Back to Journals » OncoTargets and Therapy » Volume 12
Role of Olaparib as Maintenance Treatment for Ovarian Cancer: The Evidence to Date
Authors Montemorano L, Lightfoot MDS , Bixel K
Received 10 September 2019
Accepted for publication 2 December 2019
Published 27 December 2019 Volume 2019:12 Pages 11497—11506
DOI https://doi.org/10.2147/OTT.S195552
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Leo Jen-Liang Su
Lauren Montemorano,1 Michelle DS Lightfoot,2 Kristin Bixel2
1The Ohio State University Wexner Medical Center, Department of Obstetrics and Gynecology, Columbus, OH 43210, USA; 2The Ohio State University Wexner Medical Center, James Cancer Hospital, Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Columbus, OH 43210, USA
Correspondence: Kristin Bixel
The Ohio State University Wexner Medical Center, James Cancer Hospital, Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Columbus, OH 43210, USA
Email [email protected]
Abstract: PARP inhibitors have shown significant promise in the treatment of ovarian cancer. Olaparib is a PARP inhibitor that has been approved for maintenance for BRCA-mutated ovarian cancer in the recurrent and front-line setting as well as for treatment of BRCA-mutated ovarian cancer in patients who have received multiple prior lines of chemotherapy. In this review, we focus on the use of olaparib in the maintenance setting including the evidence to date, ongoing research, and future directions.
Keywords: olaparib, PARP inhibitor, ovarian cancer, maintenance
Introduction
Ovarian cancer is the seventh most common cancer in women worldwide and is the leading cause of death from gynecologic cancers in high-income countries.1,2 The five year survival rate in the United States is 48% and the proportion of women dying from their disease has not improved substantially over time as compared to other prevalent cancers.3 Standard treatment for newly diagnosed advanced ovarian cancer consists of cytoreductive surgery and platinum-based chemotherapy with or without concurrent and maintenance bevacizumab, a vascular endothelial growth factor (VEGF) A inhibitor.4 The majority of women with epithelial ovarian cancer respond well to first-line platinum-based chemotherapy however there is a high rate of recurrence with a chemotherapy-free interval before disease progression ranging from 10 to 26 months.5–9 Response to subsequent therapies is variable and often short-lived, underscoring the need for novel effective treatment options to improve long-term disease control for women with ovarian cancer.10,11
Homologous recombination (HR) is a DNA repair process crucial for the accurate repair of DNA damage. BRCA1/2 mutations are known to lead to defective HR and ultimately results in risk for malignant transformation of cells.12 BRCA mutations, both germline and somatic, are thought to occur in up to 25% of patients with newly diagnosed serous ovarian cancer.13 While BRCA1/2 mutations were initially thought to be responsible for the majority of hereditary epithelial ovarian cancers, further investigation has shown that compromise of the HR pathway can occur by several other potential mechanisms.14,15 Thus, it is thought that approximately 50% of high-grade serous ovarian cancers have a deficiency in HR.16
There have been several studies investigating the role of maintenance therapy in ovarian cancer which until recently have not been found to significantly prolong survival.6,17 However, poly (ADP-ribose) polymerase (PARP) inhibitors have shown significant promise with several clinical trials demonstrating a survival improvement in women with newly diagnosed and recurrent ovarian cancer without a substantial increase in adverse effects.18–25 The antitumor effects of PARP inhibitors rely on an exploitation of the defective DNA damage repair in cancer cells with dysfunctional HR. Olaparib is a PARP inhibitor that has several approved indications for use in ovarian cancer and has demonstrated a progression-free survival (PFS) advantage in several trials.19–22
Here, we review the use of olaparib as maintenance treatment for ovarian cancer. We will summarize the evolution of its use, current approved indications, and evidence with respect to its clinical safety and efficacy. Finally, we will provide guidance on treatment decisions with olaparib for patients with ovarian cancer as well as commentary regarding ongoing research and future directions.
