved follicle growth and viability in a dose-dependent manner, suggesting that the number of MSCs influences culture outcomes.192
Generation of oocytes from embryonic and induced pluripotent stem cells
Hübner et al reported for the first time derivation of oocyte-like cells from mouse ESCs.193 In 2012, Hayashi et al demonstrated that it was possible to differentiate female ESCs and iPSCs into primordial germ cell–like cells (PGCLCs) and that their aggregation with ovarian somatic cells allowed reconstitution of an ovarian structure in which the PGCLCs exhibited meiotic potential.194 Moreover, transplantation of such reconstituted ovaries under the mouse ovarian bursa resulted in maturation of PGCLCs to vesicle-stage oocytes that were fertilized following IVM (Figure 4). Offspring were generated after in vitro fertilization of PGCLC-derived oocytes and embryo transfer to foster-mother mice, but epigenetic abnormalities were observed in half the generated eggs. The entire cycle of mouse oogenesis was later reproduced in vitro from ESCs and iPSCs, although a low success rate of full-term development was reported for ESC-derived embryos.195 However, with regard to hESCs, development of structures resembling primary ovarian follicles was the most advanced stage of differentiation that could be reached.196
Conclusion
Development of methods to preserve and restore fertility of patients subjected to gonadotoxic therapies has become an urgent matter in these last few decades. On the male side, SSCs constitute a pool of SCs able to differentiate into spermatozoa. Restoration of male fertility with SSCs is still at the research stage, but experiments in animals suggest that autotransplantation of propagated and selected SSCs into the rete testis or autografting of ITT will be possible in future. In vitro differentiation of human spermatozoa with the aim of using in vitro matured sperm in ART can also be an option, especially when there is a risk of malignant contamination of ITT but needs further development with regard to efficiency of haploid-cell generation, completion of spermatogenesis and safety issues. The classical scheme that the female germ-cell pool is fixed after birth is under debate. Several studies lean toward the existence of SCs, but it cannot be excluded that FGSCs derive from dedifferentiated cells. Development of germ cells from other sources of SCs such as ESCs and iPSCs has also been proposed to restore fertility in both males and females, but the genetic stability of the cells and capacity to generate healthy offspring is uncertain. Finally, the use of MSCs to act against follicular loss in grafts or restore the damaged male or female somatic germ-cell environment has shown promising results, but long-term risks associated with MSC transplantation or culture still need to be evaluated.
Abbreviation list
ART, assisted reproductive technology; ASC, adipose-derived stem cell; bFGF, basic fibroblast growth factor; BMP4, bone morphogenic protein 4; BMSC, bone marrow-derived stem cell; ESC, embryonic stem cell; FACS, fluorescence-activated cell sorting; FGSC, female germline stem cell; FP, fertility preservation; hiPSC, human-induced pluripotent stem cell; HLA, human leukocyte antigen; HUPVC, human umbilical perivascular cell; HUPVC, human umbilical perivascular mesenchymal stem cell; ITT, immature testicular tissue; IVF, in vitro fertilization; IVM, In vitro maturation; LIF, leukemia inhibitory factor; Lin, lineage; MEF, mouse embryonic fibroblast; MenSC, menstrual blood-derived endometrial mesenchymal stem cell; MSC, mesenchymal stem cell; PDMS, polydimethylsiloxane; PGCLC, primordial germ cell-like cell; POF, premature ovarian failure; SSC, spermatogonial stem cell; SSEA-4, stage-specific embryonic antigen-4; ST, seminiferous tubule; TCS, testicular cell suspension; UC-MSC, umbilical cord-derived mesenchymal stem cell.
Acknowledgments
Some experiments reported here were supported by grants from the Fonds National de la Recherche Scientifique de Belgique and Fondation Salus Sanguinis.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jensen JR, Morbeck DE, Coddington CC
2. Vassilakopoulou M, Boostandoost E, Papaxoinis G, et al. Anticancer treatment and fertility: effect of therapeutic modalities on reproductive system and functions. Crit Rev Oncol Hematol. 2016;97:328–334. doi:10.1016/j.critrevonc.2015.08.002
3. Gidoni Y, Holzer H, Tulandi T, et al. Fertility preservation in patients with non-oncological conditions. Reprod Biomed Online. 2008;16(6):792–800.
4. Giudice MG, Del Vento F, Wyns C. Male fertility preservation in DSD, XXY, pre-gonadotoxic treatments – update, methods, ethical issues, current outcomes, future directions. Best Pract Res Clin Endocrinol Metab. 2019. doi:10.1016/j.beem.2019.01.002
5. Wyns C, Curaba M, Petit S, et al. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Human Reprod. 2011;26(4):737–747.
6. Curaba M, Poels J, van Langendonckt A, et al. Can prepubertal human testicular tissue be cryopreserved by vitrification? Fertil Steril. 2011;95(6):2123e2129–2112.
7. Keros V, Hultenby K, Borgstrom B, et al. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Human Reprod. 2007;22(5):1384–1395.
