Back to Journals » Clinical Epidemiology » Volume 15
Role of Serum Lipids, Blood Glucose and Blood Pressure in Breast Cancer Risk for Women with Type 2 Diabetes Mellitus
Authors Zhang F , de Bock GH , Denig P , Landman GW, Zhang Q , Sidorenkov G
Received 16 August 2022
Accepted for publication 5 December 2022
Published 24 January 2023 Volume 2023:15 Pages 109—121
DOI https://doi.org/10.2147/CLEP.S386471
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Henrik Sørensen
Fan Zhang,1– 3 Geertruida H de Bock,1 Petra Denig,4 Gijs W Landman,5 Qingying Zhang,2 Grigory Sidorenkov1
1Department of Epidemiology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands; 2Department of Preventive Medicine, Shantou University Medical College, Shantou, People’s Republic of China; 3Oncology Research Laboratory, Cancer Hospital of Shantou University Medical College, Shantou, People’s Republic of China; 4Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands; 5Department of Internal Medicine, Gelre Hospital, Apeldoorn, the Netherlands
Correspondence: Grigory Sidorenkov, Department of Epidemiology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands, Email [email protected]
Purpose: Women with type 2 diabetes mellitus (T2DM) have an increased risk of breast cancer. We aimed to determine the contribution of lipids, glucose and blood pressure to this risk based on the multifactorial nature of T2DM.
Patients and Methods: This population-based cohort study used data from a Dutch database (the Groningen Initiative to Analyse Type 2 Diabetes Treatment) for the period 2004– 2013. The cohort included women diagnosed with T2DM, aged 30– 80 years, with no history of breast cancer and with follow-up data for at least 1 year. We used Cox proportional hazards models to estimate the associations of exposures with breast cancer occurrence, reporting adjusted hazard ratios (aHR) with 95% confidence intervals (CI). Exposures of interest included total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, glycated hemoglobin A (HbA1c) and systolic blood pressure (SBP).
Results: During a median of 4.45 years’ follow-up, 183 of 10,183 included women received a breast cancer diagnosis. We observed U-shaped associations with breast cancer incidence for total cholesterol and HDL-C at baseline. Compared with moderate elevations, women had significantly higher breast cancer risks associated with high total cholesterol (aHR, 95% CI: 1.72, 1.15– 2.55) and HDL-C (aHR, 95% CI: 1.74, 1.18– 2.58) levels, while low total cholesterol (aHR, 95% CI: 1.43, 0.94– 2.19) and HDL-C (aHR, 95% CI: 1.44, 0.95– 2.17) levels produced marginal effects without significance. Women with high LDL-C levels more often received a breast cancer diagnosis than those with medium levels (aHR, 95% CI: 1.56, 1.03– 2.35).
Conclusion: This real-world dataset highlights the importance of balancing lipid profiles, particularly total cholesterol and HDL-C. Dysregulation of the lipid profile, not the glucose or blood pressure profiles, may increase the risk of breast cancer in women with T2DM.
Keywords: cholesterol, triglycerides, glycated hemoglobin A, systolic blood pressure, breast cancer
Plain Language Summary
Type 2 diabetes mellitus (T2DM) may increase the risk of breast cancer in women, but we know little about the causes. Although researchers initially thought that raised blood sugar levels offered a plausible explanation, the subsequent literature has not shown a clear association. Focus has since shifted to the roles of disordered fat levels in the blood (dyslipidemia) and raised blood pressure (hypertension), because we know that women with T2DM suffer from these more often than healthy women. Many observational studies have examined the relationships between these disorders and breast cancer risk, but these have not confirmed clear associations in women with T2DM. The present research examined the associations between different fat levels (total cholesterol, high- and low-density lipoprotein cholesterol, and triglycerides), blood pressure, and blood sugar control (glycated hemoglobin A) with the risk of breast cancer in women with T2DM. This study revealed U-shaped associations with breast cancer incidence for two fats (total cholesterol and high-density lipoprotein cholesterol) and a detrimental role for another (raised levels of low-density lipoprotein cholesterol). These findings improve our understanding of the roles of several modifiable risk factors for the occurrence of breast cancer, and they emphasize a need to closely monitor the lipid profiles of women with T2DM.
Introduction
The incidence of female breast cancer has increased globally over the last three decades, reaching 2.26 million cases in 2020, when it overtook lung cancer as the most commonly diagnosed malignancy.1,2 Breast cancer has multifaceted genetic, lifestyle and disease-related risk factors, and shares many with type 2 diabetes mellitus (T2DM). Consistent with this, several reviews have even indicated that women with T2DM have a 14%–25% increased risk of breast cancer.3–5 Although obesity may intuitively contribute to this excess risk, research has revealed an increased obesity-related cancer incidence in women both after a diabetes diagnosis and as early as 5 years before this diagnosis.6 Thus, the mechanisms underlying the relationship between T2DM and breast cancer require further study.
