Back to Journals » Cancer Management and Research » Volume 12
Role of Multimodal Treatment in Urothelial Carcinoma Spinal Metastasis: 15 Patients’ Experiences in a Single Center
Authors Wang T, Gao X, Zhang K, Yang J, Wu Z, Liu T, Jia Q, Xiao J
Received 16 April 2020
Accepted for publication 21 August 2020
Published 24 September 2020 Volume 2020:12 Pages 9003—9012
DOI https://doi.org/10.2147/CMAR.S258429
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Tao Wang,1,* Xin Gao,1,* Kun Zhang,1,* Jian Yang,1,* Zheyu Wu,1,2 Tielong Liu,1 Qi Jia,1 Jianru Xiao1
1Department of Orthopaedic Oncology, Spinal Tumor Center, Shanghai Changzheng Hospital, Second Military Medical University, Shanghai 200003, People’s Republic of China; 2Department of Orthopaedics, Zhongnan Hospital, Wuhan University, Wuhan, Hubei 430071, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianru Xiao; Qi Jia Tel +86-021-81886999
Email [email protected]; [email protected]
Purpose: Spinal metastasis from urothelial carcinoma (UC) is relatively uncommon. The aim of the present study is to explore the clinicopathological features, surgical treatments and outcomes of this rare disease.
Patients and Methods: Fifteen patients with UC spinal metastasis who received surgery in our center between 2009 and 2018 were retrospectively investigated. Clinical data, treatment options, and outcomes were analyzed.
Results: For the 15 patients (9 men and 6 women), the primary tumors were located in the upper urothelial tract in ten and lower urothelial tract in five. UC mainly metastasized to the lumbar spine in seven cases, followed by the thoracic spine in five. Pathologic fracture and soft tissue mass with dura mater compression were observed in 66.7% and 93.3% cases, respectively. Palliative resection was performed in nine cases and excisional resection in six. Eleven patients received postoperative chemotherapy, including three with a preoperative ECOG score > 2. Bisphosphonates were administered in all patients. Pain was relieved remarkably in all patients, and both the neurological function and general status were improved significantly after surgery. The median overall survival was 14 months. Log rank test showed that patients receiving postoperative chemotherapy survived longer than those without chemotherapy (p=0.037). WHO grade 3 was also correlated with poorer prognosis (p=0.012).
Conclusion: Pathological fracture and soft tissue mass with dura mater compression is frequently observed on radiological images in patients with UC spinal metastasis. Surgery is useful to prevent deterioration of performance status and improve quality of life, which provide an opportunity for further systematic therapy. Multimodal treatments, including surgery, postoperative chemotherapy and bisphosphonates are recommended. WHO grade 2 and receiving postoperative chemotherapy were favorable prognostic factors for the overall survival of patients with UC spinal metastasis.
Keywords: urothelial carcinoma, upper tract urothelial carcinoma, spine metastasis, bisphosphonates, prognosis, WHO grade
Introduction
Urothelial carcinoma (UC) is a malignant tumor originating from the urothelium throughout the urinary tract. UC is generally divided into upper tract (including renal pelvis and ureter) urothelial carcinoma (UTUC) and lower tract (including bladder and urethra) urothelial carcinoma (LTUC). Urothelial bladder cancer is the most common form of UC, while UTUC is relatively rare, accounting for approximately 5%.1 According to the World Health Organization (WHO), UC can be classified into low-grade (grade 1) and high-grade (grade 2 and 3) lesions, depending on their oncologic behaviors.2
The reported metastasis rate for UC is about 10.1%, which is considered as a devastating, almost uniformly fatal disease.3 The predominant metastatic site is lymph node, followed by the liver, lung, bone, and peritoneum.3–6 Spinal metastasis from the genitourinary system is relatively uncommon, accounting for about 58.3% of all UC bone metastasis.7 The importance of a multidisciplinary approach involving a team of specialists in the areas of orthopedics, oncology and radiotherapy has been emphasized for patients with bone metastases.8 Patients with UC spinal metastasis deserve special attention because of the high rate of skeletal-related events causing severe spinal cord compression, intractable pain, spinal instability, and poor quality of life,7 which in return, restrict the application of chemotherapy and radiotherapy.9 Previous studies anecdotally reported the beneficial role of metastasectomy in selected patients with metastatic UC.10,11 Larkin et al reported laminectomy and debulking resection for thoracic metastasis from the ureter.12 However, the clinical features, treatments and outcomes of UC spinal metastasis remain unclear.
