Back to Journals » Drug Design, Development and Therapy » Volume 15
Role of Oxidative Stress and Reduced Endogenous Hydrogen Sulfide in Diabetic Nephropathy
Authors Hussain Lodhi A, Ahmad FUD , Furwa K, Madni A
Received 11 November 2020
Accepted for publication 15 February 2021
Published 5 March 2021 Volume 2021:15 Pages 1031—1043
DOI https://doi.org/10.2147/DDDT.S291591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Anastasios Lymperopoulos
Arslan Hussain Lodhi,1 Fiaz-ud-Din Ahmad,1 Kainat Furwa,1 Asadullah Madni2
1Department of Pharmacology, Faculty of Pharmacy, The Islamia University of Bahawalpur, Bahawalpur, Pakistan; 2Department of Pharmaceutics, Faculty of Pharmacy, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
Correspondence: Fiaz-ud-Din Ahmad
Department of Pharmacology, Faculty of Pharmacy, The Islamia University of Bahawalpur, Khawaja Fareed Campus, Railway Road, Bahawalpur, 63100, Pakistan
Tel +92-320-8402376
Email [email protected]
Purpose: Persistent hyperglycemia lead towards depletion of hydrogen sulfide (H2S) resulting in generation of oxidative stress and diabetic nephropathy. The aim of the current study was to explore the antioxidant potential of H2S and captopril, a -SH containing compound in streptozotocin (STZ)-induced diabetic nephropathy.
Methods: Fifty four Wistar-Kyoto (WKY) rats male (200– 250g) were divided into nine groups (n=6) with each group injected once with STZ (60mg/kg i.p) except normal control. After 3 weeks of induction of diabetes, groups were assigned as normal control, diabetic control, diabetic-captopril, diabetic-NaHS, diabetic-captopril-NaHS, diabetic-spironolactone, diabetic-metformin, diabetic-metformin-NaHS and diabetic-vitamin-c. All the animals were served with normal saline (N/S 4mL/kg p.o), captopril (50mg/kg/day p.o), sodium hydrosulfide (NaHS) (56μmol/kg i.p), spironolactone (50mg/kg/day s.c), metformin (500mg/kg/day p.o) and vitamin-c (50mg/kg p.o) on daily basis for next 4 weeks, respectively. Metabolic studies, H2S levels, renal hemodynamics and oxidative stress markers were analyzed at 0, 14 and 28 days followed by histopathological analysis of renal tissues.
Results: The results showed decreased H2S levels, body weight, sodium to potassium ratio, glutathione (GSH), superoxide dismutase (SOD), total antioxidant assay (T-AOC) with malondialdehyde (MDA) and blood glucose levels significantly increased among diabetic rats. Treatment with captopril, NaHS, metformin, spironolactone and vitamin C showed significant improvement among renal hemodynamics and oxidative stress markers, respectively. But treatment groups like NaHS in combination with captopril and metformin showed more pronounced effects.
Conclusion: The observations suggest that H2S mediated protective effects on STZ-induced diabetic nephropathy may be associated with reduced oxidative stress via augmenting the antioxidant effect.
Keywords: captopril, diabetic nephropathy, oxidative stress, renal tissue, streptozotocin, sodium hydrosulfide
Introduction
Diabetic nephropathy (DN) is the “long term” major complication of diabetes with its incidence being increased annually worldwide leaving an open window for the exploration of new therapies.1 Hyperglycemia, hypertension, smoking, hyper-filtration and high protein diet are considered to be the major risk factors involved in the pathogenesis of diabetic nephropathy. Oxidative stress is considered to be the major culprit involved in the onset and progression of renal damage. Consistent hyperglycemia increases the polyol pathway which was considered to be an important factor in the development of nephropathy.2,3 The increased glucose level directly increases lipid peroxidation among glomerulus and hydrogen peroxide production among mesangial cells leading towards oxidative stress and the ultimate depletion of H2S.4,5 H2S has been considered as the third novel gasotransmitter after carbon monoxide (CO) and nitric oxide (NO) with rotten eggs smell. Increased H2S concentration exerts protective effects in multiple organ systems including kidney and heart by decreasing the renin circulation, left ventricular and renal fibrosis respectively.6 The pathophysiological role of H2S consists of insulin secretion, neurotransmission, vascular relaxation, cell proliferation and apoptosis. H2S production in mammalian tissue is catalyzed by two enzymes, cystathionine β-synthase (CBS) and Ɣ-lyase (CSE) through transsulfuration pathway. The “up regulation” of the renin-angiotensin-aldosterone system also involves the production of inflammation, oxidative stress and apoptosis, which affects the utilization of glucose in diabetes.7,8 Chemical agents such as alloxan and STZ, imparts selective damage to pancreatic β-cells leading towards hyperglycemia are reliable methods of producing animal models of diabetic nephropathy.9,10 STZ functions as a toxic glucose analogue which enters the pancreatic β-cells through GLUT2 glucose transporter mechanism. It destroys β-cells due to its alkylating nature and ability to generate the reactive oxygen species ultimately produces oxidative stress, necrosis