Background: Homologous Recombination and PARP Inhibitors
HR is a high-fidelity DNA repair process for double-strand DNA breaks and BRCA1 and BRCA2 are key proteins required for the formation of the repair complex at the site of DNA damage. Germline or somatic mutations in the BRCA1 and BRCA2 genes results in dysfunction of their protein product, which creates genetic instability and thus a predilection of affected cells for malignant transformation. Other genetic aberrations can occur in the HR pathway including mutations in other homologous recombination genes and epigenetic changes such as inactivation of BRCA1/2 or methylation of promoters.14,15
PARP enzymes are involved in detecting single-strand DNA breaks and act as signal transducers via catalytic activity to recruit DNA repair proteins. Ultimately, PARP enzymes are released from the site of single-stranded breaks and repair ensues.26 PARP inhibitors are theorized to work by two potential mechanisms: 1) allowing the persistence of spontaneously occurring single-strand breaks due to a loss of enzymatic function, and 2) preventing the release of PARP from DNA (termed PARP trapping). Both mechanisms lead to persistent single-strand breaks, collapsed replication forks, and resultant double-strand breaks. Repair of double-strand breaks can occur by either homologous recombination or non-homologous end-joining (NHEJ). Homologous recombination repairs DNA with high-fidelity while NHEJ is an error-prone repair process that causes genetic instability.26 In normal cells with intact HR pathways, PARP inhibition is inconsequential given the accurate repair of double-stranded breaks with homologous recombination.
In cells with BRCA1/2 mutations or other abnormalities in HR, PARP inhibition results in a process termed “synthetic lethality” whereby two mechanisms of DNA repair are functionally terminated leading to a reliance on NHEJ and subsequently, cell death.27,28 In this way, PARP inhibitors are unique in that they exploit an underlying defective process in cancer cells. PARP inhibitors are the first Food and Drug Administration (FDA)-approved therapy for ovarian cancer based on the underlying mechanism of malignancy.29 There are currently three PARP inhibitors FDA-approved for use in women with ovarian cancer: olaparib, rucaparib, and niraparib. Their FDA-approved indications are listed in Table 1.30–32
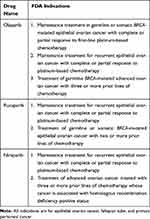 |
Table 1 PARP Inhibitor FDA Indications for Ovarian Cancer |
Background: Olaparib
Olaparib (Lynparza®) is an oral PARP inhibitor developed by AstraZeneca Pharmaceuticals LP. Based on available data, the standard dosing of olaparib is 300 mg tablet twice daily or 400 mg twice daily in capsule form.19–22 The primary adverse events noted in these trials include nausea, fatigue, vomiting, and anemia. A summary of grade 3–4 adverse events is provided in Table 2.19–22 Rare but serious adverse events associated with olaparib use include a risk of developing a secondary malignancy such as myelodysplastic syndrome, acute myeloid leukemia (AML), or chronic myelomonocytic leukemia (CML). Trials have shown that <1.5% of patients using olaparib develop these conditions, with the majority of events having a fatal outcome.22,30 This is roughly equivalent to the rates of AML or CML seen with the use of other PARP inhibitors.23,33 Risk of bone marrow neoplasia has been found to be lower in other oncologic populations after chemotherapy, with one study demonstrating a 10-year cumulative risk of approximately 0.5% among breast cancer patients who received chemotherapy which was significantly different compared to patients who did not receive adjuvant chemotherapy.34
 |
Table 2 Percentage of Patients Experiencing G3-4 Toxicities in Phase III Studies of Olaparib |
Olaparib was initially FDA-approved in the United States (US) in 2014 for women with recurrent ovarian cancer who harbored a germline BRCA mutation (gBRCAm) and had received three or more prior lines of chemotherapy (see Figure 1 Timeline of Approval). This approval was based on Study 42 which demonstrated an objective response rate of >30% with olaparib monotherapy in a heavily pretreated patient population.35 Around the same time as the initial FDA-approval for olaparib in 2014, the European Medicines Agency (EMA) approved olaparib as maintenance monotherapy for patients with platinum-sensitive relapsed (PSR) BRCA1/2-mutated high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer, based on Study 19 which will be discussed in detail below.19,20,36 In 2017, the FDA broadened its approval of olaparib to include maintenance monotherapy for patients with platinum-sensitive recurrent ovarian cancer regardless of BRCA mutation status.30 The EMA followed suit shortly thereafter with an approval that matched these indications in 2018.37 And most recently, olaparib was approved for front-line maintenance therapy after a phase III trial (SOLO1) showed significant improvement in PFS among women with germline or somatic BRCA mutations who received olaparib maintenance therapy following platinum-based chemotherapy when compared to placebo (HR 0.30, 95% CI 0.23–0.41).21
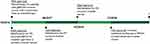 |
Figure 1 Olaparib timeline of approval. |
In the remainder of this article, we will review evidence for current approved indications for olaparib as maintenance treatment for ovarian cancer and comment on critical ongoing trials that have the potential to expand its use in this arena.