Patients with T2DM often suffer from multiple metabolic disorders. Logically, we might assume that hyperglycaemia increases tumour growth in cancer cells.7,8 However, the literature has not supported this hypothesis,9–11 especially for glycated hemoglobin A (HbA1c), indicating the involvement of mechanisms other than hyperglycaemia alone. As a common comorbidity of diabetes, dyslipidaemia has been studied extensively in relation to breast cancer, albeit with inconsistent findings. Whereas biological experiments have implicated cholesterol as a risk factor for breast cancer,12 research in general populations has uncovered an inverse association between total cholesterol, high-density lipoprotein cholesterol (HDL-C) and breast cancer risk.13 Nevertheless, questions of reverse causality surround that finding,14 and more recent Mendelian randomisation studies have consistently suggested that increased HDL-C levels have a positive association with increased breast cancer risk.15–17 By contrast, research into low-density lipoprotein cholesterol (LDL-C) and triglycerides has revealed less conclusive findings.13,15–20 Inconsistent results in the literature possibly result from the use of different age groups, producing inconsistent changes relative to age.21,22 Intriguingly, diabetes per se could also complicate this relationship by inducing quantitative and qualitative changes in serum lipids and lipoproteins.23–25 Although dyslipidaemia is routinely measured in patients with T2DM,26 this has been the focus of only one study to our knowledge. This found no association between HDL-C and breast cancer, though the analysis relied on an unrepresentative sample of mostly low-income residents.27 Finally, hypertension is a common comorbidity in adults with T2DM.28 Although studies have considered the relationship between blood pressure and breast cancer in general populations,29–31 this remains unclear in women with T2DM.
The contributions of total cholesterol, HDL-C, LDL-C, triglycerides, and systolic blood pressure (SBP) to the risk of breast cancer in women with T2DM warrant further research. Given that clinicians routinely collect data on these variables for patients with T2DM, we aimed to use a primary care database to clarify their contribution to the excess risk of breast cancer. The results could support the development of screening strategies for women with T2DM.
Materials and Methods
Study Design, Setting and Population
This retrospective population-based cohort study included women diagnosed with T2DM who received routine treatment in Dutch primary care and had at least one HbA1c or fasting plasma glucose (FPG) measurement between January 2004 and December 2013. The diagnosis of T2DM was established by general practitioners (GPs). Usually, this relied on elevated plasma glucose levels or HbA1c levels following recommendations of Dutch guideline for T2DM management.32 Patients were excluded if they met the following criteria at baseline: (1) age <30 or >80 years, (2) history of breast cancer, or (3) a follow-up period of <12 months after the inclusion date. We allowed a latency period of 12 months between patient inclusion and breast cancer diagnosis.
Data were obtained from the Groningen Initiative to Analyse Type 2 Diabetes Treatment (GIANTT) database, which comprises a cohort of approximately 60,000 patients with type 2 diabetes in primary care in Groningen, the Netherlands. More information about the database is available in the Supplementary Materials and online (https://www.giantt.nl/, accessed on 18th January 2022). This research required no ethics committee approval (see Supplementary Materials for explanations).
Baseline Characteristics
Given the potential for delays in the diagnosis of T2DM, we defined the baseline index date as the first date of HbA1c or FPG measurement within a period from 1 year before the registered date of T2DM diagnosis to 1 year before the end of follow-up. Baseline data collection proceeded with different strategies to limit missing data, as follows: age (at the index date); body mass index (BMI) (first record within 1 year before the index date and the end of 2012); smoking status (most recent record up to the end of 2012); total cholesterol, HDL-C, LDL-C, triglycerides and SBP (first record within one year around the index date); medications (all records per drug within one year around the index date); and a history of diseases (all records before and within one year around the index date). Further details are included in Figure S1 and the Supplementary Materials.
Explanatory and Outcome Variables
The exposures of interest were the total cholesterol, HDL-C, LDL-C, triglyceride (mmol/L), HbA1c (%) and SBP (mmHg) at baseline. Data were collected from the electronic medical records of general practices. For laboratory measurements, certified labs performed the sample analyses. Blood pressure was measured in general practice according to a recommended procedure (Het Nederlands Huisartsen Genootschap [The Dutch College of General Practitioners]. Protocol Ambulante bloeddrukmeting [Protocol for measuring blood pressure], 30-03-2016, accessed on 22 October 2022. Url: https://www.nhg.org/downloads/protocol-ambulante-bloeddrukmeting). LDL-C was routinely calculated using the Friedewald equation.33
The outcome of interest was time to breast cancer diagnosis, as assessed by patient follow-up to diagnosis, death, drop-out, or the end of data collection (December 2013), whichever came first. GPs recorded the breast cancer diagnosis in medical records according to the International Classification of Primary Care,34 typically based on communication from medical specialists who have established the diagnosis. We defined drop-out as the absence of measurements or prescriptions (eg, antihyperglycemics, antihyperlipidemics, antihypertensives, antiplatelets, antidepressants, oral contraceptives, glucocorticoids and aspirin) to 31st December 2012.
