Back to Journals » OncoTargets and Therapy » Volume 10
Role of Nrf2 signaling pathway in the radiation tolerance of patients with head and neck squamous cell carcinoma: an in vivo and in vitro study
Authors Wang T, Hu P, Li B, Zhang J, Cheng Y, Liang Y
Received 20 September 2016
Accepted for publication 21 December 2016
Published 23 March 2017 Volume 2017:10 Pages 1809—1819
DOI https://doi.org/10.2147/OTT.S122803
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
This paper has been retracted.
Tao Wang, Peng Hu, Bo Li, Jun-Peng Zhang, Yu-Feng Cheng, Ye-Min Liang
Department of Radiotherapy, Qilu Hospital of Shandong University, Jinan, People’s Republic of China
Abstract: We aimed to investigate the relationship between the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway and the radiation tolerance of patients with head and neck squamous cell carcinoma (HNSCC). From January 2015 to January 2016, 117 patients with HNSCC were enrolled in our study and assigned into the sensitive and tolerance groups based on curative effect. Immunohistochemistry (IHC) was conducted to measure protein expressions of Nrf2, heme oxygenase-1 (HO1), NADPH quinine oxidoreductase 1 (NQO1) and glutathione S-transferase (GST). Human squamous cell carcinoma cell line, HSC-4, was induced by radiation to construct the HSC-4-radiation resistance (RR) cell line. HSC-4 and HSC-4-RR were also assigned into the blank, negative control (NC) and Nrf2 siRNA groups. Cell Counting Kit-8 (CCK-8), quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting were employed to detect cell viability, mRNA expression and protein expression, respectively, of Nrf2, HO1, NQO1 and GST. A total of 40 nude mice were equally assigned into the untreated, Nrf2 siRNA, radiation therapy (RT) and RT + Nrf2 siRNA groups. Compared with the sensitive group, patients in the tolerance group had upregulated Nrf2, HO1, NQO1 and GST expressions. HSC-4-RR cell line had improved cell viability and higher protein and mRNA expressions of Nrf2, HO1, NQO1 and GST compared with HSC-4 cell line. Compared with the HSC-4-NC and HSC-4-blank groups, the HSC-4-Nrf2 siRNA group had downregulated cell viability. Compared with the HSC-4-RR-NC and HSC-4-RR-blank groups, the HSC-4-RR-Nrf2 siRNA group had lower cell viability. However, the HSC-4-RR-Nrf2 siRNA group had elevated cell viability than the HSC-4-Nrf2 siRNA group. Tumor volume and tumor weight in the RT and RT + Nrf2 siRNA groups decreased evidently. The RT + Nrf2 siRNA group exhibited decreased tumor volume and tumor weight in comparison with the RT group. Our data demonstrated that downregulation of HO1, NQO1 and GST via inhibiting Nrf2 signaling pathway reduces the radiation tolerance of patients with HNSCC.
Keywords: nuclear factor erythroid 2-related factor 2, head and neck squamous cell carcinoma, radiation tolerance, signaling pathway, heme oxygenase-1, NADPH quinine oxidoreductase 1, glutathione S-transferase
Introduction
Head and neck squamous cell carcinoma (HNSCC), known as a morbid, common and frequently lethal malignancy, ranks the sixth most frequent non-skin cancer around the world, with a prevalence of >600,000 cases each year.1 Most of the patients are in the age range of 50–70 years, but the occurrence of this cancer could also be detected in older patients.2 The classic risk factors for HNSCC are alcohol consumption and tobacco use, while it is also associated with the infection of high-risk types of human papilloma viruses (HPVs).3 The most commonly used treatments include surgery, radiation and chemotherapy, with/without both biological and targeted therapies in which cooperation between different specialists is required.4 The outcome of patients with HNSCC who present a more advanced stage is extremely poor, with a 5-year survival rate of only 30%.5 Meanwhile, conventional treatments often lead to side effects that could affect normal physiological functions, such as swallowing, speech and physical appearance.4 Accordingly, radiotherapy has become a commonly accepted alternative for HNSCC treatment.6 Unfortunately, some patients with HNSCC develop chemo- and radioresistance, and only 50%–60% of them treated with radiation and chemotherapy are cured of their disease.7 According to a recent research, the 1-, 2- and 3-year overall survival rates in 26 cases of patients with advanced HNSCC treated by intensity-modulated radiotherapy were 76%, 61% and 47%, respectively.8 Radiation therapy (RT) in the treatment of HNSCC remains ineffective, and new strategies are in urgent need.