and persistent hyperglycemia.11 Captopril (a -SH containing compound) acts as an antagonist of angiotensin-converting enzyme, ultimately decreases the tissue levels of angiotensin-II. Captopril neutralizes reactive oxygen species and lipid peroxidation due to its powerful antioxidant activity. In addition, the previous studies showed that captopril increases insulin secretion, improved glycemic control and pancreatic cell blood flow respectively.12 Similarly, spironolactone (aldosterone antagonist) also ameliorates renal fibrosis by inhibition of TGF- β1, PAI-1 and macrophages infiltration. Furthermore, exogenous sodium hydrosulfide NaHS (an H2S donor), vitamin C and metformin (standard antioxidant) supplementation produce a scavenging effect on the generation of reactive oxygen species (ROS).13 Therefore, this study aims to explore the nephroprotective potential of H2S against streptozotocin-induced renal insult which triggers a major role in ameliorating the tubular lesions and oxidative stress. Similarly, it should be noticed that the possible underlying molecular mechanisms of H2S in diabetic nephropathy are yet to be explored. Therefore, in-depth knowledge of interaction between H2S and its downstream targeted genes will surely help to develop H2S-mediated novel therapies which may reverse the diabetic nephropathy.14
Methodology
Chemicals
2, 2-diphenyl-1-picrylhydrazyl radical (DPPH), butylated hydroxytoluene (BHT), N,N-2 dimethyl-p-phenylenediamine sulfate, zinc acetate dihydrate and ethanol were purchased from Sigma Aldrich (Germany). Trichloroacetic acid (BDH, Prolab), phosphate buffer saline (Honeywell, Germany), ferrous chloride (BDH, Prolab), streptozotocin (Bioshop, Canada), sodium hydrosulfide (Daejing Chemicals, Korea), and ketamine (Global Pharmaceuticals) of research grade were purchased and used during the study. Creatinine, urea, uric acid, and albumin assay kits were purchased from Human Diagnostics (Germany). Oxidative stress and antioxidant markers including total superoxide dismutase (T-SOD), glutathione (GSH), malondialdehyde (MDA) and total antioxidant capacity (TAO-C) assay kits were purchased from Elabscience Biotechnology (USA).
In vitro Assay
DPPH Free Radical Neutralizing Assay
DPPH method is the most widely employed assay used to assess the free radical neutralizing potential of natural compounds. It works as a free radical marked to be stable, due to the availability of unpaired valence electron at one of the bridges of a nitrogen atom. It majorly acts as a trap for other free radicals that produce violet color upon oxidation. Due to deficiency of electron in DPPH, it accepts an electron from antioxidants ie, electron rich species, which upon neutralization becomes yellow. NaHS among different concentrations (3.125–100µM) were fused with 2mL of methanolic solution of DPPH (90 µM) with slight modification. At room temperature, incubation of all the serial dilutions were conducted for 30 minutes and then analyzed with the help of 96-well plate reader at 517nm. The percentage of inhibition for the positive control butylated hydroxytoluene (BHT) was also calculated with the same protocol as employed for NaHS.15,16 All the above mentioned protocol was performed in thrice and results averaged:
Inhibition % = (A Control – A Sample/AControl) ×100
A control= Absorbance of Control A sample= Absorbance of Sample
In vivo Assay
Experimental Animals
Fifty four Wistar-Kyoto (WKY) rats were divided into nine groups (male, n=6), at the 8th week of age weighing (200–250g) were obtained and placed in animal house of Research Lab of Pharmacology, Faculty of Pharmacy, the Islamia University of Bahawalpur. All the animals (6 to 8/cage) were housed in polycarbonate cages fed with standard diet and water ad libitum.
Induction of Diabetic Nephropathy
All the study design was reviewed and approved by Pharmacy Animal Ethics Committee with approval number (PAEC/2020/26). In research lab, standard housing conditions such as humidity> 65% and temp: 25±3◦C, alternative light and dark circle after 12 hours with saw dust of cages replaced after every 2 days were provided. All animals were treated as per assigned protocols of Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Following starvation for 16 hours, the animals were injected once with STZ (60mg/kg i.p) mixed in sodium citrate buffer (1mL/kg). After injection, drinking water mixed with sucrose (15 g/liter) for the next two days was served, to deplete the remaining insulin reserves and limit early mortality. The duration of experiment was almost two months and animal behaviour including their physical condition were monitored on daily basis to minimize the distress.17 After 1st week, hyperglycemia was measured in a fasting state after pricking the tail vein of the animal. The rats with a fasting glucose value of over (280–300mg/dl) were included in the diabetic nephropathy model. To avoid the development of ketonuria, diabetic rats were injected with long acting insulin (Protophane, 2–4 units/rat s.c) to restrict the glucose level (300–600mg/dl) for the next 3weeks respectively.