Statistical Analyses
All data analyses were performed using R version 4.1.0. Descriptive statistics are presented as means and standard deviations or counts and percentages. The baseline characteristics of patients with and without a diagnosis of breast cancer were compared using independent t-tests (continuous variables) or chi-square tests (categorical variables; not considering missing values). Due to missing data, we used multiple imputation with a chained equations procedure, generating 25 imputed datasets with the “mice” R-package (see Supplementary Materials).35
Cox proportional hazards models were conducted to test the associations of total cholesterol, HDL-C, LDL-C, triglycerides, SBP and HbA1c with breast cancer occurrence. We present the results as adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) and include crude cumulative incidence curves of breast cancer over time. The parameters were included as categorical variables in three levels (high, medium and low), with restricted cubic splines (RCS) used to establish the cut-off limits (see Supplementary Materials and Figure S2). For HbA1c, we used the low level as the reference because the RCS curves showed a nearly monotone decreasing trend; by contrast, we used the medium level as the reference for lipids and SBP. Age (cut-off values: 55 and 65 years) and BMI (cut-off values: 25 and 30 kg/m2) were categorised into three levels, using the low level as the reference. History of disease (yes/no) and medication usage (yes/no) were input as dichotomous variables.
Multivariate analyses were performed as follows. Model 1 was adjusted for age and BMI. Model 2 was adjusted for total cholesterol, HDL-C, LDL-C, triglycerides, SBP and HbA1c, other than the targeted explanatory variable, in addition to Model 1. Model 3 was adjusted for metformin, non-metformin glucose-lowering medications, angiotensin converting enzyme inhibitors/angiotensin-receptor blockers (ACE-i/ARB), non-ACE-i/ARB anti-hypertension medications, and statins, in addition to Model 1. Model 4 was adjusted for all covariates with a P-value <0.2 in the univariate analyses. See Supplementary Materials for more details. The proportional hazards assumption was examined by the score test, which revealed that only one variable (non-metformin anti-diabetic medications; P < 0.05) went against the assumption.36 Therefore, we used this variable as a stratification factor in Model 3.
Two sensitivity analyses were conducted separately in both the univariate and multivariable models. First, we assessed the bias of reverse causality by extracting subsets of imputed datasets that only included patients with follow-up data for at least 2 years and fitted the Cox proportional hazards models. Second, we accounted for the competing risk of all-cause death by using the Fine-Gray model on the imputed datasets with a follow-up of at least 1 year.37 To assess detection bias, we also conducted stratified analyses on the imputed datasets by age (≥65 and <65 years) and BMI (≥30 and <30 kg/m2), with the other included as a continuous variable (BMI or age, respectively). Finally, we performed an analysis stratified by statin use at baseline (yes/no).
Data Availability
The University Medical Centre Groningen maintains individual data, which the GIANTT steering committee can make available upon request. This manuscript reports aggregate data only.
Results
Cohort Characteristics
We identified 12,176 women with T2DM who had at least one blood glucose measurement recorded between 2004 and 2013; of these, 10,183 met all the eligibility criteria with a median follow-up of 4.45 years (Figure 1). Breast cancer was detected in 183 subjects, corresponding to a crude incidence rate of 3.69 per 1000 person-years. Compared to women without breast cancer, those who received a diagnosis were older (63.57 vs 61.79 years, P = 0.010), had lower BMIs (30.26 vs 31.37 kg/m2, P = 0.007), and slightly higher HDL-C (mean: 1.38 vs 1.30 mmol/L, P=0.029) at baseline (Table 1). Although total cholesterol did not differ as a continuous variable with breast cancer diagnosis, women without breast cancer more often had a total cholesterol level of 4.7–6.1 mmol/L, whereas those with breast cancer more often had a total cholesterol <4.7 mmol/L (P = 0.003). We found no statistical significance for other exposures and covariates.
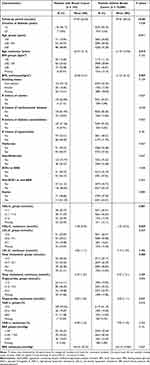 |
Table 1 Baseline Characteristics by Breast Cancer Diagnosis in Women with Type 2 Diabetes Mellitus |
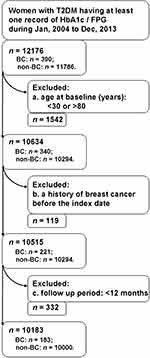 |
Figure 1 Flow chart of patient selection. Abbreviations: BC, breast cancer; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A; n, sample size. |
Association of Lipids with Breast Cancer
Univariate analyses of the imputed and complete datasets indicated that women with high total cholesterol, HDL-C or LDL-C levels at baseline had an increased risk of breast cancer compared to those with medium levels (Figure S3 and Table S1). The final multivariable-adjusted model based on imputed datasets for follow-up ≥1 year in Model 4 (Figure 2 and Table S2) revealed aHR values of 1.72 (95% CI, 1.15–2.55) for total cholesterol (≥6.1 versus ≥4.7 to <6.1 mmol/L), 1.74 (95% CI, 1.18–2.58) for HDL-C (≥1.6 versus ≥1.1 to <1.6 mmol/L), and 1.56 (95% CI, 1.03–2.35) for LDL-C (≥4.1 versus ≥2.6 to <4.1 mmol/L). Additionally, the risk of breast cancer increased with a low total cholesterol (<4.7 vs ≥4.7 to <6.1 mmol/L) and low HDL-C (<1.1 vs ≥1.1 to <1.6 mmol/L) compared with the medium level, whereas the risk was less for low triglyceride levels (<1.3 versus ≥1.3 to <2.4 mmol/L); however, the 95% CIs in Model 4 did not reach significance for the respective aHR values of 1.43 (95% CI, 0.94–2.19), 1.44 (95% CI, 0.95–2.17), and 0.66 (95% CI, 0.42–1.05) (Figure 2 and Table S2). Considering each lipid measure as a continuous variable, Figure S2 depicts the estimates for the HRs and 95% CIs in the crude RCS, which shows a U-shaped pattern for both total cholesterol and HDL-C with nadir hazard ratios at approximately 4.9 mmol/L and 1.1 mmol/L, respectively. The crude cumulative incidence curves are shown in Figure S4.