Nuclear factor erythroid 2-related factor 2 (Nrf2) acts as a significant transcription factor regulating the antioxidant response through inducing the gene expressions to bear an antioxidant response element (ARE) in regulatory regions.9 Due to the tight regulation by Keap1, Nrf2 is ubiquitously expressed at low constitutive levels in all human organs, which is a substrate adaptor protein for the Cullin3-based E3 ubiquitin ligase.10 It is reported that many Nrf2 target genes, such as drug transporters, conjugating enzymes and drug-metabolizing enzymes, play an important role in the determination of drug response and resistance.11 Moreover, Nrf2 could also regulate many genes except for the classic cytoprotective ones, especially in terms of cell differentiation and proliferation.12 Nrf2 signaling pathway is considered the most crucial pathway in the cell for the protection of cells against oxidative stress.13 Moreover, the Nrf2 signaling pathway has several protumorigenic effects, including promotion of metabolic activities and inhibition of apoptosis that support cell proliferation and radioresistance.14 Previous studies have demonstrated that elevated Nrf2 leads to the poor prognosis of squamous cell carcinoma and downregulation of Nrf2 contributes to sensitivity restoration to oxidative stress and chemotherapy.15,16 An increase in Nrf2 expression is found in ~91.5% of tumors, and Keap1, which regulates Nrf2 expression is frequently elevated in HNSCC tumors compared with the normal mucosa, thus making Nrf2 a potential biomarker candidate for the diagnosis and prognosis of HNSCC.17 However, few studies have focused on the concrete mechanism of Nrf2 signaling pathway in the radiation tolerance of HNSCC. Therefore, the aim of this study was to explore the role of Nrf2 signaling pathway in the radiation tolerance of patients with HNSCC to achieve a more effective treatment strategy.
Materials and methods
Ethics statement
All patients were informed about the procedures and signed an informed consent. The study was approved by the ethics committee of Qilu Hospital of Shandong University.
Subjects
From November 2014 to January 2016, 117 patients with HNSCC (aged 9–86 years with a mean age of 49.63±10.23 years) who received RT in Qilu Hospital of Shandong University were selected as the subjects. The inclusion criteria were as follows: 1) patients who did not receive any treatment prior to admission; 2) patients without other malignancies; 3) patients who received RT in Qilu Hospital of Shandong University with complete clinical data and 4) patients without mental disorders or disturbance of consciousness. The exclusion criteria were as follows: 1) patients with recurrence after the first course of treatment; 2) patients with multiple primary malignant tumors, as well as having metastases from other sites; 3) patients in pregnancy or lactation period and 4) patients abusing drugs or with operational contraindication such as liver and kidney dysfunction. According to the seventh edited tumor node metastasis (TNM) staging of the American Joint Committee on Cancer (AJCC),18 there were 20 patients in stage I, 18 patients in stage II, 44 patients in stage III and 35 patients in stage IV.
Evaluation of RT efficacy
According to the World Health Organization’s (WHO) criteria for the evaluation of efficacy of solid tumors,19 the efficacy of RT in patients with HNSCC was evaluated 2 months later, and the patients were then divided into groups of complete remission (CR; 18 cases), partial remission (PR; 30 cases), stable disease (SD; 30 cases) and progression disease (PD; 42 cases). CR + PR was deemed to be sensitive to RT and assigned into the sensitive group (48 cases), while SD + PD was considered to be insensitive to RT and assigned into the tolerance group (69 cases).
Immunohistochemistry (IHC)
The tissue samples of patients with HNSCC were embedded with conventional paraffin with the continuous slice thickness of 4 μm. The anti-rabbit Nrf2 antibody (1:200; ab62352; Abcam Inc., Cambridge, MA, USA), the anti-rabbit heme oxygenase-1 (HO1) antibody (1:800; ab13248; Abcam Inc.), the anti-rabbit NADPH quinine oxidoreductase 1 (NQO1) antibody (1:800; ab34173; Abcam Inc.), the anti-rabbit glutathione S-transferase (GST) antibody (1:800; ab19256; Abcam Inc.), biotin-goat anti-rabbit IgG and diaminobenzidine (DAB) were all purchased from DAKO (Glostrup, Denmark). The slices were sealed with bovine serum albumin (BSA) after dewaxing, added with primary antibody, placed at room temperature for 1 h, added with secondary antibody, stained with DAB and then re-dyed with hematoxylin. After the differentiation of hydrochloric acid and alcohol, the slices were processed by dehydration, transparentization, mounting and microscopy. After the experiment, the double-blind method was employed to evaluate the results; two pathologists completely unaware of the clinical data of patients independently observed each tissue slice a few times, and yellow or brown yellow granules that appeared in the tumor tissue was considered to be positive expression. A total of 1,000 cells were randomly selected from each slice in the six fields of view under the microscope, and the percentages of Nrf2, HO1, NQO1 and GST protein-positive cells were counted.