In this study, we reported 15 consecutive patients with UC spinal metastasis who received surgical treatment in our center, in an attempt to elucidate the clinical features, treatment options and prognostic factors of this rare disease.
Patients and Methods
Fifteen consecutive patients with UC spinal metastasis who received surgical treatment in our center between Aug 2009 and Dec 2018 were retrospectively investigated. The pathological diagnosis and WHO grade were confirmed by two pathologists independently according to the WHO (2004) classification.2 Relevant clinical data were obtained through a review of the medical records, including age, gender, tumor location, treatment history, interval from initial to confirmed diagnosis of spinal metastasis, details of surgery, adjuvant therapy, and outcomes. This study was approved by the Shanghai Changzheng hospital ethics committee, and informed consent was obtained from each patient or family members of those who had passed away.
All patients were evaluated by X-ray, CT, and MRI of the spine. PET-CT and chest/abdominal CT scans were performed to evaluate systemic metastasis. The surgical indications included a life expectancy of at least 3 months, neurological deficits, refractory pain, radiographic instability, and tumor progression despite chemotherapy and radiation. The neurological status, performance status and degree of pain of the patients were evaluated by Frankel score,13 the Eastern Cooperative Oncology Group performance score (ECOG-PS)14 and visual analog scale (VAS) scores respectively. Surgery was performed in all patients using palliative or excisional surgery according to Weinstein–Boriani–Biagini system15 and revised Tokuhashi score16 by the same surgery team. Patients with a spinal instability neoplastic score >7 were considered unstable and thus underwent spinal stabilization.17
The patients were followed up on the outpatient basis or via telephone interviews every month for the first 3 months, and every 3 months for the next year. The length of the follow-up period was defined as the date of surgery to the date of disease-related death or November 2019.
The equality of variances for the two data groups was determined with the Levene’s test and continuous variables were compared by Student’s t-test. The overall survival (OS) rates were estimated by the Kaplan–Meier method, and the Log rank test was applied to compare the difference. All statistical analyses were performed using IBM SPSS statistics (version 21.0, IBM Corp.), with p < 0.05 considered statistically significant.
Results
Patient Features
Included in this study were nine men and six women, with a median age of 66.5 (range 59–80) years (Table 1). The lesions were mainly located in the lumbar spine (7, 46.7%), followed by the thoracic spine (5, 33.3%), cervical spine (2, 13.3%) and sacrum (1, 6.7%). Eleven patients (73.3%) suffered from multiple-level spinal metastases and four patients had a single-level spinal lesion. Only bone metastases were observed in five patients, spine metastasis alone was found in three patients, ilium or rib was also involved in the other two patients. Multiple extra-bone metastases were detected in 10 patients, including visceral metastasis (lung in two and liver in two, and one patient metastasized to both) in five patients, lymph nodes in eight patients, and the psoas major muscles in two patients. Localized pain was the most consistent complaint in 14 patients, with a median duration of 9 (range 1–24) months. Neurological symptoms, varying from simple and slight radicular pain to paraparesis, occurred in 10 patients.