Treatment Protocol
All the experimental animals were subjected to treatment protocol for next 4 weeks, which was as followed: Group-I normal control (N/S 4mL/kg), Group-II diabetic control (60mg/kg i.p), Group-III diabetic-captopril (60mg/kg i.p and 50mg/kg/day p.o), Group-IV diabetic-NaHS (60mg/kg i.p and 56µmol/kg i.p), Group-V diabetic-captopril-NaHS (60mg/kg i.p, 50mg/kg/day p.o and 56µmol/kg i.p), Group-VI diabetic-spironolactone (60mg/kg i.p and 50mg/kg/day s.c), Group-VII diabetic-metformin (60mg/kg i.p and 500mg/kg/day p.o), Group-VIII diabetic-metformin-NaHS (60mg/kg i.p, 500mg/kg/day p.o and 56µmol/kg i.p) and Group-IX diabetic-vitamin-c (60mg/kg i.p and 50mg/kg p.o) were treated. All the animals were subjected to overnight fasting (12-hours), weighed and dissected using anesthetic mixture xylazine and ketamine (1:10) at a dose of 0.2 mL/100g.18 The kidneys were excised immediately after dissection, washed with fresh normal saline followed by storage at −20◦C for future analysis.19
Collection of Metabolic Data and Plasma
Samples of metabolic data and plasma were collected from all groups on day 0, 14 and 28 of the treatment protocol. Animals were placed in metabolic cages for 24 hours after which food intake, water intake, body weight and urine output was calculated. Similarly, retro-orbital puncture technique was employed for the collection of blood samples in heparinized centrifuge tubes. Retro-orbital technique can be used for greater number of animals within a short period of time with sterile hematocrit capillary tube followed by complete tissue repair within 10 days for repeated sampling.20 All the tubes were then centrifuged for 15-minutes at 3000 rpm followed by collection of plasma.21 The plasma and urine samples were stored at −20◦C in refrigerator for future analysis.
Biochemical Analysis
Blood glucose, renal parameters and oxidative stress and antioxidant markers including total superoxide dismutase (T-SOD), glutathione (GSH), malondialdehyde (MDA) and total antioxidant assay (T-AOC) were calculated by kit method.
Preparation of Whole-Kidney Homogenate
The whole kidney tissue was separatedand cleaned for any adherent fatty tissues after washing with fresh normal saline. Kidney extract was prepared by homogenization after taking 1g of kidney tissue dissolved in 10 volumes of chilled phosphate buffer saline solution having pH=7.4.22 The whole- kidney tissue homogenate was then centrifuged at 10,000 rpm at 4°C for 10-minutes and the supernatant was isolated and stored at −20°C in refrigerator for analysis.23
Histopathological Analysis
A kidney section was removed and placed in formalin solution (10%) for 3 days. The tissues were subjected to standard histopathological methods by applying graded ethanol, fixed with xylene and then kept in paraffin wax, stained with eosin and hematoxylin for further analysis.
Measurement of Plasma and Urinary H2S Concentration
To measure the plasma and urinary concentration through spectrophotometric method, 100µL of sample solution were fused with 50 µL of distilled water in micro centrifuge tubes. In it, 300 µL of zinc acetate (1%w/v) were added sequentially to trap the H2S. After 5 minutes, the reaction was terminated by the addition of 200 µL N,N-2 dimethyl-p-phenylenediamine sulfate (20millimolar mixed with 7.2 M HCl). Immediately after this, 200 µL of FeCl3 (30millimolar mixed with 1.2 M HCl). Then the mixture was placed in the dark for 20 minutes. After this, 150 µL of trichloroacetic acid (10% w/v) was added to precipitate the concerned substance from the sample. The mixture was then centrifuged at 10,000rpm for 10 minutes at 4°C and the resulting supernatant were separated. The sample separated was assessed in a 96-well plate reader at 670nm in duplicates. A calibration curve was drawn and the concentrations of H2S among samples were calculated from it.7
Statistical Analysis
The data obtained were expressed as mean ± SEM and statistical significance between different experimental groups were analyzed by applying one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test. Values of p<0.05 were considered as statistically significant.