Olaparib Maintenance Monotherapy for Platinum-Sensitive Recurrent Ovarian Cancer
Following the initial FDA-approval in 2014 for olaparib as monotherapy for recurrent gBRCAm ovarian cancer, several studies sought to demonstrate benefit in the maintenance setting. Study 19 and its subsequent analyses found that olaparib maintenance monotherapy significantly improved PFS in women with platinum-sensitive recurrent ovarian cancer who had received two or more platinum-based regimens and had a complete or partial response demonstrated to the most recent platinum-based chemotherapy, particularly in patients with germline and somatic BRCA mutations.19,20 In this randomized, double-blind, placebo-controlled phase II study, 256 women were enrolled including 129 in the placebo group and 136 in the olaparib group. Patients randomly assigned to the olaparib group received 400 mg twice daily (capsule formulation). Results showed a median PFS advantage of 8.4 months with olaparib versus 4.8 months with placebo (p<0.001). Olaparib was generally well-tolerated, with the most common adverse events reported compared to placebo including nausea (68% versus 35%), fatigue (49% versus 38%), and vomiting (32% versus 14%). Grade 3 and 4 toxicities occurred in 35% of patients who received olaparib versus 20% of patients who received placebo.19
A planned subsequent retrospective analysis of Study 19 was completed in 2014 and sought to explore the hypothesis that women with BRCA mutations would have the greatest benefit with olaparib maintenance treatment. BRCA status was known for approximately 95% of the patients enrolled. Seventy-four patients (56%) in the olaparib group had a known or suspected deleterious or somatic BRCA mutation versus 62 (50%) in the placebo group. For patients with a gBRCAm, PFS was significantly longer for women receiving olaparib compared to placebo (11.2 months vs 4.3 months, p<0.0001). Additionally, there was a statistically significant two month survival advantage in this group (7.4 months versus 5.5 months, p=0.0075).20
SOLO2 was a phase III trial that substantiated the findings of Study 19 and also validated the new tablet formulation of olaparib (versus the capsule formulation used in Study 19). This was a double-blind, randomized, placebo-controlled trial that evaluated olaparib maintenance in platinum-sensitive relapsed ovarian cancer patients with a BRCA1/2 mutation who had received at least two prior lines of chemotherapy. Patients were randomly assigned 2:1 to olaparib 300 mg twice daily or placebo, and randomization was stratified by response to previous platinum chemotherapy (complete versus partial) and length of platinum-free interval (>6–12 months versus >12 months). There were 196 patients randomly assigned to receive olaparib and 99 to receive placebo. The median PFS was significantly longer for women treated with olaparib compared to placebo (19.1 months versus 5.5 months, p<0.0001). Secondary endpoints including time to first subsequent therapy and median time to second progression were significantly improved in the olaparib group when compared to placebo. Additionally, quality of life measures showed no appreciable difference for patients receiving olaparib compared with those receiving placebo. The most common adverse event in the olaparib group was anemia. The rate of serious adverse events was 18% in patients receiving olaparib versus 8% in patients in the placebo group. The PFS benefit seen in SOLO2 substantially exceeded that seen in Study 19 and provided additional data confirming a manageable safety profile of olaparib.22
Olaparib maintenance monotherapy has also been studied after using it in combination with chemotherapy irrespective of BRCA1/2 status. In a phase II trial by Oza et al, women with platinum-sensitive recurrent high-grade serous ovarian cancer who had received up to three previous courses of platinum-based chemotherapy were randomized to receive olaparib in combination with chemotherapy followed by olaparib maintenance monotherapy versus chemotherapy alone. Patients in the combination group (n=81) received paclitaxel (175 mg/m2 on day 1) and carboplatin (AUC 4 mg/mL per min on day 1) plus olaparib (200 mg twice daily on days 1–10 of each 21-day cycle), followed by olaparib monotherapy (400 mg twice daily). Patients in the chemotherapy only group (n=75) received paclitaxel (175 mg/m2 on day 1) and carboplatin (AUC 6 mg/mL per min on day 1) and then no maintenance treatment. The combination chemotherapy and maintenance group had a significantly improved PFS compared to the chemotherapy only group, 12.2 months versus 9.6 months (p=0.0012). However, it is important to note that patients in the combination chemotherapy group had more frequent adverse events during treatment.38 Thus, it is not clear based on these results whether there is any benefit to adding olaparib to cytotoxic chemotherapy prior to olaparib maintenance therapy. Additional investigation would be warranted before this strategy could be recommended as standard of care.