Human squamous cell carcinoma (HSC-4)-radiation resistance (RR) cell line preparation
The cervical lymph node metastasis in the HSC-4 cell lines of HNSCC used in our study was preserved in liquid nitrogen. After recovery, the HSC-4 cells were subcultured at 37°C in a 5% CO2 constant temperature incubator with culture medium of minimum essential medium (MEM) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin and streptomycin. During the subculture, the cells were screened with puromycin at a final concentration of 2 μg/mL. The cell lines with radiation tolerance were prepared, and then radiation induction was performed. The cells then had conventional irradiation of 2 Gy/day with continuous irradiation for 15 days, and the morphology of the cells under the microscope was observed. The screened radiation-tolerant cell line, HSC-4-RR, was used for further experiment.
Cell transfection
Nrf2 siRNA and the negative control (NC) plasmid fragments were purchased from Qiagen (Hilden, Germany). Invitrogen Lipofectamine 2000 (Lipo2000; Invitrogen, Inc., Carlsbad, CA, USA) was used to transfect HSC-4 and HSC-4-RR cells. The cells were seeded into 96-well culture plates, with 5,000 cells in each well. These cells were divided into three groups: the blank group, the negative control (NC) group and the Nrf2 siRNA group (each group had five replicates). Solution A (25 μL serum-free MEM added with 200 ng Nrf2 siRNA or NC plasmid) prepared in the eppendorf (EP) tube was gently shaken to mix well, and solution B (25 μL serum-free MEM) was added with 0.15 μL Lipo2000. The mixture of solution A and solution B was placed at room temperature for 15 min, and then the mixture was added into the wells and placed in an incubator at 37°C for 72 h.
Cell Counting Kit-8 (CCK-8) assay
According to the instructions of CCK-8 (Item No C0038; Beyotime Institute of Biotechnology, Haimen, Jiangsu, People’s Republic of China), the cells in the logarithmic growth phase were digested to make a suspension of 3×104 cells/mL. For the 96 wells, each well was added with 100 μL suspension, and three replicates were set for the cells in each well. The viability of HSC-4 and HSC-4-RR cells was detected at 24, 48, 72 and 96 h. In addition, the difference between the cell viability of HSC-4 and HSC-4-RR in different transfected groups was detected after transfected with Nrf2 siRNA.
Quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR was used to detect mRNA expressions of related proteins in Nrf2 signaling pathway in HSC-4 and HSC-4-RR cells. The cells seeded in the 96-well plates were scratched off. Each well was added with 1 mL Trizol and was shaken thoroughly. The mixture was placed for 10 min, added with 200 μL chloroform and shaken for 10 s. Then, the suspension was centrifuged at 12,000× g for 5 min at 4°C. The supernatant was obtained. The cells were added with 200 μL avantin, shaken to obtain a homogeneous mixture, placed for 10 min and centrifuged at 12,000× g for 10 min at 4°C. The supernatant was abandoned. The cells were added with 1 mL 75% ice ethanol, gently washed and centrifuged at 12,000 g for 10 min at 4°C, and the supernatant was again abandoned. After the liquid in the tube was dried, diethyl pyrocarbonate (DEPC) was added to dissolve the precipitation for 15 min. The absorbance of RNA was detected for its concentration. The reaction system of PCR process was as follows: 1 μL cDNA, 1 μL each of the upstream and downstream primers, 10 μL mixture and 8 μL DEPC. The reaction conditions were: at 94°C for 5 min, at 94°C for 30 s, annealing for 30 s, at 72°C for 1 min, repeated for 35 cycles, and at 72°C for 5 min. The primer sequences are shown in Table 1.
Western blotting
The cells were scratched off from ice, and centrifuged at 3,000× g at 4°C. Protein was cleaved using radioimmunoprecipitation assay (RIPA) lysis buffer in the tube at a ratio of 1:5, placed in the refrigerator at 4°C for 1 h, and centrifuged at 10,000× g/min for 10 min in the cryogenic centrifugal machine. The supernatant was transferred to the new EP tube for storage. Bicinchoninic acid (BCA) kit (Univ-bio, Shanghai, China) was used to determine the concentration of protein. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used for the 50 μg total protein at 70 V for 120 min. The protein was transferred to polyvinylidene fluoride (PVDF) membrane and sealed for 1.5 h at room temperature with 5% dried skimmed milk. Nrf2 antibody (1:100, ab62352; Abcam, Inc.), HO1 antibody (1:1,000, ab13248; Abcam, Inc.), NQO1 antibody (1:2,000, ab28947; Abcam, Inc.), GST antibody (1:2,000, ab19256; Abcam, Inc.) and GAPDH antibody (1:2,000, ab9485; Abcam, Inc.) were incubated at room temperature for 2 h and overnight at 4°C. The membrane was washed with Tris-Buffered Saline and Tween 20 (TBST) (pH 7.4) for three times, and added with horseradish peroxidase-labeled sheep anti-mouse IgG (1:2,000; Amersham Pharmacia Biotech, Buckinghamshire, UK) for incubation at room temperature for 1 h. Following the development by Western blotting chemiluminescence reagent, photos were scanned and gray-level image was analyzed. The expression of relative protein was calculated as: the gray level of target protein/the gray level of reference protein. The results were calculated three times.