Results
In vitro Assay
DPPH Free Radical Neutralizing Assay
The free radical neutralizing assay of butylated hydroxy toluene (BHT) and sodium hydrosulfide (NaHS) at multiple graded concentrations (3.125–100 µM) were determined by the DPPH assay method. At 3.125 μM BHT and NaHS showed (42.67±0.56 and 37.00±0.57), at 50 μM concentration (64.33±0.57 and 61.33±0.33) and at 100 μM concentration (ie, 80.33±0.58 and 76.00±0.57) following percentage of inhibition were observed as shown in Figure 1.
 |
Figure 1 Percentage of inhibition of (A) butylated hydroxytoluene (BHT), (B) sodium hydrosulfide (NaHS) on the DPPH free radical scavenging assay. The results are shown as mean ± SEM in triplicate. |
In vivo Assay
Induction of Diabetes
The animals were intoxicated once with STZ (60mg/kg i.p) followed by significant hyperglycemia (p<0.05), polyuria, polydipsia and decreased body weight among diabetic animals in comparison to normal control. At the 28th day, statistically non-significant difference was observed in body weight and blood glucose concentrations among treatment and normal animals. At the end of the 28th day, all treatment groups showed (p<0.05) significant reduction in blood glucose levels when compared to diabetic control as shown in Table 1. The diabetic-spironolactone group showed a lesser reduction (p>0.05) in comparison to all other treated animals. Marked reduction in body weights of animals were observed after STZ-injection as compared to normal control, but all the treatment groups significantly (p<0.05) prevent the loss of body weight at the end of the 28th day as compared to diabetic control as shown in Table 1.
 |
Table 1 Effect of Treatment on Blood Glucose and Body Weight of STZ-Induced WKY Rats |
Metabolic data including food, water intake and urinary flow rate were also observed on day 0, 14 and 28, respectively. During study, the food intake at 0th, 14th and 28th day increased significantly (p<0.05) among diabetic animals (ie, 24.67±0.33, 25.63±0.42, 25.65±0.46) as compared to normal control (ie, 18.00±1.38, 17.00±0.81, 18.54±023) g/day, respectively. But the treatment groups at 0th, 14th and 28th day showed significant results (p<0.05) by increasing the food intake. Dia+capto (ie, 20.12±0.71, 25.00±0.43, 28.12±0.51), dia+NaHS (ie, 20.12±0.21, 25.43±0.25, 27.61±0.34), dia+capto+NaHS (ie, 21.12±0.35, 25.00±0.43, 31.00±0.89), dia+SLN (ie, 21.12±0.35, 25.43±0.53, 27.43±0.71.25), dia+ met (ie, 18.12±0.35, 25.70±0.43, 30.00±0.96), dia+met+NaHS (ie, 19.12±0.35, 26.12±0.43, 31.00±.0.91), and dia+vit C (ie, 22.12±0.35, 26.76±0.43, 29.87±1.01) g/day, respectively. Similarly, the water intake increased significantly (p<0.05) among diabetic animals (ie, 54.17±0.74, 49.82±0.1.16, 49.50±8.99) as compared to normal control (ie, 41.17±0.79, 42.17±1.62, 43.17±0.60) mL/day, respectively. But the treatment groups at 0th, 14th and 28th day showed significant results (p<0.05) by increasing the water intake. Dia+capto (ie, 48.33±0.88, 57.50±0.42, 63.00±0.44), dia+NaHS (ie, 49.67±0.49, 55.83±1.13, 64.83±0.65), dia+capto+NaHS (ie, 51.67±0.88, 62.83±0.65, 69.17.00±0.40), dia+SLN (ie, 51.00±1.09, 53.43±1.53, 57.33.±1.05), dia+ met (ie, 49.50±0.76, 58.00±0.83, 63.17±0.99), dia+met+NaHS (ie, 52.50±0.35, 59.83.±0.88, 60.50±1.31), and dia+vit C (ie, 53.17±0.60, 54.33±0.88, 60.50±1.31) mL/day respectively. Similarly, the urine flow rate increased significantly (p<0.05) among diabetic animals (ie, 7.50±0.54, 8.50±0.67, 11.67±0.42) as compared to normal control (ie, 2.74±0.21, 2.79±0.21, 3.55±0.32) (μL/min/100g body weight) respectively. But the treatment groups at 0th, 14th and 28th day showed significant results (p<0.05) by increasing the urine flow rate dia+capto (ie, 9.88±0.27, 11.27±0.42, 15.83±0.30), dia+NaHS (ie, 12.11±0.49, 13.50±0.36, 15.67±0.65), dia+capto+NaHS (ie, 14.67±0.28, 16.00±0.25, 18.17.00±0.30), dia+SLN (ie, 13.00±.0.41, 14.43±0.53, 15.33.±0.44), dia+ met (ie, 10.50±0.46, 13.00±0.83, 14.17.±0.85.), dia+met+NaHS (ie, 11.50±0.35, 14.83.