To summarize, these studies demonstrated the efficacy and safety of olaparib as maintenance monotherapy for platinum-sensitive recurrent ovarian cancer irrespective of BRCA mutation status but with a more substantial benefit in patients with BRCA-mutated ovarian cancer (see Table 3 for a summary). Study 19 and SOLO2 were the basis for the 2017 FDA approval for olaparib for platinum-sensitive relapsed ovarian cancer regardless of BRCA mutation status. Given the positive results of these studies and the progression free survival advantage olaparib conferred, new studies sought to evaluate the efficacy of olaparib as maintenance therapy in other settings such as women with newly diagnosed advanced ovarian cancer.
 |
Table 3 Published Trials Evaluating Olaparib Maintenance Therapy |
Olaparib as First-Line Maintenance Therapy
As olaparib maintenance therapy was found to benefit women in the setting of platinum-sensitive relapsed ovarian cancer, use in the frontline setting was investigated. SOLO1 was a phase III randomized, placebo-controlled, double-blind study that sought to evaluate the efficacy of olaparib as maintenance monotherapy in patients with high-grade serous or endometrioid ovarian cancer, primary peritoneal cancer, or fallopian-tube cancer and a BRCA1/2 mutation (germline or somatic) who had a complete or partial response to platinum-based chemotherapy. Patients were assigned in a 2:1 ratio to receive olaparib tablets 300 mg twice daily or placebo.21
This study demonstrated a substantial PFS benefit with the use of olaparib maintenance therapy. The risk of disease progression or death was 70% lower with olaparib than with placebo after a median follow-up of 41 months (hazard ratio for disease progression or death, 0.28; 95% CI, 0.20 to 0.39; p<0.001). While the median PFS was not yet met for the olaparib group, a sensitivity analysis of investigator-assessed PFS was performed to assess for attrition bias and showed that the median PFS was approximately 36 months longer in the olaparib group compared to the placebo group. Moreover, the median PFS was 13.8 months in the placebo group, which is consistent with other studies of women with BRCA1/2 mutations with newly diagnosed advanced ovarian cancer who received only carboplatin and paclitaxel, thus indicating that the magnitude of PFS benefit is not exaggerated by the poor performance of the placebo group.21 Interim analysis also demonstrated favorable findings for other secondary end points. The median time to first subsequent therapy or death was 51.8 months in the olaparib group and 15.1 months in the placebo group. The estimate of the rate of freedom from the use of second subsequent therapy and from death at three years was 74% in the olaparib group and 56% in the placebo group (hazard ratio for the use of a second subsequent therapy or death, 0.45; 95% CI, 0.32 to 0.63). Measures of health-related quality of life were similar among the olaparib and placebo group. The most common adverse events that occurred during the trial intervention or up to 30 days after discontinuation included nausea, fatigue, vomiting, and anemia. Anemia was the most common serious adverse event, occurring in 7% of patients in the olaparib group compared to no patients in the placebo group.
SOLO1 has provided evidence that PFS advantage can be achieved after frontline therapy particularly in women with BRCA1/2 mutated ovarian cancer. Future research will focus on confirming this benefit and demonstrating efficacy among other populations.