Sensitivity analyses performed with the imputed datasets of women followed-up for at least 2 years produced similar findings, except for a marginal and insignificant association between a high LDL-C level and breast cancer risk in Model 4 (aHR, 1.56; 95% CI, 0.97–2.50; Table S3). The Fine-Gray models to account for the competing risk of death also produced results comparable to those of the primary analyses (Table S4).
Subgroup analyses (Table S5) indicated a positive association between higher total cholesterol levels and the risk of breast cancer, particularly for older (Model 3: aHR, 2.15; 95% CI, 1.21–3.80) and non-obese (Model 3: aHR: 2.13; 95% CI: 1.26–3.62) women, but not for younger (Model 3: aHR, 1.36; 95% CI, 0.79–2.34) or obese (Model 3: aHR, 1.31; 95% CI, 0.73–2.37) peers. Statin prescriptions did not alter this relationship.
Association of Blood Glucose and Blood Pressure with Breast Cancer
Neither HbA1c nor SBP had a significant univariable or multivariable association with the risk of breast cancer in the imputed datasets for women who received at least 1 year of follow-up (Figures 2, S3 and Tables S1 and S2). Figures S2 and S4 show the corresponding crude RCS and cumulative incidence curves, while Tables S3–S4 show that the two sensitivity analyses produced similar results.
Subgroup analysis by age (Table S5) revealed a significant association between low SBP levels and the risk of breast cancer in Model 3 for women aged ≥65 years (HR, 2.06; 95% CI, 1.27–3.35), but not for those aged <65 years (HR, 1.03; 95% CI, 0.64–1.65). We also observed this association for women with a BMI ≥30 kg/m2 in Model 3 (HR, 1.87; 95% CI, 1.13–3.10), but not for those with a BMI <30 kg/m2 (HR, 1.05; 95% CI, 0.65–1.69).
Discussion
Summary
This study identified U-shaped associations between total cholesterol and HDL-C at baseline with the risk of breast cancer in women with T2DM, with increased risk at high (significant) and low (non-significant) levels compared with medium levels. Women with high LDL-C levels at baseline were also more likely to be diagnosed with breast cancer than those with medium levels. These associations appeared to be independent of statin therapy. Of note, we also observed no relationship between either glycemic control or blood pressure and the risk of breast cancer.
Breast Cancer Risk Associated with Circulating Lipids
Total Cholesterol, HDL-C and LDL-C
Cholesterol may affect breast cancer risk by altering the properties of membranes to facilitate lipid raft formation and increase signalling events. Moreover, levels affect the metabolite 27-hydroxycholesterol, which might act as an oestrogen receptor agonist that promotes associated cancer cell line proliferation.12,38 Despite the lack of direct evidence for breast-specific cancer in women with diabetes, a low total cholesterol level appears to be associated with increased all-site cancer risk.39 Intriguingly, we observed a U-shaped relationship between total cholesterol and breast cancer risk in both the primary and sensitivity analyses, notably for women aged ≥65 years, but unexpectedly for women with a BMI <30 kg/m2. Given that total cholesterol increases around age 50 years in women,21,22 we further adjusted the models with age as a continuous variable, and the observed associations remained.
Statins, which inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, efficiently reduce plasma cholesterol.40 Although in vitro and in vivo evidence suggests that statins have antiproliferative effects on various cancer, clinical evidence has failed to reveal an association with breast cancer risk.41 To account for the possible effect of statins, we nevertheless included their use as a covariate in Model 3 and as a stratification factor in the stratified analyses. The positive association between high total cholesterol levels and breast cancer risk persisted in patients not receiving statins.
Existing evidence suggests that HDL-C has concentration-dependent biphasic effects: at low concentrations it is associated with an increased risk of atherosclerotic cardiovascular disease42 and at high levels it is associated with increased risks of death from cardiovascular disease, cancer and other causes.43 Although we observed a similar U-shaped association between HDL-C and breast cancer, we do not understand the underlying mechanism. It may be that patients with T2DM develop alterations in HDL metabolism, composition and structure, such as the replacement of cholesteryl esters with triglycerides or the glycation or oxidative modification of HDL apolipoproteins or enzymes. In turn, these changes may cause functional HDL deficiencies and even the acquisition of detrimental abilities that induce cancer cell proliferation.44,45 The Louisiana State University Health Care Services Division (LSUHCSD) cohort found a non-linear association between HDL-C and all-site cancer risk (but not for breast-specific risk) under T2DM conditions.27 This non-linearity was also observed in a prospective cohort of patients with T2DM from the Chinese Hong Kong Diabetes Registry.39 LDL-C may also induce the proliferation of some breast cancer subtypes,46,47 with a Hong Kong cohort showing elevated risk for high LDL-C levels compared to medium levels on a combined outcome of breast, bone, connective tissue and skin cancer.48 In the GIANTT cohort, given the imbalanced sample sizes and overlapping CIs of HRs in subgroups, we cannot state that statins influence the association of HDL-C or LDL-C with breast cancer risk.