8. Poels J, Abou-Ghannam G, Herman S, et al. In search of better spermatogonial preservation by supplementation of cryopreserved human immature testicular tissue xenografts with N-acetylcysteine and testosterone. Front Surg. 2014;1:47.
9. Poels J, Van Langendonckt A, Many MC, et al. Vitrification preserves proliferation capacity in human spermatogonia. Human Reprod. 2013;28(3):578–589.
10. Wyns C, Curaba M, Martinez-Madrid B, et al. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Human Reprod. 2007;22(6):1603–1611. doi:10.1093/humrep/dem062
11. Wyns C, Van Langendonckt A, Wese FX, et al. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Human Reprod. 2008;23(11):2402–2414. doi:10.1093/humrep/den272
12. Poels J, Van Langendonckt A, Dehoux JP, et al. Vitrification of non-human primate immature testicular tissue allows maintenance of proliferating spermatogonial cells after xenografting to recipient mice. Theriogenology. 2012;77(5):1008–1013. doi:10.1016/j.theriogenology.2011.10.015
13. Jahnukainen K, Ehmcke J, Nurmio M, et al. Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res. 2012;72(20):5174–5178. doi:10.1158/0008-5472.CAN-12-1317
14. Baert Y, Van Saen D, Haentjens P, et al. What is the best cryopreservation protocol for human testicular tissue banking? Human Reprod. 2013;28(7):1816–1826. doi:10.1093/humrep/det100
15. Kadam P, Van Saen D, Goossens E. Can mesenchymal stem cells improve spermatogonial stem cell transplantation efficiency? Andrology. 2017;5(1):2–9. doi:10.1111/andr.12304
16. Rienzi L, Gracia C, Maggiulli R, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–155. doi:10.1093/humupd/dmw038
17. Edgar DH, Gook DA. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum Reprod Update. 2012;18(5):536–554. doi:10.1093/humupd/dms016
18. Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32(8):1167–1170. doi:10.1007/s10815-015-0544-9
19. Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52(1):198–236. doi:10.1152/physrev.1972.52.1.198
20. Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17(2):121–155. doi:10.1210/edrv-17-2-121
21. Johnson J, Canning J, Kaneko T, et al. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. doi:10.1038/nature02316
22. Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290(2):193–200.
23. Muller J, Skakkebaek NE. Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys who suffered from sudden death. Int J Androl. 1983;6(2):143–156.
24. Hermann BP, Sukhwani M, Lin CC, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25(9):2330–2338. doi:10.1634/stemcells.2007-0143
25. Nagano MC. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol Reprod. 2003;69(2):701–707. doi:10.1095/biolreprod.103.016352
26. de Michele F, Poels J, Vermeulen M, et al. Haploid germ cells generated in organotypic culture of testicular tissue from prepubertal boys. Front Physiol. 2018;9:1413. doi:10.3389/fphys.2018.01413
27. Kanatsu-Shinohara M, Ogonuki N, Inoue K, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69(2):612–616. doi:10.1095/biolreprod.103.017012
28. Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101(47):16489–16494. doi:10.1073/pnas.0407063101
29. Meng X, Lindahl M, Hyvonen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287(5457):1489–1493.
30. Kanatsu-Shinohara M, Inoue K, Ogonuki N, et al. Leukemia inhibitory factor enhances formation of germ cell colonies in neonatal mouse testis culture. Biol Reprod. 2007;76(1):55–62. doi:10.1095/biolreprod.106.055863
31. Anjamrooz SH, Movahedin M, Tiraihi T, et al. In vitro effects of epidermal growth factor, follicle stimulating hormone and testosterone on mouse spermatogonial cell colony formation. Reprod Fertil Dev. 2006;18(6):709–720.
32. Sadri-Ardekani H, Mizrak SC, van Daalen SK, et al. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302(19):2127–2134. doi:10.1001/jama.2009.1689
33. Sadri-Ardekani H, Akhondi MA, van der Veen F, et al. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305(23):2416–2418. doi:10.1001/jama.2011.791
34. Akhondi MM, Mohazzab A, Jeddi-Tehrani M, et al. Propagation of human germ stem cells in long-term culture. Iran J Reprod Med. 2013;11(7):551–558.
35. Baert Y, Braye A, Struijk RB, et al. Cryopreservation of testicular tissue before long-term testicular cell culture does not alter in vitro cell dynamics. Fertil Steril. 2015;104(5):
36. Conrad S, Azizi H, Hatami M, et al. Differential gene expression profiling of enriched human spermatogonia after short- and long-term culture. Biomed Res Int. 2014;2014:138350. doi:10.1155/2014/138350
37. Goharbakhsh L, Mohazzab A, Salehkhou S, et al. Isolation and culture of human spermatogonial stem cells derived from testis biopsy. Avicenna J Med Biotechnol. 2013;5(1):54–61.