Model establishment of nude mice and index detection
A total of 40 BALB/c male nude mice (3–4 weeks, and 14–18 g) raised by specific pathogen-free (SPF) laboratory and provided by the Mode Animal Centre of Nanjing University (Jiangsu, China) were recruited for our experiment. Daily activities of the nude mice such as eating and drinking were observed. HSC-4 cells in the logarithmic growth phase were selected and digested to make a single cell suspension with a density of 3×106/mL. A total of 1×106 tumor cells were subcutaneously injected through the back of the nude mice. Two weeks after tumor formation, the selected nude mice were randomly assigned into the untreated group, the Nrf2 siRNA group, the RT group and the RT + Nrf2 siRNA group, with 10 nude mice in each group. The nude mice in the Nrf2 siRNA group and the RT + Nrf2 siRNA groups were injected intravenously with 20 μL nucleic acid in solution containing 30 nM of Nrf2 siRNA to the solid tumor, while the untreated group and the RT group were injected with the same volume of normal saline. Approximately 48 h after injection, the RT group and the RT + Nrf2 siRNA group received RT, with radiation dose of 6 Gy. After 10 days of continuous irradiation, the nude mice were sacrificed for the experimental study. The growth inhibition rate was calculated as follows: (1 - tumor weight of the RT group or the RT + Nrf2 siRNA group/tumor weight of the untreated group) ×100%.
Statistical analysis
All data were analyzed by SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). Count data were expressed as case and percentage, and comparisons were performed using chi-square test. Measurement data were expressed as mean ± standard deviation, and the comparisons between two groups were performed using unpaired t-test, while the comparisons among three or more groups were conducted using one-way analysis of variance (ANOVA). Bilateral P<0.05 was considered as statistically significant.
Results
Comparisons of baseline characteristics between the sensitive and tolerance groups
Among the 117 patients with HNSCC, there were 48 cases (mean age: 49.24±11.93 years) in the sensitive group and 69 cases (mean age: 50.18±7.21 years) in the tolerance group. No significant difference was found regarding the age between the two groups (P>0.05). The mean weights of patients in the tolerance group and in the sensitive group were 59.04±6.11 and 58.38±9.54 kg, respectively (P>0.05). Moreover, no significant difference was found in TNM staging, as well as primary lesion and maximum tumor diameter between the sensitive and tolerance groups (all P>0.05), suggesting good homogeneity (Table 2).
  | Table 2 Comparisons of baseline characteristics between the sensitive and tolerance groups |
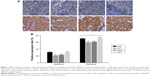
Comparisons of expressions of Nrf2 signaling pathway-related proteins in tumor tissue sections between the sensitive and tolerance groups
Positive expressions were found in Nrf2 and its downstream genes including HO1, NQO1 and GST in tissues of 117 patients with HNSCC. The positive expression rates of Nrf2, HO1, NQO1 and GST in the tolerance group were 70.83%, 60.42%, 62.50% and 72.92%, respectively, and in the sensitive group were 28.99%, 21.74%, 24.64% and 31.88%, respectively (Figure 1). Compared with patients in the sensitive group, patients in the tolerance group had elevated Nrf2, HO1, NQO1 and GST expressions (all P<0.05).
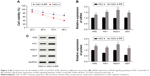
Comparisons of cell viability and mRNA and protein expressions of Nrf2 signaling pathway in HSC-4 and HSC-4-RR cell lines
CCK-8 (Figure 2A) showed that the viability of HSC-4-RR cell line had improved (96.84%, 82.63%, 71.92% and 66.24%) after radiation (2 Gy/day) for 24, 48, 72 and 96 h compared with the wild-type HSC-4 cell line (86.31%, 70.14%, 52.65% and 39.12%), suggesting the success of cell line construction (all P<0.05). Compared with the wild-type HSC-4 cell line, HSC-4-RR cell line had higher mRNA and protein expressions of Nrf2, HO1, NQO1 and GST (all P<0.05; Figure 2B and C).