Triglycerides
A previous study showed a possible association between low triglyceride levels and increased cancer risk in patients with T2DM.49 After adjusting for other lipid measures and covariates, we found a marginal and insignificant association between low triglyceride levels and a lower risk of breast cancer in women with T2DM. Although the underlying mechanisms are still unclear, lipid droplets may play a role in the resistance of cancer cells to lipotoxic stress.50,51
Possible Explanations for Discrepancies
Compared with other population-based studies, three broad explanations may explain some of the discrepancies in the association between lipid measures and breast cancer risk in the current research.
First, our population had a different comorbidity status. Dyslipidemia induces both qualitative and quantitative changes in patients with diabetes. Glycated and oxidised HDLs cause a loss of physiological function, which results in a stronger ability to promote cell proliferation, thereby increasing the risk of breast cancer.52,53 Furthermore, glycated LDL is more prone to oxidation than native LDL54 and shows decreased LDL receptor-mediated catabolism.55 Serum oxidised LDL levels are known to be associated with an increased risk of breast cancer.46 Thus, diabetes might alter the association between lipid metabolism and breast cancer risk.
Second, we used different methodologies to analyse the data. Observations in both this and previous studies27,39 suggest the possibility of a non-linear association between lipids (especially total cholesterol and HDL-C) and breast cancer risk. Consequently, earlier research that has often used the lowest or highest level as the reference group may have obscured the true associations.27,56
Third, our population used different medications, notably glucose-lowering and lipid-lowering drugs, which could have positively or negatively affected lipids and lipoproteins.55 Thus, disease severity may have differed. For example, the LSUHCSD cohort relied on hospital-based data,27 whereas our cohort relied on GP-based data for recently diagnosed women. Thus, we might expect different disease severity and medication use for women with T2DM in these cohorts, which may offset or obscure the impact of lipids on breast cancer risk.
Breast Cancer Risk Associated with Blood Glucose or Blood Pressure
In vitro biological evidence suggests that hyperglycemia creates a fertile ground for tumour growth by promoting reliance on aerobic glycolysis (known as the Warburg effect) and increasing glucose consumption in cancer cells. However, this outcome depends partly on the experimental conditions used, including the assay type, glucose concentration and incubation time.57,58 Most women in the current study had a recent diagnosis of T2DM and relatively good control at baseline (HbA1c < 7.0%). The resulting failure to demonstrate a relationship between HbA1c levels and breast cancer risk is consistent with a recent review demonstrating that improved glycemic control through the use of glucose-lowering drugs or other treatment may not confer reduced cancer risk for patients with T2DM.59 Another review of observational studies found similar results.10
Regarding SBP, shared dysregulation of the immune function may explain the biological link between hypertension and cancer.60,61 However, further investigation is needed because we only observed an increased risk of breast cancer with a very low SBP compared to a medium SBP in women aged ≥65 years or with a BMI ≥30 kg/m2. This pattern may instead reflect the higher prevalence of hypertension and the longer history of antihypertensive treatment in older or obese patients. Although an association may exist between antihypertensive medications and the risk of breast cancer, research has not produced consistent findings.62
Strengths and Limitations
This population-based cohort of women with T2DM (over 95% diagnosed recently) benefitted from a relatively large sample size with detailed information about laboratory tests, physical examinations, medications and disease history. The dataset extracted from electronic records also used real-world data from routine GP care.63 However, several limitations require careful consideration.
First, by using routinely collected data from electronic medical records, some variation may have existed between general practices in how the measurements and diagnoses were obtained. Nevertheless, we do not expect this to cause specific bias.
Second, we may not have used a sufficiently long latency period to allow the detection of breast cancer.64,65 However, this should be considered in the context that abnormal lipid levels might have persisted for years in patients who develop diabetes. This study still uncovered significant associations between lipids and breast cancer risk, identifying groups at above-average risk of developing breast cancer.
Third, the results could suffer from reverse causality bias. To reduce this, we performed sensitivity analyses that excluded patients censored or diagnosed with breast cancer during the first 2 years of follow-up. Most of the associations persisted in these analyses.
Fourth, some patients not using statins may have received ezetimibe. We do not believe that this affected our findings because few women received this treatment (around 2%).
Fifth, we experienced a moderate number of missing values, especially for the lipid profile at baseline (15%–25%). Sticking to the complete dataset would have caused a substantial loss of patients and a reduction of power, leaving the multivariable analysis only feasible with a limited number of covariates. Thus, we applied multiple imputation by chained equation to create multiple predictions for each missing value based on all available information about a patient, which is considered superior to complete case analysis.35 The broad comparability of the univariable analysis results with the imputed and complete datasets indicates that imputation caused trivial inference.