38. Guo Y, Liu L, Sun M, et al. Expansion and long-term culture of human spermatogonial stem cells via the activation of SMAD3 and AKT pathways. Exp Biol Med (Maywood). 2015;240(8):1112–1122. doi:10.1177/1535370215590822
39. Koruji M, Shahverdi A, Janan A, et al. Proliferation of small number of human spermatogonial stem cells obtained from azoospermic patients. J Assist Reprod Genet. 2012;29(9):957–967. doi:10.1007/s10815-012-9817-8
40. Mirzapour T, Movahedin M, Koruji M, et al. Xenotransplantation assessment: morphometric study of human spermatogonial stem cells in recipient mouse testes. Andrologia. 2015;47(6):626–633. doi:10.1111/and.12310
41. Mirzapour T, Movahedin M, Tengku Ibrahim TA, et al. Evaluation of the effects of cryopreservation on viability, proliferation and colony formation of human spermatogonial stem cells in vitro culture. Andrologia. 2013;45(1):26–34. doi:10.1111/j.1439-0272.2012.01302.x
42. Mirzapour T, Movahedin M, Tengku Ibrahim TA, et al. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia. 2012;44(Suppl 1):41–55. doi:10.1111/j.1439-0272.2010.01135.x
43. Nickkholgh B, Mizrak SC, Korver CM, et al. Enrichment of spermatogonial stem cells from long-term cultured human testicular cells. Fertil Steril. 2014;102(2):558–565. e555. doi:10.1016/j.fertnstert.2014.04.022
44. Nickkholgh B, Mizrak SC, van Daalen SK, et al. Genetic and epigenetic stability of human spermatogonial stem cells during long-term culture. Fertil Steril. 2014;102(6):1700–1707. e1701. doi:10.1016/j.fertnstert.2014.08.022
45. Piravar Z, Jeddi-Tehrani M, Sadeghi MR, et al. In vitro culture of human testicular stem cells on feeder-free condition. J Reprod Infertil. 2013;14(1):17–22.
46. Sadri-Ardekani H, Homburg CH, van Capel TM, et al. Eliminating acute lymphoblastic leukemia cells from human testicular cell cultures: a pilot study. Fertil Steril. 2014;101(4):1072–1078. e1071. doi:10.1016/j.fertnstert.2014.01.014
47. Zheng Y, Thomas A, Schmidt CM, et al. Quantitative detection of human spermatogonia for optimization of spermatogonial stem cell culture. Human Reprod. 2014;29(11):2497–2511. doi:10.1093/humrep/deu232
48. Kokkinaki M, Djourabtchi A, Golestaneh N. Long-term culture of human SSEA-4 positive Spermatogonial Stem Cells (SSCs). J Stem Cell Res Ther. 2011;2:2.
49. Lim JJ, Sung SY, Kim HJ, et al. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. 2010;43(4):405–417. doi:10.1111/j.1365-2184.2010.00691.x
50. Shiva R, Ghasem S, Masoud H, et al. Comparison of colony formation of human spermatogonial stem cells (SSCs) with and without collagen. J Pak Med Assoc. 2016;66(3):285–291.
51. Bhang DH, Kim BJ, Kim BG, et al. Testicular endothelial cells are a critical population in the germline stem cell niche. Nat Commun. 2018;9(1):4379.
52. Cai H, Wu JY, An XL, et al. Enrichment and culture of spermatogonia from cryopreserved adult bovine testis tissue. Anim Reprod Sci. 2016;166:109–115.
53. Gat I, Maghen L, Filice M, et al. Initial germ cell to somatic cell ratio impacts the efficiency of SSC expansion in vitro. Syst Biol Reprod Med. 2017;64:1–12.
54. Gat I, Maghen L, Filice M, et al. Optimal culture conditions are critical for efficient expansion of human testicular somatic and germ cells in vitro. Fertil Steril. 2017;107(3):595–605. e597.
55. Medrano JV, Rombaut C, Simon C, et al. Human spermatogonial stem cells display limited proliferation in vitro under mouse spermatogonial stem cell culture conditions. Fertil Steril. 2016;106(6):1539–1549. e1538.
56. Dovey SL, Valli H, Hermann BP, et al. Eliminating malignant contamination from therapeutic human spermatogonial stem cells. J Clin Invest. 2013;123(4):1833–1843.
57. Gassei K, Ehmcke J, Dhir R, et al. Magnetic activated cell sorting allows isolation of spermatogonia from adult primate testes and reveals distinct GFRa1-positive subpopulations in men. J Med Primatol. 2010;39(2):83–91.
58. Hermann BP, Sukhwani M, Salati J, et al. Separating spermatogonia from cancer cells in contaminated prepubertal primate testis cell suspensions. Human Reprod. 2011;26(12):3222–3231.
59. Smith JF, Yango P, Altman E, et al. Testicular niche required for human spermatogonial stem cell expansion. Stem Cells Transl Med. 2014;3(9):1043–1054.
60. Sa R, Miranda C, Carvalho F, et al. Expression of stem cell markers: OCT4, KIT, ITGA6, and ITGB1 in the male germinal epithelium. Syst Biol Reprod Med. 2013;59(5):233–243.
61. von Kopylow K, Schulze W, Salzbrunn A, et al. Isolation and gene expression analysis of single potential human spermatogonial stem cells. Mol Hum Reprod. 2016;22(4):229–239.
62. Zohni K, Zhang X, Tan SL, et al. CD9 is expressed on human male germ cells that have a long-term repopulation potential after transplantation into mouse testes. Biol Reprod. 2012;87(2):27.