Comparisons of cell viability in HSC-4 and HSC-4-RR cell lines after transfection in each group
As shown in Figure 3, no significant difference was found in cell viability at different time points between the HSC-4-NC and HSC-4-blank groups and between the HSC-4-RR-NC and HSC-4-RR-blank groups (all P>0.05). However, the HSC-4-RR-NC and HSC-4-RR-blank groups had upregulated cell viability at different time points compared with the HSC-4-NC and HSC-4-blank groups (all P<0.05). Compared with the HSC-4-NC and HSC-4-blank groups, the HSC-4-Nrf2 siRNA group had downregulated cell viability (both P<0.05), which revealed that transfection with Nrf2 siRNA enhanced the sensitivity of HSC-4 cells to radiation. Compared with the HSC-4-RR-NC and HSC-4-RR-blank groups, the HSC-4-RR-Nrf2 siRNA group had lower cell viability (both P<0.05). However, the HSC-4-RR-Nrf2 siRNA group had elevated cell viability than the HSC-4-Nrf2 siRNA group (P<0.05) and no significant difference was found between the HSC-4-RR-Nrf2 siRNA group and the HSC-4-NC and HSC-4-blank groups (all P>0.05), indicating that after transfection with Nrf2 siRNA, HSC-4-RR cells recovered its sensitivity to radiation.
Comparisons of expressions of Nrf2 signaling pathway-related proteins in HSC-4 and HSC-4-RR cell lines after transfection in each group
Western blotting and qRT-PCR showed that protein and mRNA expressions of Nrf2, HO1, NQO1 and GST in the HSC-4-NC group were higher than those in the HSC-4-Nrf2 siRNA group but lower than those in the HSC-4-RR-NC group (all P<0.05). Compared with the HSC-4-RR-NC group, cells in the HSC-4-RR-Nrf2 siRNA group had elevated protein and mRNA expressions of Nrf2, HO1, NQO1 and GST (all P<0.05). The protein and mRNA expressions of Nrf2, HO1, NQO1 and GST in the HSC-4-RR-Nrf2 siRNA group were upregulated compared with those in the HSC-4-Nrf2 siRNA group (all P<0.05). Meanwhile, no significant difference was found in protein and mRNA expressions of Nrf2, HO1, NQO1 and GST between the HSC-4-blank and HSC-4-NC groups and between the HSC-4-RR-blank and HSC-4-RR-NC groups (all P>0.05; Figure 4).
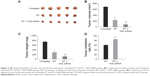
Successful construction of HNSCC nude mice models
After the injection of tumor cells, the rate of tumor formation in nude mice was 100%. As shown in Figure 5A and B, no obvious difference in the tumor volume was found between the Nrf2 siRNA group and the untreated group (P>0.05). While the tumor volume in the RT and RT + Nrf2 siRNA groups evidently decreased (both P<0.05), the RT + Nrf2 siRNA group had smaller tumor volume compared with that of the RT group (P<0.05). The results suggested that RT could inhibit tumor growth, and nude mice were more radiation sensitive after transfected with Nrf2 siRNA. Tumor weight (Figure 5C) of nude mice in the untreated group was the heaviest among the four groups, and no noticeable difference was observed between the untreated and Nrf2 siRNA groups (P>0.05). The tumor weight of nude mice in the RT and RT + Nrf2 siRNA groups decreased significantly, and the tumor weights of nude mice in the RT + Nrf2 siRNA group reduced evidently compared with those in the RT group (all P<0.05). Tumor growth inhibition rate was calculated based on the tumor weight of nude mice. Compared with the nude mice in the RT group, tumor growth inhibition of nude mice in the RT + Nrf2 siRNA group was more obvious (84.57% vs 68.91%, P<0.05; Figure 5D).
Discussion
In our in vivo and in vitro study, we investigated the relationship and possible mechanism between the Nrf2 signaling pathway and the radiation tolerance of patients with HNSCC. Finally, we concluded that downregulation of the downstream antioxidant genes HO1, NQO1 and GST via inhibiting Nrf2 signaling pathway reduces the radiation tolerance of patients with HNSCC.