±0.88, 19.50±.1.31), and dia+vit C (ie, 11.17±0.60, 13.33±0.88, 16.50±0.91) (μL/min/100g body weight), respectively. A significant difference (p<0.05) was observed between plasma creatinine levels of diabetic when compared to normal control. The treatment groups indicated a marked reduction (p<0.05) in creatinine levels on the 14th and 28th day respectively as shown in Table 2. Similarly, the diabetic group exhibited increased creatinine clearance when compared to normal control as shown in Table 2. On the other hand, all the treatment groups showed a marked increase (p<0.05) in the creatinine clearance as compared to diabetic and control groups as shown in Table 2. Diabetic groups showed a marked increase in blood urea nitrogen levels as compared to control groups (p<0.05). Dia-Capto-NaHS, Dia-Met-NaHS, vitamin c and captopril groups significantly greater effects (p<0.05) as compared to the spironolactone group. Captopril, NaHS, Metformin and Vitamin C groups also showed a non-significant difference (p>0.05) from other treatment groups as shown in Table 3. Plasma uric acid levels were significantly increased (p<0.05) among diabetic animals as compared to normal control. Similarly at the 14th and 28th day, treatment groups showed a significant decrease in uric acid concentration (p<0.05) as compared to the diabetic groups. Capto-NaHS, Met-NaHS, metformin and vitamin c groups showed significant effects (p<0.05) as compared to diabetic group as shown in Table 3. Plasma albumin levels were also observed on the 0, 14th and the 28th day and diabetic groups showed a significant difference (p<0.05) among values when compared to normal control. All the treatment groups improved the albumin values (p<0.05) at the end of the 28th day when compared to the control. The urinary sodium-to-potassium ratio is significantly decreased among diabetic groups (p<0.05) when compared to the normal control. All the treated groups showed significant improvement when compared to diabetic animals (Table 4). Dia-Capto-NaHS and Dia-Met-NaHS showed marked improvement as compared to other groups.
 |
Table 2 Effect of Treatment on Plasma Creatinine and Creatinine Clearance of STZ-Induced WKY Rats |
 |
Table 3 Effect of Treatment on Blood Urea Nitrogen (BUN) and Plasma Uric Acid of STZ-Induced WKY Rats |
 |
Table 4 Effect of Treatment on Plasma Albumin and Urinary Na/K Ratio of STZ-Induced WKY Rats |
Effect on Whole Kidney Tissue
Renal glutathione was also assessed after preparing the whole kidney tissue homogenate. The diabetic animals showed a significant reduction (p<0.05) in glutathione values as compared to normal control as shown in Table 5. At the end of the 28th day, all the treatment groups showed marked improvement in renal glutathione. The groups administered with exogenous hydrogen sulfide showed massive improvements as compared to other treatment groups. Similarly, significantly higher concentrations of malondialdehyde were seen among diabetic groups (p<0.05) as compared to normal control group as shown in Table 5. All the treatment groups showed a marked reduction in malondialdehyde levels in kidney tissues. Superoxide dismutase and total antioxidant capacity T-AOC was also measured at the 0th, 14th and 28th day and diabetic groups depicted significant reduction (p<0.05) in SOD levels when compared to normal control. All the treatment groups showed a marked improvement in SOD and T-AOC levels as compared to diabetic groups. Dia-Capto-NaHS and Dia-Met-NaHS showed marked improvement (p<0.05) as compared to other groups as shown in Table 6.
 |
Table 5 Effect of Treatment on Reduced Glutathione (GSH) and Malondialdehyde (MDA) in Renal Tissues |
 |
Table 6 Effect of Treatment on Total Superoxide Dismutase (T-SOD) and Total Antioxidant Capacity (T-AOC) in Renal Tissues |
Plasma and Urinary H2S
Plasma and urinary H2S were significantly lowered among diabetic animals (p<0.05) as compared to control group on the 0th, 14th and 28th day of the study. All the exogenously administered groups significantly increased H2S (p<0.05) in plasma and urine on 14th and the 28th day of the treatment protocol when compared to the diabetic group as shown in Figure 2 and Figure 3.
Histopathological Analysis
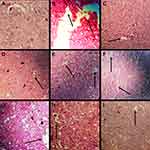
Histopathological examination of the kidney tissues obtained from normal control (A) showed the normal texture of glomerulus, no signs of glomerulosclerosis and mesangial lining expansion were seen. (B) On the other hand, sections from the diabetic group showed chronic inflammatory cells with mild infiltration, tubular atrophy, mesangial expansion and fibrosis were shown. (C-I) All the treatment groups showed protection against the renal necrosis induced by streptozotocin as shown in Figure 4.