PAOLA-1 (NCT02477644/ENGOT-ov25) is the second phase III trial evaluating the efficacy of olaparib as front-line maintenance therapy and also provides insight regarding concomitant use of olaparib with bevacizumab. Participants received first-line platinum chemotherapy plus bevacizumab and were randomized to maintenance placebo or olaparib plus maintenance bevacizumab regardless of BRCA status. Preliminary results demonstrated a median PFS of 22.1 in the olaparib and bevacizumab group versus 16.6 months in the placebo and bevacizumab group (p<0.0001).39 Of note, sub-analyses showed that the PFS benefit was only demonstrated in those with BRCA mutations or homologous recombination deficiency. Unfortunately no trial arm evaluated olaparib maintenance therapy without bevacizumab, therefore the additional benefit of adding bevacizumab remains unclear.
Ongoing Research and Future Directions
Here we have provided the evidence to date supporting the use of olaparib as first-line maintenance treatment for women with BRCAm ovarian cancer as well as maintenance therapy following treatment for platinum-sensitive recurrent disease. In addition to olaparib, rucaparib and niraparib have FDA and EMA indications for use for maintenance treatment for ovarian cancer.31,32 Studies involving other PARP inhibitors including veliparib and talazoparib have shown promising clinical results and may lead to approvals in the near future (NCT01472783, NCT02470585, NCT01540565, NCT01286987).
The role of olaparib in ovarian cancer continues to expand and there are many questions left to be answered about how to optimize its use. Ongoing studies are evaluating the role of olaparib as maintenance therapy in patients without germline or somatic BRCA mutations, in patients previously treated with a PARP inhibitor, in combination with other targeted therapies, and in the setting of PARP resistance.
BRCA mutations result in homologous recombination deficiency (HRD) and confer sensitivity to PARP inhibition. While only about a quarter of patients with ovarian cancer have germline or somatic BRCA mutations, studies have demonstrated that approximately half have homologous recombination deficient tumors.13–16 This suggests that the population that may derive benefit from olaparib could extend beyond those with BRCA mutations. Data from Study 19 indicate there is likely a benefit, albeit less than for BRCA-mutated patients. This concept is also supported by data from the NOVA trial demonstrating a 9 month improvement in PFS with the use of niraparib as maintenance therapy after treatment for platinum-sensitive recurrent ovarian cancer in non-gBRCA patients with HRD.33 Phase III studies on olaparib maintenance monotherapy in non-BRCAm patients are ongoing (OPINION/NCT03402841).
The advancement of olaparib into front-line maintenance also raises questions regarding the role of subsequent PARP treatment, or the role of PARP after PARP. While trials are ongoing to assess the efficacy of a PARP after prior PARP therapy (OReO, NCT03106987), small retrospective studies have shown that some patients may experience a partial response or stable disease from repeat PARP.40
There is also great interest in the potential benefits of olaparib in combination with other targeted therapies in an effort to overcome PARP resistance and exploit opportunities for additive efficacy. Tumors with BRCA mutations or homologous recombination deficiencies exhibit significantly higher mutational and neoantigen loads and higher PD-L1 expression than BRCA1/2 wild-type or homologous recombination repair intact tumors.41 As such several trials are investigating the role of checkpoint inhibitors in combination with PARP inhibitors. DUO-O (NCT03737643) is an actively-recruiting phase III trial evaluating durvalumab (an anti-PD-L1 antibody) in combination with chemotherapy and bevacizumab followed by maintenance durvalumab, bevacizumab and olaparib. While not a study of olaparib maintenance therapy, MEDIOLA is a phase I/II trial investigating durvalumab in combination with olaparib in a platinum-sensitive BRCAm population (NCT02734004). Emerging clinical data will help establish the efficacy of combination therapy with olaparib and immune check point inhibitors in women with and without BRCA or homologous recombination deficiencies.