Sixth, residual confounding could not be excluded, even in the analyses adjusted for an extensive set of confounders, because of the many unmeasured factors (eg, physical activity, dietary factors and advanced glycation end products). Based on the four models with adjusted covariates, however, we observed no notable differences in the estimates. Additionally, we could not exclude selection bias. Among patients without a breast cancer diagnosis, a few baseline characteristics showed statistical differences between those with <12 and ≥12 months’ follow-up (data not shown). The large numbers may explain this finding, though differences in the absolute numbers may not be clinically significant.
Conclusions
Dysregulation of the lipid profile, and not the glucose or blood pressure profiles, appears to be associated with the increased risk of breast cancer in women with T2DM in a real-world setting. This supports not only the importance of balancing total cholesterol and HDL-C levels but also the detrimental role of LDL-C on the occurrence of breast cancer in these women. Checking the lipid profile regularly may help to identify women with T2DM at an increased risk of breast cancer.
Future Perspectives
The results of this study require validation in cohorts with longer follow-up periods. Other research should now investigate the roles of lipoproteins, their glycated and oxidated forms, and cholesterol metabolites in the molecular mechanism of breast cancer. Such research will provide a more complete understanding of the association between lipids and the risk of breast cancer.
Abbreviations
ACE-i/ARB, angiotensin converting enzyme inhibitors/angiotensin-receptor blockers; aHRs, Adjusted hazard ratios; BMI, Body mass index; CIs, Confidence intervals; FPG, Fasting plasma glucose; GPs, general practitioners; HbA1c, Glycated hemoglobin A; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LSUHCSD, Louisiana State University Health Care Services Division; Ref., reference; RCS, Restricted cubic splines; SBP, systolic blood pressure; T2DM, Type 2 diabetes mellitus.
Acknowledgments
We thank Dr Robert Sykes for the language editing.
Disclosure
Fan Zhang received from the Graduate School of Medical Sciences, University of Groningen, University Medical Center Groningen: a PhD position grant. The authors report no other conflicts of interest in this work.
References
1. Hu K, Ding P, Wu Y, Tian W, Pan T, Zhang S. Global patterns and trends in the breast cancer incidence and mortality according to sociodemographic indices: an observational study based on the global burden of diseases. BMJ Open. 2019;9(10):e028461. doi:10.1136/bmjopen-2018-028461
2. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–789. doi:10.1002/ijc.33588
3. Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121(4):856–862. doi:10.1002/ijc.22717
4. Liao S, Li J, Wei W, et al. Association between diabetes mellitus and breast cancer risk: a meta-analysis of the literature. Asian Pac J Cancer Prev. 2011;12(4):1061–1065.
5. Starup-Linde J, Karlstad O, Eriksen SA, et al. CARING (CAncer Risk and INsulin analoGues): the association of diabetes mellitus and cancer risk with focus on possible determinants - a systematic review and a meta-analysis. Curr Drug Saf. 2013;8(5):296–332. doi:10.2174/15748863113086660071
6. Schrijnders D, Hendriks SH, Kleefstra N, et al. Sex differences in obesity related cancer incidence in relation to type 2 diabetes diagnosis (ZODIAC-49). PLoS One. 2018;13(1):e0190870. doi:10.1371/journal.pone.0190870
7. Brown KA. Metabolic pathways in obesity-related breast cancer. Nat Rev Endocrinol. 2021;17(6):350–363. doi:10.1038/s41574-021-00487-0
8. Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23(1):27–47. doi:10.1016/j.cmet.2015.12.006
9. Boyle P, Koechlin A, Pizot C, et al. Blood glucose concentrations and breast cancer risk in women without diabetes: a meta-analysis. Eur J Nutr. 2013;52(5):1533–1540. doi:10.1007/s00394-012-0460-z
10. Macacu A, Pizot C, Boyle P, Autier P. Risk of Breast Cancer According to Glycaemia and HbA1c Concentration — a Meta-analysis of Prospective Studies. Diabetes. 2018;67:1944–P. doi:10.2337/db18-1944-P
11. Jung SY, Mancuso N, Han S, Zhang ZF. The Role of Genetically Determined Glycemic Traits in Breast Cancer: a Mendelian Randomization Study. Front Genet. 2020;11:540724. doi:10.3389/fgene.2020.540724
12. Xu H, Zhou S, Tang Q, Xia H, Bi F. Cholesterol metabolism: new functions and therapeutic approaches in cancer. Biochim Biophys Acta Rev Cancer. 2020;1874(1):188394. doi:10.1016/j.bbcan.2020.188394
13. Touvier M, Fassier P, His M, et al. Cholesterol and breast cancer risk: a systematic review and meta-analysis of prospective studies. Br J Nutr. 2015;114(3):347–357. doi:10.1017/S000711451500183X
14. Ganjali S, Banach M, Pirro M, Fras Z, Sahebkar A. HDL and cancer - causality still needs to be confirmed? Update 2020. Semin Cancer Biol. 2021;73:169–177. doi:10.1016/j.semcancer.2020.10.007