63. Hermann BP, Sukhwani M, Simorangkir DR, et al. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Human Reprod. 2009;24(7):1704–1716.
64. Valli H, Sukhwani M, Dovey SL, et al. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil Steril. 2014;102(2):566–580. e567.
65. Izadyar F, Wong J, Maki C, et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Human Reprod. 2011;26(6):1296–1306.
66. Maghen L, Shlush E, Gat I, et al. Human umbilical perivascular cells: a novel source of MSCs to support testicular niche regeneration. Reproduction. 2016;153(1):85–95.
67. Wyns C, Curaba M, Vanabelle B, et al. Options for fertility preservation in prepubertal boys. Hum Reprod Update. 2010;16(3):312–328.
68. Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91(24):11303–11307.
69. Ganguli N, Wadhwa N, Usmani A, et al. An efficient method for generating a germ cell depleted animal model for studies related to spermatogonial stem cell transplantation. Stem Cell Res Ther. 2016;7(1):142.
70. Hamra FK, Gatlin J, Chapman KM, et al. Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proc Natl Acad Sci U S A. 2002;99(23):14931–14936.
71. Honaramooz A, Behboodi E, Megee SO, et al. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69(4):1260–1264.
72. Trefil P, Micakova A, Mucksova J, et al. Restoration of spermatogenesis and male fertility by transplantation of dispersed testicular cells in the chicken. Biol Reprod. 2006;75(4):575–581.
73. Herrid M, Olejnik J, Jackson M, et al. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod. 2009;81(5):898–905.
74. Hermann BP, Sukhwani M, Winkler F, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11(5):715–726.
75. Izadyar F, Den Ouden K, Stout TA, et al. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126(6):765–774.
76. Mikkola M, Sironen A, Kopp C, et al. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod Domest Anim. 2006;41(2):124–128.
77. Kim Y, Turner D, Nelson J, et al. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008;136(6):823–831.
78. Wu X, Goodyear SM, Abramowitz LK, et al. Fertile offspring derived from mouse spermatogonial stem cells cryopreserved for more than 14 years. Human Reprod. 2012;27(5):1249–1259.
79. Wang X, Ding Q, Zhang Y, et al. Two allogeneic descendents derived from the high-dose busulfan-treated infertile mouse model after freeze-thawed spermatogonial stem cell transplantation. Fertil Steril. 2008;90(4 Suppl):1538–1549.
80. Yuan Z, Hou R, Wu J. Generation of mice by transplantation of an adult spermatogonial cell line after cryopreservation. Cell Prolif. 2009;42(2):123–131.
81. Goossens E, Bilgec T, Van Saen D, et al. Mouse germ cells go through typical epigenetic modifications after intratesticular tissue grafting. Human Reprod. 2011;26(12):3388–3400.
82. Goossens E, de Vos P, Tournaye H. Array comparative genomic hybridization analysis does not show genetic alterations in spermatozoa and offspring generated after spermatogonial stem cell transplantation in the mouse. Human Reprod. 2010;25(7):1836–1842.
83. Mulder CL, Catsburg LAE, Zheng Y, et al. Long-term health in recipients of transplanted in vitro propagated spermatogonial stem cells. Human Reprod. 2018;33(1):81–90.
84. Faes K, Lahoutte T, Hoorens A, et al. In search of an improved injection technique for the clinical application of spermatogonial stem cell transplantation. Reprod Biomed Online. 2017;34(3):291–297.
85. Schlatt S, Rosiepen G, Weinbauer GF, et al. Germ cell transfer into rat, bovine, monkey and human testes. Human Reprod. 1999;14(1):144–150.
86. Radford J. Restoration of fertility after treatment for cancer. Horm Res. 2003;59(Suppl 1):21–23.
87. Jahnukainen K, Hou M, Petersen C, et al. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001;61(2):706–710.
88. Fujita K, Tsujimura A, Miyagawa Y, et al. Isolation of germ cells from leukemia and lymphoma cells in a human in vitro model: potential clinical application for restoring human fertility after anticancer therapy. Cancer Res. 2006;66(23):11166–11171.
89. Fujita K, Ohta H, Tsujimura A, et al. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J Clin Invest. 2005;115(7):1855–1861.
90. Geens M, Van de Velde H, De Block G, et al. The efficiency of magnetic-activated cell sorting and fluorescence-activated cell sorting in the decontamination of testicular cell suspensions in cancer patients. Human Reprod. 2007;22(3):733–742.
91. Ogawa T, Ohmura M, Ohbo K. The niche for spermatogonial stem cells in the mammalian testis. Int J Hematol. 2005;82(5):381–388.
92. Hou M, Andersson M, Eksborg S, et al. Xenotransplantation of testicular tissue into nude mice can be used for detecting leukemic cell contamination. Human Reprod. 2007;22(7):1899–1906.
93. Shinohara T, Inoue K, Ogonuki N, et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Human Reprod. 2002;17(12):3039–3045.