The combination of olaparib and anti-angiogenesis therapy is also being explored. It has been theorized that hypoxia leads to downregulation of homologous recombination repair genes.42 As previously discussed, results from PAOLA-1 demonstrated a PFS benefit in women who received olaparib and bevacizumab for frontline maintenance as compared to those who received placebo and bevacizumab. Given that no arm evaluated olaparib maintenance therapy without bevacizumab, its contribution to the PFS benefit is unclear. ICON9 (NCT03278717) is actively recruiting and aims to compare olaparib maintenance treatment with and without cediranib in platinum-sensitive relapsed ovarian cancer. Table 4 lists the active phase III trials utilizing olaparib maintenance therapy.
 |
Table 4 Active Phase III Clinical Trials Utilizing Olaparib as Maintenance Therapy |
While no phase III trials are directly evaluating the role of PARP inhibitors in platinum-resistant disease, early studies show there may still be a role for PARP treatment in this population. A dose-escalation phase 1b study of alpelisib (a PI3K inhibitor) and olaparib demonstrated that among 28 women with epithelial ovarian cancer, 82% of whom had platinum-resistant disease, 36% had a partial response (median 5.5 months) and 50% had stable disease.43 It should be noted that this was not the primary endpoint of the study. However, these results indicate that the applications of PARP inhibitors, especially in combination with other targeted therapies, may play an important role in an even broader cohort of patients with ovarian cancer. Ongoing Phase II studies including ROLANDO and BAROCCO (NCT03161132, NCT03314740) are investigating the role of combination therapies in platinum-resistant ovarian cancer with olaparib and pegylated liposomal doxorubicin and olaparib, paclitaxel, and cediranib, respecitvely. Future studies could focus on maintenance treatment in this group.
Increased utilization of PARP inhibitors portends a need to better understand PARP inhibitor resistance. The most widely accepted mechanism of PARP inhibitor resistance is the restoration of BRCA function or HR activity via secondary mutations.44 Therefore many strategies for overcoming or preventing PARP inhibitor resistance focus on therapies that downregulate BRCA function or increase the degree of HR deficiencyIt is likely that studies on the horizon will continue to evaluate targeted and combination therapies that increase tumor sensitivity to PARP inhibition. Additionally, efforts to understand characteristics and mechanisms involved in patients with durable responses to olaparib are also underway and will likely provide valuable information (OLALA/NCT02489058).
Conclusion
The advent of PARP inhibitors is an unprecedented advancement in the treatment of women with ovarian cancer. Current FDA approved indications for olaparib use include maintenance for BRCA-mutated ovarian cancer in both the recurrent and front-line setting, as well as for treatment of gBRCAm ovarian cancer in patients who have received multiple prior lines of chemotherapy. With the publication of the results from SOLO1 and SOLO2, the role of olaparib maintenance therapy for women with gBRCAm has been solidified. Importantly, olaparib is the only PARP inhibitor FDA approved for front-line maintenance therapy in BRCA-mutated patients. Ongoing studies will further delineate the role of olaparib in ovarian cancer and likely expand indications for use.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. doi:10.1016/j.bpobgyn.2016.08.006
2. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.v61:2
3. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2016. Bethesda, MD: National Cancer Institute. Available from: https://seer.cancer.gov/csr/1975_2016/.
4. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, ovarian cancer version 1; 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian_blocks.pdf.