Discussion
The main objective of the study was to explore the nephroprotective potential of exogenous H2S in the pathophysiological implications of diabetic nephropathy. Among in vitro assay, DPPH method is the most widely accepted protocol used to assess the free radical neutralizing potential of natural compounds. DPPH has a single unpaired valence electron attached to one of the nitrogen atom bridge which declared it a stable radical. The assay is based on fusion of antioxidant that decolorizes the purple color of DPPH solution to yellow at a wavelength of 517 nm.23 The decrease in absorbance of DPPH solution with graded concentration of NaHS showed their greater tendency to donate electron and neutralize the DPPH free radical. The free radical neutralizing property of NaHS is directly linked to its antioxidant potential.24 The diabetic animals showed significant hyperglycemia, polyuria, polydipsia and decreased body weight following STZ injection. STZ preferentially accumulates in pancreatic β-cells, imparts DNA alkylation, ROS production and ultimately necrosis.25,26 In recent studies, H2S levels had been directly affiliated with the diabetic kidney disease. The pathophysiological role of H2S is reported to be in insulin secretion, vascular relaxation, neurotransmission, apoptosis and cell proliferation respectively. The recent findings suggest that increasing H2S levels inside the body with the help of exogenous donors plays a vital role in major implications of diabetic nephropathy. NaHS significantly reduced blood glucose and preventedweight loss among diabetic animals which may be due to its antioxidant effect and ability to augment the H2S levels by improving insulin sensitivity inside the diabetic animals.7,14,16
Captopril also decreased blood glucose and prevented weight loss as compared to diabetic group which may be due to its ability to improve glycemic control, insulin secretion and increased pancreatic blood flow and ability to neutralize the free radical species.27 All other treatment groups metformin, spironolactone and vitamin C also showed significant reduction which may be due to their tendency to neutralize the free radicals and restoration of pancreatic β-cells as evidenced from histopathological analysis.28 During treatment, food intake was significantly increased at the 14th and 28th day which may be due to hypoglycemia produced as a result of improved insulin sensitivity and secretion promoting decrease in blood glucose. Similarly, water intake and urine flow rate were also increased during the treatment at 14th and 28th day. The increased urine flow rate may be due to increased urine volume in dose dependant manner with the increased H2S levels in renal artery.7 Among renal parameters, creatinine, blood urea nitrogen, and uric acid were significantly increased after STZ administration which can damage the nephron and are considered as potential biomarkers of renal dysfunction.29 Similarly, creatinine clearance, albumin and sod-potassium-ratio were significantly decreased as a result of STZ-induced renal necrosis. At the end of the 14th and 28th day, all the treatment groups significantly reversed the above mentioned parameters which may be due to their anti-oxidant potential to ameliorate the oxidative stress and hence tubular necrosis.30 It has also been suggested that decreased plasma creatinine and increased creatinine clearance among treatment groups may be due to the vasodilating effect provided by exogenous H2S in pre-glomerular arterioles.31 The abnormalities in re-absorption of sodium and excretion have been previously linked to development of diabetic nephropathy. Hence, sodium excretion was increased by exogenous H2S due to inhibition of Na+/K+-2Cl transport mechanism and ultimately Na+/K+-ATPase activity. The increased H2S concentration may lead to increase in urine flow rate which can be associated with the phenomena of natriuresis which showed water passively followed the sodium which may result in increased urine output.7 The renin angiotensin aldosterone system had a wide implication in the pathophysiology of diabetic nephropathy. As glycemic level increases, the production of reactive oxygen species up-regulates the RAAS system. Diabetic nephropathy is independently linked to aldosterone activity which is inversely proportional to sodium to potassium ratio. Among diabetic animals, the decreased urinary sodium to potassium ratio is a marked indicator of increased aldosterone activity due to the up-regulation of RAAS as a result of hyperglycemia. The increased urinary sodium to potassium ratio showed by all the treatment groups, especially spironolactone, may be linked to their ability to block the aldosterone activity.7 Among renal tissues, STZ-administration decreased the concentration of nephroprotective enzymes such as glutathione and total superoxide dismutase, total antioxidant capacity followed by increased malondialdehyde levels produced as a result of lipid peroxidation.32 All the treatment groups reversed the protective enzymes in renal tissues which may be due to their ability to ameliorate the oxidative stress by neutralizing the reactive oxygen species. NaHS functions as a donor of exogenous H2S and potent antioxidant during the in vivo assay. NaHS modulates H2S levels inside the body which implicated various functions like neurotransmission, vascular relaxation and improved insulin sensitivity. Captopril exerts its antioxidant and anti-inflammatory effect via inhibition of free radical species and angiotensin-II which imparts oxidative stress in pancreatic beta cells. Similarly, metformin and vitamin C were considered to be powerful antioxidants reported to have protective role in lipid peroxidation, cardiac fibrosis and multiple sclerosis.7,12,27,29 Spironolactone (direct aldosterone antagonist) also ameliorates renal fibrosis by inhibition of TGF- β1, PAI-1 and macrophages infiltration and inhibits aldosterone. Direct antagonism of aldosterone provides a potential therapeutic target in the prevention of diabetic nephropathy.33 Hence, Capto-NaHS and Met-NaHS when administered in combination produced augmented effects as compared to all other treatment groups. All the treatment groups ultimately increase plasma and urinary H2S levels which acts as an antioxidant to prevent the renal damage. In the histopathological study, diabetic animals showed chronic inflammatory cells with mild infiltration, mesangial lining expansion with differentiated glomerulosclerosis was observed. But all the treatment groups protected against the renal tissue damage by neutralizing the free radical at the 14th and 28th day.