94. Kaneko H, Kikuchi K, Nakai M, et al. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS One. 2013;8(7):e70989.
95. Liu J, Cheng KM, Silversides FG. Production of live offspring from testicular tissue cryopreserved by vitrification procedures in Japanese quail (Coturnix japonica). Biol Reprod. 2013;88(5):124.
96. Liu Z, Nie YH, Zhang CC, et al. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Res. 2016;26(1):139–142.
97. Sato Y, Nozawa S, Yoshiike M, et al. Xenografting of testicular tissue from an infant human donor results in accelerated testicular maturation. Human Reprod. 2010;25(5):1113–1122.
98. Van Saen D, Goossens E, Bourgain C, et al. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Human Reprod. 2011;26(2):282–293.
99. Van Saen D, Goossens E, De Block G, et al. Regeneration of spermatogenesis by grafting testicular tissue or injecting testicular cells into the testes of sterile mice: a comparative study. Fertil Steril. 2009;91(5 Suppl):2264–2272.
100. Luetjens CM, Stukenborg JB, Nieschlag E, et al. Complete spermatogenesis in orthotopic but not in ectopic transplants of autologously grafted marmoset testicular tissue. Endocrinology. 2008;149(4):1736–1747. doi:10.1210/en.2007-1325
101. Wistuba J, Luetjens CM, Wesselmann R, et al. Meiosis in autologous ectopic transplants of immature testicular tissue grafted to Callithrix jacchus. Biol Reprod. 2006;74(4):706–713. doi:10.1095/biolreprod.105.048793
102. Fayomi AP, Peters K, Sukhwani M, et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science. 2019;363(6433):1314–1319. doi:10.1126/science.aav2914
103. Huleihel M, Nourashrafeddin S, Plant TM. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J Androl. 2015;17(6):972–980. doi:10.4103/1008-682X.154994
104. Abofoul-Azab M, AbuMadighem A, Lunenfeld E, et al. Development of postmeiotic cells in vitro from spermatogonial cells of prepubertal cancer patients. Stem Cells Dev. 2018;27(15):1007–1020. doi:10.1089/scd.2017.0301
105. Perrard MH, Sereni N, Schluth-Bolard C, et al. Complete human and rat ex vivo spermatogenesis from fresh or frozen testicular tissue. Biol Reprod. 2016;95(4):1–10. doi:10.1095/biolreprod.116.142240
106. Sun M, Yuan Q, Niu M, et al. Efficient generation of functional haploid spermatids from human germline stem cells by three-dimensional-induced system. Cell Death Differ. 2018;25:747–764. doi:10.1038/s41418-017-0015-1
107. Sato T, Katagiri K, Gohbara A, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471(7339):504–507. doi:10.1038/nature09850
108. Reda A, Hou M, Winton TR, et al. In vitro differentiation of rat spermatogonia into round spermatids in tissue culture. Mol Hum Reprod. 2016. doi:10.1093/molehr/gaw047
109. de Michele F, Poels J, Weerens L, et al. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Human Reprod. 2017;32(1):32–45. doi:10.1093/humrep/dew300
110. de Michele F, Poels J, Giudice MG, et al. In-vitro formation of the blood-testis barrier during long-term organotypic culture of human prepubertal tissue: comparison with a large cohort of pre/peripubertal boys. Mol Hum Reprod. 2018. doi:10.1093/molehr/gay012
111. Komeya M, Kimura H, Nakamura H, et al. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci Rep. 2016;6:21472. doi:10.1038/srep21472
112. Komeya M, Hayashi K, Nakamura H, et al. Pumpless microfluidic system driven by hydrostatic pressure induces and maintains mouse spermatogenesis in vitro. Sci Rep. 2017;7(1):15459. doi:10.1038/s41598-017-15799-3
113. Yamanaka H, Komeya M, Nakamura H, et al. A monolayer microfluidic device supporting mouse spermatogenesis with improved visibility. Biochem Biophys Res Commun. 2018;500(4):885–891. doi:10.1016/j.bbrc.2018.04.180
114. Nayernia K, Nolte J, Michelmann HW, et al. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11(1):125–132. doi:10.1016/j.devcel.2006.05.010
115. Zhou Q, Wang M, Yuan Y, et al. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell. 2016;18(3):330–340. doi:10.1016/j.stem.2016.01.017
116. Clark AT, Bodnar MS, Fox M, et al. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum Mol Genet. 2004;13(7):727–739. doi:10.1093/hmg/ddh088
117. Eguizabal C, Montserrat N, Vassena R, et al. Complete meiosis from human induced pluripotent stem cells. Stem Cells. 2011;29(8):1186–1195. doi:10.1002/stem.672
118. Easley C, Phillips BT, McGuire MM, et al. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012;2(3):440–446. doi:10.1016/j.celrep.2012.07.015
119. Irie N, Weinberger L, Tang WW, et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160(1–2):253–268. doi:10.1016/j.cell.2014.12.013
120. Medrano JV, Ramathal C, Nguyen HN, et al. Divergent RNA-binding proteins, DAZL and VASA, induce meiotic progression in human germ cells derived in vitro. Stem Cells. 2012;30(3):441–451. doi:10.1002/stem.1012