15. Johnson KE, Siewert KM, Klarin D, et al. The relationship between circulating lipids and breast cancer risk: a Mendelian randomization study. PLoS Med. 2020;17(9):e1003302. doi:10.1371/journal.pmed.1003302
16. Beeghly-Fadiel A, Khankari NK, Delahanty RJ, et al. A Mendelian randomization analysis of circulating lipid traits and breast cancer risk. Int J Epidemiol. 2020;49(4):1117–1131. doi:10.1093/ije/dyz242
17. Nowak C, Arnlov J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat Commun. 2018;9(1):3957. doi:10.1038/s41467-018-06467-9
18. Orho-Melander M, Hindy G, Borgquist S, et al. Blood lipid genetic scores, the HMGCR gene and cancer risk: a Mendelian randomization study. Int J Epidemiol. 2018;47(2):495–505. doi:10.1093/ije/dyx237
19. Ni H, Liu H, Gao R. Serum Lipids and Breast Cancer Risk: a Meta-Analysis of Prospective Cohort Studies. PLoS One. 2015;10(11):e0142669. doi:10.1371/journal.pone.0142669
20. Ma HQ, Cui LH, Li CC, Yu Z, Piao JM. Effects of Serum Triglycerides on Prostate Cancer and Breast Cancer Risk: a Meta-Analysis of Prospective Studies. Nutr Cancer. 2016;68(7):1073–1082. doi:10.1080/01635581.2016.1206582
21. Ambroz M, de Vries ST, Vart P, et al. Sex Differences in Lipid Profile across the Life Span in Patients with Type 2 Diabetes: a Primary Care-Based Study. J Clin Med. 2021;10(8):1775. doi:10.3390/jcm10081775
22. Balder JW, de Vries JK, Nolte IM, Lansberg PJ, Kuivenhoven JA, Kamphuisen PW. Lipid and lipoprotein reference values from 133,450 Dutch Lifelines participants: age- and gender-specific baseline lipid values and percentiles. J Clin Lipidol. 2017;11(4):1055–1064e6. doi:10.1016/j.jacl.2017.05.007
23. Goldberg IJ. Clinical review 124: diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab. 2001;86(3):965–971. doi:10.1210/jcem.86.3.7304
24. Farbstein D, Levy AP. HDL dysfunction in diabetes: causes and possible treatments. Expert Rev Cardiovasc Ther. 2012;10(3):353–361. doi:10.1586/erc.11.182
25. Mancini GBJ, Hegele RA, Leiter LA; Diabetes Canada Clinical Practice Guidelines Expert C. Dyslipidemia. Can J Diabetes. 2018;42(Suppl 1):S178–S185. doi:10.1016/j.jcjd.2017.10.019
26. Authors/Task Force M, Guidelines ESCCfP, Societies ESCNC. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi:10.1016/j.atherosclerosis.2019.08.014
27. Zhao W, Guan J, Horswell R, et al. HDL cholesterol and cancer risk among patients with type 2 diabetes. Diabetes Care. 2014;37(12):3196–3203. doi:10.2337/dc14-0523
28. Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes. 2013;6:327–338. doi:10.2147/DMSO.S51325
29. Seretis A, Cividini S, Markozannes G, et al. Association between blood pressure and risk of cancer development: a systematic review and meta-analysis of observational studies. Sci Rep. 2019;9(1):8565. doi:10.1038/s41598-019-45014-4
30. Yang Y, Lynch BM, Hodge AM, et al. Blood pressure and risk of breast cancer, overall and by subtypes: a prospective cohort study. J Hypertens. 2017;35(7):1371–1380. doi:10.1097/HJH.0000000000001372
31. Christakoudi S, Kakourou A, Markozannes G, et al. Blood pressure and risk of cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2020;146(10):2680–2693. doi:10.1002/ijc.32576
32. Rutten G, De Grauw W, Nijpels G, et al. NHG-Standaard Diabetes mellitus type 2 (derde herziening). Huisarts Wet. 2013;56(10):512–525.
33. de Vries FM, Voorham J, Hak E, Denig P. Adherence to standard-dose or low-dose statin treatment and low-density lipoprotein cholesterol response in type 2 diabetes patients. Curr Med Res Opin. 2015;31(12):2197–2206. doi:10.1185/03007995.2015.1092126
34. Lamberts H, Wood M, eds. International Classification of Primary Care (ICPC). Oxford:: Oxford University Press; 1987.
35. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi:10.1002/sim.4067
36. Grambsch P. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. doi:10.1093/biomet/81.3.515
37. Fine JP. A proportional hazards model for the subdistribution of a competing risk. JASA. 1999;94:496–509. doi:10.1080/01621459.1999.10474144
38. Nelson ER, Chang CY, McDonnell DP. Cholesterol and breast cancer pathophysiology. Trends Endocrinol Metab. 2014;25(12):649–655. doi:10.1016/j.tem.2014.10.001
39. Yang X, So WY, Ma RC, et al. Predicting values of lipids and white blood cell count for all-site cancer in type 2 diabetes. Endocr Relat Cancer. 2008;15(2):597–607. doi:10.1677/ERC-07-0266
40. Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5(4):378–387. doi:10.1111/j.1582-4934.2001.tb00172.x
41. Islam MM, Yang HC, Nguyen PA, et al. Exploring association between statin use and breast cancer risk: an updated meta-analysis. Arch Gynecol Obstet. 2017;296(6):1043–1053. doi:10.1007/s00404-017-4533-3
42. Di Angelantonio E, Sarwar N. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi:10.1001/jama.2009.1619
43. Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38(32):2478–2486. doi:10.1093/eurheartj/ehx163