HNSCC, an immunosuppressive malignancy with high morbidity, is the sixth most common cancer worldwide.20,21 Recent studies demonstrate that despite advances in surgery, chemotherapy, radiotherapy and combinations of treatment modalities, the long-term survival rate of patients with HNSCC has remained at 50% for the past 30 years.22,23 Genomic and proteomic studies tried to provide insight into the molecular drivers of HNSCC, which may help us design targeted therapeutic agents for it.24
In our study, we first investigated the expressions of Nrf2 signaling pathway-related proteins between the sensitive and tolerance groups; the results showed that compared with patients in the sensitive group, patients in the tolerance group had elevated Nrf2, HO1, NQO1 and GST expressions. A previous study demonstrated that dihydroquercetin (DHQ) induced the downregulation of HO1 and NQO1 expressions through Nrf2 signaling pathway.25 RT affects cell viability and changes the cell cycle by damaging the DNA, which is the most important cause for cell apoptosis.26,27 The mechanism of radiation tolerance in human tumors is mainly related to the repair of damaged DNA, cell cycle regulation, cell apoptosis, proliferation and other genes and transduction pathway; cancer stem cells can promote the tolerance of cancer cells to radiotherapy by activating the DNA damage response system of the cell itself.28 Nrf2 is proved to strongly influence intrinsic resistance to oxidative stress as well as control adaptive responses to various environmental stressors.29 Previous evidence also showed that Nrf2 expression was evaluated in HNSCC, and Nrf2 expression may be a possible HNSCC candidate biomarker.17 Upregulation of Nrf2 leads to Keap1 loss that contributes to lung squamous cell carcinomas (LSCC),30 and Nrf2 activity has also been indicated to confer RT in LSCC.31 Induced by multiple kinds of oxidative agents, HO1 is a stress-responsive enzyme that plays an oncogenic role in cancerous or transformed human cells and elevated HO1 expression was detected in malignant tumors, including gastric cancer and breast cancer.32,33 Nrf2 as well as its induction by sulforaphane is vital for the expression of NQO1.34 It was also reported that NQO1 expression is closely correlated with the progression and prognosis of patients with HNSCC, and high expression of NQO1 may be used as an important indicator for poor prognosis of patients with HNSCC.35 GSTs were also reported to play a crucial role in detoxifying carcinogenic metabolites, and they can also catalyze the connection of glutathione into many kinds of organic compounds, such as carcinogens and oxidative stress, to form water-soluble products.36 A study conducted by Zafereo et al37 also demonstrated that GSTs have been reported to confer increased risk for HNSCC. Consistent with our study, Shibata et al38 investigated the role of Nrf2 mutation in the resistance of therapy in esophageal squamous cancer (ESC) and found that downregulation of mutant Nrf2 is very likely to enhance radiation sensitivity in ESC cells.
Meanwhile, after the construction of HSC-4-RR, we also found that in comparison with the wild-type HSC-4 cell line, HSC-4-RR cell line had higher protein and mRNA expressions of Nrf2, HO1, NQO1 and GST. Furthermore, our study also provides evidence that HSC-4-RR cell line had improved viability after radiation compared with the wild-type HSC-4 cell line. Genotoxic agents generated either in the environment or intracellularly, such as ultraviolet (UV) light, ionizing radiation and reactive oxygen species, continuously damage DNA.39 A growing body of evidence suggests that dysregulation of DNA repair genes affects the response of cells to DNA-damaging anticancer treatment and upregulation of DNA damage response, as well as repair genes can cause cancer development by the increasing cancer cells, and also resulting in the increasing resistance to chemotherapy and radiotherapy.40,41
We found that while the HSC-4-RR-NC and HSC-4-RR-blank groups had upregulated cell viability compared with that of the HSC-4-NC and HSC-4-blank groups, the HSC-4-NC and HSC-4-blank groups had higher cell viability than the HSC-4-Nrf2 siRNA group (P<0.05), which showed that transfection with Nrf2 siRNA enhanced the sensitivity of HSC-4 cells to radiation. At the same time, our results also found that the HSC-4-RR-Nrf2 siRNA group had downregulated cell viability compared with the HSC-4-RR-NC and HSC-4-RR-blank groups and that the HSC-4-RR-Nrf2 siRNA group had elevated cell viability than the HSC-4-Nrf2 siRNA group (P<0.05), which indicated that after transfection with Nrf2 siRNA, HSC-4-RR cells recovered its sensitivity to radiation. Moreover, the current study also demonstrated that after transfected with Nrf2 siRNA, the protein and mRNA expressions of Nrf2, HO1, NQO1 and GST and cell viability of HSC-4-RR-Nrf2 siRNA was lower than that of the HSC-4-RR-NC group but higher than that of the HSC-4-Nrf2 siRNA. Nrf2 is a receptor of electrophiles and adapter for Cul3 ubiquitin ligase, which is also negatively regulated by specific suppressor protein Keap1.42 Previous study clarifies that Nrf2 is often in high expression in tumor and tumor cells seem to be able to hijack Nrf2 signaling pathway and enhance their ability to antioxidant stress, thereby increasing the tolerance of radiotherapy and chemotherapy.43 RNA interference is described as a response to double-stranded RNA leading to sequence-specific posttranscriptional gene silencing, and siRNA is incorporated into a nuclease complex called RNA-induced silencing complex (RISC) that targets and cleaves mRNA complementary to the siRNA and thus lowers its expression.44,45 Hence, after transfected with Nrf2 siRNA, the protein and mRNA expressions of Nrf2, HO1, NQO1 and GST and cell viability of HSC-4-RR-Nrf2 siRNA were decreased.