121. Panula S, Medrano JV, Kee K, et al. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet. 2011;20(4):752–762.
122. Park TS, Galic Z, Conway AE, et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells. 2009;27(4):783–795. doi:10.1002/stem.13
123. Sasaki K, Yokobayashi S, Nakamura T, et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell. 2015;17(2):178–194. doi:10.1016/j.stem.2015.06.014
124. Sugawa F, Arauzo-Bravo MJ, Yoon J, et al. Human primordial germ cell commitment in vitro associates with a unique PRDM14 expression profile. Embo J. 2015;34(8):1009–1024. doi:10.15252/embj.201488049
125. Zhao Y, Ye S, Liang D, et al. In vitro modeling of human germ cell development using pluripotent stem cells. Stem Cell Rep. 2018;10(2):509–523. doi:10.1016/j.stemcr.2018.01.001
126. Bhartiya D, Kasiviswanathan S, Unni SK, et al. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4as a stem cell marker. J Histochem Cytochem. 2010;58(12):1093–1106. doi:10.1369/jhc.2010.956870
127. Kurkure P, Prasad M, Dhamankar V, et al. Very small embryonic-like stem cells (VSELs) detected in azoospermic testicular biopsies of adult survivors of childhood cancer. Reprod Biol Endocrinol. 2015;13:122. doi:10.1186/s12958-015-0121-1
128. Danova-Alt R, Heider A, Egger D, et al. Very small embryonic-like stem cells purified from umbilical cord blood lack stem cell characteristics. PLoS One. 2012;7(4):e34899. doi:10.1371/journal.pone.0034899
129. Miyanishi M, Mori Y, Seita J, et al. Do pluripotent stem cells exist in adult mice as very small embryonic stem cells? Stem Cell Rep. 2013;1(2):198–208. doi:10.1016/j.stemcr.2013.07.001
130. Howell SJ, Radford JA, Ryder WD, et al. Testicular function after cytotoxic chemotherapy: evidence of Leydig cell insufficiency. J Clin Oncol. 1999;17(5):1493–1498. doi:10.1200/JCO.1999.17.5.1493
131. Bar-Shira Maymon B, Yogev L, Marks A, et al. Sertoli cell inactivation by cytotoxic damage to the human testis after cancer chemotherapy. Fertil Steril. 2004;81(5):1391–1394. doi:10.1016/j.fertnstert.2003.09.078
132. Shinohara T, Orwig KE, Avarbock MR, et al. Restoration of spermatogenesis in infertile mice by Sertoli cell transplantation. Biol Reprod. 2003;68(3):1064–1071. doi:10.1095/biolreprod.102.009977
133. Anand S, Bhartiya D, Sriraman K, et al. Underlying mechanisms that restore spermatogenesis on transplanting healthy niche cells in busulphan treated mouse testis. Stem Cell Rev. 2016;12(6):682–697. doi:10.1007/s12015-016-9685-1
134. Koh SH, Kim KS, Choi MR, et al. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233–248. doi:10.1016/j.brainres.2008.06.087
135. Yang RF, Liu TH, Zhao K, et al. Enhancement of mouse germ cell-associated genes expression by injection of human umbilical cord mesenchymal stem cells into the testis of chemical-induced azoospermic mice. Asian J Androl. 2014;16(5):698–704. doi:10.4103/1008-682X.129209
136. Schajnovitz A, Itkin T, D’Uva G, et al. CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat Immunol. 2011;12(5):391–398. doi:10.1038/ni.2017
137. Kanatsu-Shinohara M, Inoue K, Takashima S, et al. Reconstitution of mouse spermatogonial stem cell niches in culture. Cell Stem Cell. 2012;11(4):567–578. doi:10.1016/j.stem.2012.06.011
138. Karimaghai N, Tamadon A, Rahmanifar F, et al. Spermatogenesis after transplantation of adipose tissue-derived mesenchymal stem cells in busulfan-induced azoospermic hamster. Iran J Basic Med Sci. 2018;21(7):660–667. doi:10.22038/IJBMS.2018.29040.7010
139. Hsiao CH, Ji AT, Chang CC, et al. Local injection of mesenchymal stem cells protects testicular torsion-induced germ cell injury. Stem Cell Res Ther. 2015;6:113. doi:10.1186/s13287-015-0114-1
140. Kadam P, Ntemou E, Baert Y, et al. Co-transplantation of mesenchymal stem cells improves spermatogonial stem cell transplantation efficiency in mice. Stem Cell Res Ther. 2018;9(1):317. doi:10.1186/s13287-018-1065-0
141. Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11(5):631–636. doi:10.1038/ncb1869
142. Zhang C, Wu J. Production of offspring from a germline stem cell line derived from prepubertal ovaries of germline reporter mice. Mol Hum Reprod. 2016;22(7):457–464. doi:10.1093/molehr/gaw030
143. Wu C, Xu B, Li X, et al. Tracing and characterizing the development of transplanted female germline stem cells in vivo. Mol Ther. 2017;25(6):1408–1419. doi:10.1016/j.ymthe.2017.04.019
144. Xiong J, Lu Z, Wu M, et al. Intraovarian transplantation of female germline stem cells rescue ovarian function in chemotherapy-injured ovaries. PLoS One. 2015;10(10):e0139824. doi:10.1371/journal.pone.0139824