44. Kontush A, Chapman MJ. Why is HDL functionally deficient in type 2 diabetes?. Curr Diab Rep. 2008;8(1):51–59.
45. Mazzuferi G, Bacchetti T, Islam MO, Ferretti G. High density lipoproteins and oxidative stress in breast cancer. Lipids Health Dis. 2021;20(1):143. doi:10.1186/s12944-021-01562-1
46. Cedo L, Reddy ST, Mato E, Blanco-Vaca F, Escola-Gil JC. HDL and LDL: potential New Players in Breast Cancer Development. J Clin Med. 2019;8(6):853. doi:10.3390/jcm8060853
47. Gallagher EJ, Zelenko Z, Neel BA, et al. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene. 2017;36(46):6462–6471. doi:10.1038/onc.2017.247
48. Yang X, So W, Ko GT, et al. Independent associations between low-density lipoprotein cholesterol and cancer among patients with type 2 diabetes mellitus. CMAJ. 2008;179(5):427–437. doi:10.1503/cmaj.071474
49. Yang X, Ma RC, So WY, et al. Low triglyceride and nonuse of statins is associated with cancer in type 2 diabetes mellitus: the Hong Kong Diabetes Registry. Cancer. 2011;117(4):862–871. doi:10.1002/cncr.25455
50. Jarc E, Eichmann TO, Zimmermann R, Petan T. Lipidomic data on lipid droplet triglyceride remodelling associated with protection of breast cancer cells from lipotoxic stress. Data Brief. 2018;18:234–240. doi:10.1016/j.dib.2018.03.033
51. Munir R, Lisec J, Swinnen JV, Zaidi N. Lipid metabolism in cancer cells under metabolic stress. Br J Cancer. 2019;120(12):1090–1098. doi:10.1038/s41416-019-0451-4
52. Pan B, Ren H, Ma Y, et al. High-density lipoprotein of patients with type 2 diabetes mellitus elevates the capability of promoting migration and invasion of breast cancer cells. Int J Cancer. 2012;131(1):70–82. doi:10.1002/ijc.26341
53. Bonilha I, Zimetti F, Zanotti I, Papotti B, Sposito AC. Dysfunctional High-Density Lipoproteins in Type 2 Diabetes Mellitus: molecular Mechanisms and Therapeutic Implications. J Clin Med. 2021;10(11):2233. doi:10.3390/jcm10112233
54. Sobal G, Menzel J, Sinzinger H. Why is glycated LDL more sensitive to oxidation than native LDL? A comparative study. Prostaglandins Leukot Essent Fatty Acids. 2000;63(4):177–186. doi:10.1054/plef.2000.0204
55. Schofield JD, Liu Y, Rao-Balakrishna P, Malik RA, Soran H. Diabetes Dyslipidemia. Diabetes Ther. 2016;7(2):203–219. doi:10.1007/s13300-016-0167-x
56. His M, Zelek L, Deschasaux M, et al. Prospective associations between serum biomarkers of lipid metabolism and overall, breast and prostate cancer risk. Eur J Epidemiol. 2014;29(2):119–132. doi:10.1007/s10654-014-9884-5
57. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi:10.1126/science.123.3191.309
58. Barbosa AM, Martel F. Targeting Glucose Transporters for Breast Cancer Therapy: the Effect of Natural and Synthetic Compounds. Cancers. 2020;12(1):154. doi:10.3390/cancers12010154
59. Lin C, Cai X, Yang W, Lv F, Nie L, Ji L. Glycemic control and the incidence of neoplasm in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Endocrine. 2020;70(2):232–242. doi:10.1007/s12020-020-02376-4
60. Siedlinski M, Jozefczuk E, Xu X, et al. White Blood Cells and Blood Pressure: a Mendelian Randomization Study. Circulation. 2020;141(16):1307–1317. doi:10.1161/CIRCULATIONAHA.119.045102
61. Carnevale D, Lembo G. Immunological Aspects of Hypertension. High Blood Press Cardiovasc Prev. 2016;23(2):91–95. doi:10.1007/s40292-016-0141-8
62. Sanidas E, Velliou M, Papadopoulos D, et al. Antihypertensive Drugs and Risk of Cancer: between Scylla and Charybdis. Am J Hypertens. 2020;33(12):1049–1058. doi:10.1093/ajh/hpaa098
63. Voorham J, Denig P. Computerized extraction of information on the quality of diabetes care from free text in electronic patient records of general practitioners. J Am Med Inform Assoc. 2007;14(3):349–354. doi:10.1197/jamia.M2128
64. Olsson H, Baldetorp B, Ferno M, Perfekt R. Relation between the rate of tumour cell proliferation and latency time in radiation associated breast cancer. BMC Cancer. 2003;3:11. doi:10.1186/1471-2407-3-11
65. Diana L, Nadler IGZ. Estimating Cancer Latency Times Using a Weibull Model. Adv Epidemiol. 2014;2014:1–8. doi:10.1155/2014/746769
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