In our nude mice models, the tumor volume and tumor weight in the RT and RT + Nrf2 siRNA groups decreased evidently and the RT + Nrf2 siRNA group had smaller tumor volume than the RT group (P<0.05). Tumor growth inhibition of nude mice in the RT + Nrf2 siRNA group was more obvious compared with nude mice in the RT group (84.57% vs 68.91%, P<0.05). These results confirmed our previous presumptions that RT could inhibit tumor growth, and nude mice were more sensitive to radiation after transfected with Nrf2 siRNA.
Conclusion
It is currently established that downregulation of HO1, NQO1 and GST via inhibiting Nrf2 signaling pathway can reduce the radiation tolerance of patients with HNSCC. However, due to the different functions of Nrf2 in tumors at different periods, the development of Nrf2 signaling pathway for the treatment of patients with HNSCC requires more in-depth study.
Acknowledgments
This work was supported by the Major Research Project of Shandong Province (2015GSF121025). The authors give our sincere appreciation to the reviewers for their helpful comments on this article.
Disclosure
The authors report no conflicts of interest in this work.
References
Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. | ||
Gugic J, Strojan P. Squamous cell carcinoma of the head and neck in the elderly. Rep Pract Oncol Radiother. 2012;18(1):16–25. | ||
Rothenberg SM, Ellisen LW. The molecular pathogenesis of head and neck squamous cell carcinoma. J Clin Invest. 2012;122(6):1951–1957. | ||
Wise-Draper TM, Draper DJ, Gutkind JS, Molinolo AA, Wikenheiser-Brokamp KA, Wells SI. Future directions and treatment strategies for head and neck squamous cell carcinomas. Transl Res. 2012;160(3):167–177. | ||
Pickhard AC, Margraf J, Knopf A, et al. Inhibition of radiation induced migration of human head and neck squamous cell carcinoma cells by blocking of EGF receptor pathways. BMC Cancer. 2011;11:388. | ||
Oksuz DC, Prestwich RJ, Carey B, et al. Recurrence patterns of locally advanced head and neck squamous cell carcinoma after 3d conformal (chemo)-radiotherapy. Radiat Oncol. 2011;6:54. | ||
Sano D, Matsumoto F, Valdecanas DR, et al. Vandetanib restores head and neck squamous cell carcinoma cells’ sensitivity to cisplatin and radiation in vivo and in vitro. Clin Cancer Res. 2011;17(7):1815–1827. | ||
Yang H, Diao LQ, Shi M, et al. Efficacy of intensity-modulated radiotherapy combined with chemotherapy or surgery in locally advanced squamous cell carcinoma of the head-and-neck. Biologics. 2013;7:223–229. | ||
Chen X, Liu J, Chen SY. Over-expression of nrf2 diminishes ethanol-induced oxidative stress and apoptosis in neural crest cells by inducing an antioxidant response. Reprod Toxicol. 2013;42:102–109. | ||
Ren D, Villeneuve NF, Jiang T, et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the nrf2-mediated defense mechanism. Proc Natl Acad Sci USA. 2011;108(4):1433–1438. | ||
Villeneuve NF, Lau A, Zhang DD. Regulation of the nrf2-keap1 antioxidant response by the ubiquitin proteasome system: an insight into Cullin-ring ubiquitin ligases. Antioxid Redox Signal. 2010;13(11):1699–1712. | ||
Sporn MB, Liby KT. Nrf2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12(8):564–571. | ||
Varady J, Gessner DK, Most E, Eder K, Ringseis R. Dietary moderately oxidized oil activates the nrf2 signaling pathway in the liver of pigs. Lipids Health Dis. 2012;11:31. | ||
Martinez VD, Vucic EA, Thu KL, Pikor LA, Lam S, Lam WL. Disruption of keap1/cul3/rbx1 e3-ubiquitin ligase complex components by multiple genetic mechanisms: association with poor prognosis in head and neck cancer. Head Neck. 2015;37(5):727–734. | ||
Shibata T, Ohta T, Tong KI, et al. Cancer related mutations in nrf2 impair its recognition by keap1-cul3 e3 ligase and promote malignancy. Proc Natl Acad Sci U S A. 2008;105(36):13568–13573. | ||
Wang XJ, Sun Z, Villeneuve NF, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of nrf2. Carcinogenesis. 2008;29(6):1235–1243. | ||
Stacy DR, Ely K, Massion PP, et al. Increased expression of nuclear factor e2 p45-related factor 2 (nrf2) in head and neck squamous cell carcinomas. Head Neck. 2006;28(9):813–818. | ||
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. | ||