Conclusion
The present study showed that increased H2S concentration attenuated the STZ-induced renal dysfunction and morphologic pathology in diabetic rats. The protective effects proposed to be associated with the suppression of oxidative stress. A combination therapy with NaHS and captopril has additive effect in improving the outcome of diabetic renal insufficiency as compared to either alone. However, further studies are required which will surely help to develop H2S-mediated novel therapies in combination with antihypertensive and renoprotective drugs which may reverse the progression of diabetic nephropathy.
Limitations of the Study
Insulin, HbA1c and urine creatinine-to-albumin ratio had been enlisted into the limitations of the study.
Provision of Data
All the data relevant to the study is provided within the manuscript.
Author’s Consent
All authors consented to publish this study in a reputable journal.
Acknowledgments
Authors would like to thank the Department of Pharmacology, the Islamia University of Bahawalpur for laboratory access and uninterrupted research facilities.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
References
1. Xu S, He L, Ding K, et al. Tanshinone IIA ameliorates streptozotocin-induced diabetic nephropathy, partly by attenuating PERK pathway-induced fibrosis. Drug Design Dev Therapy. 2020;14:5773–5782. doi:10.2147/DDDT.S257734
2. Kashihara N, Haruna Y, Kondeti V, Kanwar Y. Oxidative stress in diabetic nephropathy. Curr Med Chem. 2010;17(34):4256–4269. doi:10.2174/092986710793348581
3. Safar MM, Abdelsalam RM. H2S donors attenuate diabetic nephropathy in rats: modulation of oxidant status and polyol pathway. Pharmacol Rep. 2015;67(1):17–23. doi:10.1016/j.pharep.2014.08.001
4. Ayodele OE, Alebiosu CO, Salako BL. Diabetic nephropathy--a review of the natural history, burden, risk factors and treatment.. J Natl Med Assoc. 2004;96(11):1445–1454.
5. Wang GG, Lu XH, Li W, Zhao X, Zhang C. Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Alternat Med. 2011;46(2):15–23.
6. Li Z, Organ CL, Kang J, et al. Hydrogen sulfide attenuates renin angiotensin and aldosterone pathological signaling to preserve kidney function and improve exercise tolerance in heart failure. JACC. 2018;3(6):796–809.
7. Ahmad FUD, Sattar MA, Rathore HA, et al. Exogenous Hydrogen Sulfide (H 2 S) Reduces Blood Pressure and Prevents the Progression of Diabetic Nephropathy in Spontaneously Hypertensive Rats. Ren Fail. 2012;34(2):203–210. doi:10.3109/0886022X.2011.643365
8. Yamamoto J, Sato W, Kosugi T, et al. Distribution of hydrogen sulfide (H2S)-producing enzymes and the roles of the H2S donor sodium hydrosulfide in diabetic nephropathy. Clin Exp Nephrol. 2013;17(1):32–40. doi:10.1007/s10157-012-0670-y
9. Abo-Salem OM, El-Edel RH, Harisa G, El-Halawany N, Ghonaim MM. Experimental diabetic nephropathy can be prevented by propolis: effect on metabolic disturbances and renal oxidative parameters. Pak J Pharma Sci. 2009;22(2):205–210.
10. Cam M, Yavuz Ö, Guven A, Ercan F, Bukan N, Üstündag N. Protective effects of chronic melatonin treatment against renal injury in streptozotocin-induced diabetic rats. J Pineal Res. 2003;35(3):212–220. doi:10.1034/j.1600-079X.2003.00082.x
11. Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi:10.1007/s00125-007-0886-7
12. Fouad AA, Al-Mulhim AS, Jresat I, Morsy MA. Protective effects of captopril in diabetic rats exposed to ischemia/reperfusion renal injury. J Pharm Pharmacol. 2013;65(2):243–252. doi:10.1111/j.2042-7158.2012.01585.x
13. Alhaider AA, Korashy HM, Sayed-Ahmed MM, Mobark M, Kfoury H, Mansour MA. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact. 2011;192(3):233–242. doi:10.1016/j.cbi.2011.03.014
14. Sun S, Wu WU, Cao C, et al. Hydrogen sulfide: recent progression and perspectives for the treatment of diabetic nephropathy. Molecules. 2019;24(15):2857–2869. doi:10.3390/molecules24152857
15. Ahmad A, Sattar M, Rathore HA, et al. Antioxidant Activity and Free Radical Scavenging Capacity of L-Arginine and NaHS: A Comparative In Vitro Study Acta Pol Pharm. 2015;72(2):245–252.