5. Walker JL, Brady MF, Wenzel L, et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2019;37(16):1380–1390. doi:10.1200/JCO.18.01568
6. Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Eng J Med. 2011;365(26):2473–2483. doi:10.1056/NEJMoa1104390
7. Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi:10.1056/NEJMoa052985
8. Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257. doi:10.1016/S0140-6736(14)62223-6
9. Vergote I, Coens C, Nankivell M, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018;19(12):1680–1687. doi:10.1016/S1470-2045(18)30566-7
10. Fung-Kee-Fung M, Oliver T, Elit L, et al. Optimal chemotherapy treatment for women with recurrent ovarian cancer. Curr Oncol. 2007;14(5):195–208. doi:10.3747/co.2007.148
11. Pfisterer J, Ledermann JA. Management of platinum-sensitive recurrent ovarian cancer. Semin Oncol. 2006;33(2 Suppl 6):S12–6. doi:10.1053/j.seminoncol.2006.03.012
12. Gadducci A, Guarneri V, Peccatori FA, et al. Current strategies for the targeted treatment of high-grade serous epithelial ovarian cancer and relevance of BRCA mutational status. J Ovarian Res. 2019;12(1):9. doi:10.1186/s13048-019-0484-6
13. Zhang S, Royer R, Li S, et al. Frequencies of BRCA1 and BRCA2 mutations among 1342 unselected patients with invasive ovarian cancer. Gynecol Oncol. 2011;121(2):353–357. doi:10.1016/j.ygyno.2011.01.020
14. Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer. 2016;60:49–58. doi:10.1016/j.ejca.2016.03.005
15. Kondrashova O, Topp M, Nesic K, et al. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun. 2018;9(1):3970. doi:10.1038/s41467-018-05564-z
16. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi:10.1038/nature10166
17. Copeland LJ, Brady MF, Burger RA, et al. A phase III trial of maintenance therapy in women with advanced ovarian/fallopian tube/peritoneal cancer after a complete clinical response to first-line therapy: an NRG oncology study. Gynecol Oncol. 2017;145:219. doi:10.1016/j.ygyno.2017.03.504
18. Suh DH, Kim M, Lee KH, et al. Major clinical research advances in gynecologic cancers in 2017. J Gynecol Oncol. 2018;29(2):e31. doi:10.3802/jgo.2018.29.e31
19. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–1392. doi:10.1056/NEJMoa1105535
20. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–861. doi:10.1016/S1470-2045(14)70228-1
21. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505. doi:10.1056/NEJMoa1810858
22. Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. doi:10.1016/S1470-2045(17)30469-2
23. Coleman RL, Oza AM, Lorussa D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949–1961. doi:10.1016/S0140-6736(17)32440-6
24. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164. doi:10.1056/NEJMoa1611310
25. Mirza M, Pignata S, Ledermann JA. Latest clinical evidence and further development of PARP inhibitors in ovarian cancer. Ann Oncol. 2018;29(6):1366–1376. doi:10.1093/annonc/mdy174
26. Lord CK, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481(7381):287–294. doi:10.1038/nature10760
27. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi:10.1038/nature03445
28. Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–917. doi:10.1038/nature03443
29. Liu JF, Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: current status and future promise. Gynecol Oncol. 2014;133(2):362–369. doi:10.1016/j.ygyno.2014.02.039
30. FDA label for olaparib. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208558s006lbl.pdf.
31. FDA label for rucaparib. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209115s003lbl.pdf.
32. FDA label for niraparib. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208447s014lbl.pdf.
33. Del Campo JM, Matulonis UA, Malander S, et al. Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial. J Clin Oncol. 2019;JCO1802238.
34. Wolff AC, Blackford AL, Visvanathan K, et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol. 2015;33(4):340–348. doi:10.1200/JCO.2013.54.6119
35. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. doi:10.1200/JCO.2014.56.2728
36. AstraZeneca AB Lynparza 50 mg hard capsules: EU summary of product characteristics; 2014. Available from: https://www.ema.europa.eu/documents/product-information/lynparza-epar-product-information_en.pdf.
37. AstraZeneca AB. Lynparza 100 mg and 150 mg tablet: EU CHMP post-authorisation summary of positive opinion for lynparza; 2018. Available from: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-lynparza_en.pdf.
38. Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16(1):87–97. doi:10.1016/S1470-2045(14)71135-0
39. Ray-Coquard IL, Pautier P, Pignata S, et al. Phase III PAOLA-1/ENGOT-ov25 trial: Olaparib plus bevacizumab (bev) as maintenance therapy in patients (pts) with newly diagnosed, advanced ovarian cancer (OC) treated with platinum-based chemotherapy (PCh) plus bev. Eur Soc Med Oncol. 2019. doi:10.1093/annonc/mdz394.053
40. Essel KG, Behbakht K, Lai T, et al. PARPi after PARPi in epithelial ovarian cancer.
41. Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;227(12):13587–13598.
42. Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi:10.1016/S1470-2045(14)70391-2
43. Konstantinopoulos PA, Barry WT, Birrer M, et al. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. 2019;20(4):570–580. doi:10.1016/S1470-2045(18)30905-7
44. Barber LJ, Sandhu S, Chen J, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229:422–429. doi:10.1002/path.4140
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