145. Park ES, Tilly JL. Use of DEAD-box polypeptide-4 (Ddx4) gene promoter-driven fluorescent reporter mice to identify mitotically active germ cells in post-natal mouse ovaries. Mol Hum Reprod. 2015;21(1):58–65. doi:10.1093/molehr/gau071
146. Zhou L, Wang L, Kang JX, et al. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod. 2014;20(3):271–281. doi:10.1093/molehr/gat081
147. Bai Y, Yu M, Hu Y, et al. Location and characterization of female germline stem cells (FGSCs) in juvenile porcine ovary. Cell Prolif. 2013;46(5):516–528. doi:10.1111/cpr.12058
148. Hou L, Wang J, Li X, et al. Characteristics of female germline stem cells from porcine ovaries at sexual maturity. Cell Transplant. 2018;27(8):1195–1202. doi:10.1177/0963689718784878
149. White YA, Woods DC, Takai Y, et al. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–421. doi:10.1038/nm.2669
150. Ding X, Liu G, Xu B, et al. Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci Rep. 2016;6:28218. doi:10.1038/srep28218
151. Johnson J, Bagley J, Skaznik-Wikiel M, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122(2):303–315. doi:10.1016/j.cell.2005.06.031
152. Begum S, Papaioannou VE, Gosden RG. The oocyte population is not renewed in transplanted or irradiated adult ovaries. Human Reprod. 2008;23(10):2326–2330. doi:10.1093/humrep/den249
153. Eggan K, Jurga S, Gosden R, et al. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441(7097):1109–1114. doi:10.1038/nature04929
154. Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci U S A. 2013;110(21):8585–8590. doi:10.1073/pnas.1306189110
155. Zhang H, Zheng W, Shen Y, et al. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci U S A. 2012;109(31):12580–12585. doi:10.1073/pnas.1206600109
156. Byskov AG, Hoyer PE, Yding Andersen C, et al. No evidence for the presence of oogonia in the human ovary after their final clearance during the first two years of life. Human Reprod. 2011;26(8):2129–2139. doi:10.1093/humrep/der145
157. Liu Y, Wu C, Lyu Q, et al. Germline stem cells and neo-oogenesis in the adult human ovary. Dev Biol. 2007;306(1):112–120. doi:10.1016/j.ydbio.2007.03.006
158. Reizel Y, Itzkovitz S, Adar R, et al. Cell lineage analysis of the mammalian female germline. PLoS Genet. 2012;8(2):e1002477. doi:10.1371/journal.pgen.1002477
159. Liu T, Huang Y, Zhang J, et al. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 2014;23(13):1548–1557. doi:10.1089/scd.2013.0371
160. Lai D, Wang F, Yao X, et al. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med. 2015;13:155. doi:10.1186/s12967-015-0541-x
161. Lee HJ, Selesniemi K, Niikura Y, et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25(22):3198–3204. doi:10.1200/JCO.2006.10.3028
162. Fu X, He Y, Xie C, et al. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy. 2008;10(4):353–363. doi:10.1080/14653240802035926
163. Liu J, Zhang H, Zhang Y, et al. Homing and restorative effects of bone marrow-derived mesenchymal stem cells on cisplatin injured ovaries in rats. Mol Cells. 2014;37(12):865–872. doi:10.14348/molcells.2014.0145
164. Santiquet N, Vallieres L, Pothier F, et al. Transplanted bone marrow cells do not provide new oocytes but rescue fertility in female mice following treatment with chemotherapeutic agents. Cell Reprogram. 2012;14(2):123–129. doi:10.1089/cell.2011.0066
165. Herraiz S, Buigues A, Diaz-Garcia C, et al. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018;109(5):908–918. e902. doi:10.1016/j.fertnstert.2018.01.004
166. Mohamed SA, Shalaby SM, Abdelaziz M, et al. Human mesenchymal stem cells partially reverse infertility in chemotherapy-induced ovarian failure. Reprod Sci. 2018;25(1):51–63. doi:10.1177/1933719117699705
167. Takehara Y, Yabuuchi A, Ezoe K, et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest. 2013;93(2):181–193. doi:10.1038/labinvest.2012.167
168. Su J, Ding L, Cheng J, et al. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Human Reprod. 2016;31(5):1075–1086. doi:10.1093/humrep/dew041
169. Lai D, Wang F, Chen Y, et al. Human amniotic fluid stem cells have a potential to recover ovarian function in mice with chemotherapy-induced sterility. BMC Dev Biol. 2013;13:34. doi:10.1186/1471-213X-13-34
170. Wang F, Wang L, Yao X, et al. Human amniotic epithelial cells can differentiate into granulosa cells and restore folliculogenesis in a mouse model of chemotherapy-induced premature ovarian failure. Stem Cell Res Ther. 2013;4(5):124. doi:10.1186/scrt373
171. Li J, Yu Q, Huang H, et al. Human chorionic plate-derived mesenchymal stem cells transplantation restores ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. Stem Cell Res Ther. 2018;9(1):81. doi:10.1186/s13287-018-0819-z