16. Zou Y, Lu Y, Wei D. Antioxidant Activity of a Flavonoid-Rich Extract of Hypericum perforatum L. in Vitro. J Agric Food Chem. 2004;52(16):5032–5039. doi:10.1021/jf049571r
17. Hubrecht RC, Kirkwood J. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. John Wiley & Sons; 2010.
18. Sadek KM, Shaheen H. Biochemical efficacy of vitamin D in ameliorating endocrine and metabolic disorders in diabetic rats. Pharma Biol. 2014;52(5):591–596. doi:10.3109/13880209.2013.854812
19. Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy (Methods in Renal Research). Nephrology. 2007;12(3):261–266. doi:10.1111/j.1440-1797.2007.00796.x
20. Lindstrom NM, Moore DM, Zimmerman K, Smith SA. Hematologic assessment in pet rats, mice, hamsters, and gerbils: blood sample collection and blood cell identification. Clin Lab Med. 2015;35(3):629–640. doi:10.1016/j.cll.2015.05.011
21. Elberry AA, Abdel-Naim AB, Abdel-Sattar EA, et al. Cranberry (Vaccinium macrocarpon) protects against doxorubicin-induced cardiotoxicity in rats. Food Chem Toxicol. 2010;48(5):1178–1184. doi:10.1016/j.fct.2010.02.008
22. Hillegass L, Griswold D, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24(4):285–295. doi:10.1016/0160-5402(90)90013-B
23. Li H-B, Wong -C-C, Cheng K-W, Chen F. Antioxidant properties in-vitro and total phenolic contents in methanol extracts from medicinal plants. Food Sci Tech. 2008;41(3):385–390.
24. Magalhaes LM, Segundo MA, Reis S, Lima JLFC. Methodological aspects about in vitro evaluation of antioxidant properties. Anal Chim Acta. 2008;613(1):1–19. doi:10.1016/j.aca.2008.02.047
25. Haidara MA, Mikhailidis DP, Rateb MA, et al. Evaluation of the effect of oxidative stress and vitamin E supplementation on renal function in rats with streptozotocin-induced type 1 diabetes. J Diabetes Complications. 2009;23(2):130–136. doi:10.1016/j.jdiacomp.2008.02.011
26. Taniguchi S, Kang L, Kimura T, Niki I. Hydrogen sulphide protects mouse pancreatic β-cells from cell death induced by oxidative stress, but not by endoplasmic reticulum stress. Br J Pharmacol. 2011;162(5):1171–1178. doi:10.1111/j.1476-5381.2010.01119.x
27. Sadek KM, Lebda MA, Nasr SM, Shoukry M. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed Pharmacother. 2017;92(4):1085–1094. doi:10.1016/j.biopha.2017.06.023
28. Lin S, Juriasingani S, Sener A. Is hydrogen sulfide a potential novel therapy to prevent renal damage during ureteral obstruction? Nitric Oxide. 2018;73:15–21. doi:10.1016/j.niox.2017.12.004
29. Mapanga RF, Tufts M, Shode F, Musabayane C. Renal effects of plant-derived oleanolic acid in streptozotocin-induced diabetic rats. Ren Fail. 2009;31(6):481–491. doi:10.1080/08860220902963558
30. Abouzed TK, Del Mar Contreras M, Sadek KM, et al. Red onion scales ameliorated streptozotocin-induced diabetes and diabetic nephropathy in Wistar rats in relation to their metabolite fingerprint. Diabetes Res Clin Pract. 2018;140(4):253–264. doi:10.1016/j.diabres.2018.03.042
31. Edgley AJ, Tare M, Evans RG, Skordilis C, Parkington HC. In vivo regulation of endothelium-dependent vasodilation in the rat renal circulation and the effect of streptozotocin-induced diabetes. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R829–R839. doi:10.1152/ajpregu.00861.2007
32. Srinivasan S, Pari L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chemico Biol Interactions. 2012;195(1):43–51. doi:10.1016/j.cbi.2011.10.003
33. Schrier RW, Masoumi A, Elhassan E. Aldosterone: role in edematous disorders, hypertension, chronic renal failure, and metabolic syndrome. Clin J Am Soc Nephrol. 2010;5(6):1132–1140. doi:10.2215/CJN.01410210
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.