Sasaki T. New guidelines to evaluate the response to treatment “RECIST”. Gan To Kagaku Ryoho. 2000;27(14):2179–2184. | ||
Hoesli RC, Moyer JS. Immunotherapy for head and neck squamous cell carcinoma. Curr Oral Health Rep. 2016;3(2):74–81. | ||
Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in notch1. Science. 2011;333(6046):1154–1157. | ||
Correction. Lupeol suppresses cisplatin-induced nuclear factor-kappab activation in head and neck squamous cell carcinoma and inhibits local invasion and nodal metastasis in an orthotopic nude mouse model. Cancer Res. 2016;76(7):2052–2053. | ||
Martinez-Useros J, Garcia-Foncillas J. The challenge of blocking a wider family members of EGFR against head and neck squamous cell carcinomas. Oral Oncol. 2015;51(5):423–430. | ||
Kanazawa T, Misawa K, Misawa Y, et al. Galanin receptor 2 utilizes distinct signaling pathways to suppress cell proliferation and induce apoptosis in HNSCC. Mol Med Rep. 2014;10(3):1289–1294. | ||
Liang L, Gao C, Luo M, et al. Dihydroquercetin (DHQ) induced ho-1 and nqo1 expression against oxidative stress through the nrf2-dependent antioxidant pathway. J Agric Food Chem. 2013;61(11):2755–2761. | ||
Yin Z, Zhou B, He Q, et al. Association between polymorphisms in DNA repair genes and survival of non-smoking female patients with lung adenocarcinoma. BMC Cancer. 2009;9:439. | ||
Connell PP, Kron SJ, Weichselbaum RR. Relevance and irrelevance of DNA damage response to radiotherapy. DNA Repair (Amst). 2004;3(8–9):1245–1251. | ||
Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. | ||
Hayes JD, Dinkova-Kostova AT. The nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199–218. | ||
Jeong Y, Hoang NT, Lovejoy A, et al. Role of keap1/nrf2 and tp53 mutations in lung squamous cell carcinoma development and radiation resistance. Cancer Discov. Epub 2016 Sep 23. | ||
Abazeed ME, Adams DJ, Hurov KE, et al. Integrative radiogenomic profiling of squamous cell lung cancer. Cancer Res. 2013;73(20):6289–6298. | ||
Kim DH, Song NY, Kim EH, et al. 15-deoxy-delta12,14-prostaglandin j(2) induces p53 expression through nrf2-mediated upregulation of heme oxygenase-1 in human breast cancer cells. Free Radic Res. 2014;48(9):1018–1027. | ||
Liu ZM, Chen GG, Ng EK, Leung WK, Sung JJ, Chung SC. Upregulation of heme oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene. 2004;23(2):503–513. | ||
Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the are consensus sequence. Biochem J. 2003;374(pt 2):337–348. | ||
Zhang S, Song C, Zhou J, et al. Amelioration of radiation-induced skin injury by adenovirus-mediated heme oxygenase-1 (HO-1) overexpression in rats. Radiat Oncol. 2012;7:4. | ||
Yang Y, Jin T, Liu S, et al. Prognostic significance of NADPH quinine oxidoreductase 1 overexpression in head and neck squamous cell carcinoma. Zhonghua Bing Li Xue Za Zhi. 2014;43(7):463–467. | ||
Zafereo ME, Sturgis EM, Aleem S, Chaung K, Wei Q, Li G. Glutathione s-transferase polymorphisms and risk of second primary malignancy after index squamous cell carcinoma of the head and neck. Cancer Prev Res (Phila). 2009;2(5):432–439. | ||
Shibata T, Kokubu A, Saito S, et al. Nrf2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia. 2011;13(9):864–873. | ||
Broustas CG, Lieberman HB. DNA damage response genes and the development of cancer metastasis. Radiat Res. 2014;181(2):111–130. | ||
Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8(3):193–204. | ||
Lieberman HB. DNA damage repair and response proteins as targets for cancer therapy. Curr Med Chem. 2008;15(4):360–367. | ||
Turpaev KT. Keap1-nrf2 signaling pathway: mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry (Mosc). 2013;78(2):111–126. | ||
Ji L, Wei Y, Jiang T, Wang S. Correlation of nrf2, nqo1, mrp1, cmyc and p53 in colorectal cancer and their relationships to clinicopathologic features and survival. Int J Clin Exp Pathol. 2014;7(3):1124–1131. | ||
Tinoco ML, Dias BB, Dall’Astta RC, Pamphile JA, Aragao FJ. In vivo trans-specific gene silencing in fungal cells by in planta expression of a double-stranded RNA. BMC Biol. 2010;8:27. | ||
Kim VN. RNA interference in functional genomics and medicine. J Korean Med Sci. 2003;18(3):309–318. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.