172. Selesniemi K, Lee HJ, Niikura T, et al. Young adult donor bone marrow infusions into female mice postpone age-related reproductive failure and improve offspring survival. Aging (Albany NY). 2008;1(1):49–57. doi:10.18632/aging.100002
173. Edessy M, Hosni H, Shady Y, et al. Autologous stem cells therapy, The first baby of idiopathic premature ovarian failure. Acta Medica Int. 2016;3(1):19–23. doi:10.5530/ami.2016.1.7
174. Barkholt L, Flory E, Jekerle V, et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies–bridging scientific observations and regulatory viewpoints. Cytotherapy. 2013;15(7):753–759. doi:10.1016/j.jcyt.2013.03.005
175. Jensen AK, Kristensen SG, Macklon KT, et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Human Reprod. 2015;30(12):2838–2845. doi:10.1093/humrep/dev230
176. Van der Ven H, Liebenthron J, Beckmann M, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Human Reprod. 2016;31(9):2031–2041. doi:10.1093/humrep/dew165
177. Demeestere I, Simon P, Dedeken L, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Human Reprod. 2015;30(9):2107–2109. doi:10.1093/humrep/dev128
178. Matthews SJ, Picton H, Ernst E, et al. Successful pregnancy in a woman previously suffering from beta-thalassemia following transplantation of ovarian tissue cryopreserved before puberty. Minerva Ginecol. 2018;70(4):432–435. doi:10.23736/S0026-4784.18.04240-5
179. Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod Sci. 2017;24(8):1111–1120. doi:10.1177/1933719117702251
180. Van Eyck AS, Jordan BF, Gallez B, et al. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92(1):374–381. doi:10.1016/j.fertnstert.2008.05.012
181. Xia X, Yin T, Yan J, et al. Mesenchymal stem cells enhance angiogenesis and follicle survival in human cryopreserved ovarian cortex transplantation. Cell Transplant. 2015;24(10):1999–2010. doi:10.3727/096368914X685267
182. Zhang Y, Xia X, Yan J, et al. Mesenchymal stem cell-derived angiogenin promotes primodial follicle survival and angiogenesis in transplanted human ovarian tissue. Reprod Biol Endocrinol. 2017;15(1):18. doi:10.1186/s12958-017-0235-8
183. Manavella DD, Cacciottola L, Pomme S, et al. Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen-thawed human ovarian tissue. Human Reprod. 2018;33(6):1107–1116. doi:10.1093/humrep/dey080
184. Dolmans MM, Luyckx V, Donnez J, et al. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. 2013;99(6):1514–1522. doi:10.1016/j.fertnstert.2013.03.027
185. Revel A, Revel-Vilk S, Aizenman E, et al. At what age can human oocytes be obtained? Fertil Steril. 2009;92(2):458–463. doi:10.1016/j.fertnstert.2008.07.013
186. Segers I, Mateizel I, Van Moer E, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32(8):1221–1231. doi:10.1007/s10815-015-0528-9
187. Xiao S, Zhang J, Romero MM, et al. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep. 2015;5:17323. doi:10.1038/srep17323
188. Fasano G, Dechene J, Antonacci R, et al. Outcomes of immature oocytes collected from ovarian tissue for cryopreservation in adult and prepubertal patients. Reprod Biomed Online. 2017;34(6):575–582. doi:10.1016/j.rbmo.2017.03.007
189. Anderson RA, McLaughlin M, Wallace WH, et al. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Human Reprod. 2014;29(1):97–106. doi:10.1093/humrep/det388
190. Jia Y, Shi X, Xie Y, et al. Human umbilical cord stem cell conditioned medium versus serum-free culture medium in the treatment of cryopreserved human ovarian tissues in in-vitro culture: a randomized controlled trial. Stem Cell Res Ther. 2017;8(1):152. doi:10.1186/s13287-017-0601-7
191. Rajabi Z, Yazdekhasti H, Noori Mugahi SMH, et al. Mouse preantral follicle growth in 3D co-culture system using human menstrual blood mesenchymal stem cell. Reprod Biol. 2018;18(1):122–131. doi:10.1016/j.repbio.2018.02.001
192. Xia X, Wang T, Yin T, et al. Mesenchymal stem cells facilitate in vitro development of human preantral follicle. Reprod Sci. 2015;22(11):1367–1376. doi:10.1177/1933719115578922
193. Hubner K, Fuhrmann G, Christenson LK, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300(5623):1251–1256. doi:10.1126/science.1083452
194. Hayashi K, Ogushi S, Kurimoto K, et al. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338(6109):971–975. doi:10.1126/science.1226889
195. Hikabe O, Hamazaki N, Nagamatsu G, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539(7628):299–303. doi:10.1038/nature20104
196. Jung D, Xiong J, Ye M, et al. In vitro differentiation of human embryonic stem cells into ovarian follicle-like cells. Nat Commun. 2017;8:15680. doi:10.1038/ncomms15680
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.