 |
Table 1 Treatments and Outcomes of 15 Patients with Spinal Metastasis from Urothelial Carcinoma |
Treatment of the Primary Lesions
Primary UTUC occurred in 10 cases involving the renal pelvis in three and the ureter in seven; primary LTUC in five cases, involving the bladder in four and the proximal urethra in one. A total of eleven patients underwent primary lesions radical surgery (radical cystectomy or nephroureterectomy) and subsequently developed metastasis, and the remaining four patients had distant metastasis on initial diagnosis. Among primary tumor resection patients, nine patients showed no local progression during the follow-up period, one patient (case 2) experienced local relapse and received secondary surgery, another patient (case 7) was found to have recurrence in the urinary tract and underwent surgical treatment. Six patients were classified as WHO grade 2 and the other nine patients as grade 3. The median interval between initial diagnosis of UC and spinal metastasis was 10 (mean 21, range 0–80) months, 7.5 (mean 12.5, range 0–47) months for UTUC and 36 (mean 38.0, range 0–80) months for LTUC, (p=0.044); 40 (mean 41.3, range 5–80) months for WHO grade 2 and 5 (mean 7.4, range 0–30) months for WHO grade 3, (p=0.002) (Table 2 and Figure 1).
 |
Table 2 Treatment Information of Primary Urothelial Carcinoma |
Radiologic Study
Representative images are provided in Figure 2. Pathologic fracture was observed in 10 patients (66.7%) on x-ray and CT. Bone metastasis was predominantly osteolytic in 12 cases (80%), followed by the mixed type in three cases (20%). On MRI, lesions usually show hypointense on T1W images, predominantly hypointense on T2W (8/15, 53.3%) followed by iso/hypointense (5/15, 33.3%) and hyperintense (2/15, 13.3%). Tumors showed strong inhomogeneous enhancement after gadolinium administration. Soft tissue mass was frequently observed in 14 patients (93.3%), with severe narrowing of the spinal canal and compression of the dura mater. Furthermore, soft tissue mass was observed in psoas major muscles in two patients (case 5, 14). The WBB staging, revised Tokuhashi scores and SINS scores are provided in Table 1.
Treatment and Outcomes
Surgery was performed in all cases including palliative resection in nine and excisional resection in six patients. The surgical time averaged 225 (range 100–320) minutes with a mean blood loss of 1113.3 (range 200–2000) mL. After surgery, nine (46.7%) patients received cisplatin-based chemotherapy, two patients (13.3%) received cisplatin-based chemotherapy and radiotherapy (40Gy, 20 fractions), and one (6.7%) patient received immune-checkpoint inhibitors (PD-1 antibody). The rest of the three patients received no adjuvant therapy. In addition, of the six patients whose preoperative ECOG>2, three received chemotherapy after surgery and one received PD-1 antibody. Bisphosphonates (eg, zoledronic acid) were applied in all patients.
The histology and grade of the metastatic lesions were the same as those of the primary tumors in all cases. The positive immunohistochemical markers were CK7 (n= 14, 93.3%), CK20 (n= 9, 60%), UPIII (n=7, 46.7%), p63 and GATA3 (n= 6, 40%). The Ki-67 index was > 10% positivity in 10 patients (66.7%) with a mean value of 35.9% (range 2–90%).
Pain was relieved remarkably in all patients (postoperative VAS score 0–2), and both the neurological function and the general status were improved significantly (postoperative Frankel grade D or E; postoperative ECOG score 1–2) (Table 3). Disease progression was detected in three patients, including local recurrence in one patient (case 7) and spine new-onset metastasis in two patients (case 10 and 14), both of these two patients received secondary surgery. During the follow-up period, 11 patients (73.3%) died of the disease with a median period of 12 (mean 12.7, range 8–18) months. The patient who received immune-checkpoint inhibitors (PD-1 antibody) died of 16 months after surgery.
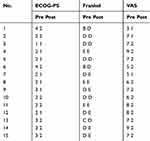 |
Table 3 The Patients’ Performance Status Before and After Operation |
Prognostic Factors
The results of univariate analysis are provided in Table 4. The median overall survival (OS) for all patients was 14 months, 15 (range 11–26) months for patients with ECOG≤2 and 11 (range 11–18) months for patients with ECOG>2, (p=0.217). The median survival time for palliative and excisional resection was 12 (range 10–26) and 14 (range 8–18) months respectively, (p=0.228). According to the log rank test, age, gender, primary tumor location, metastasis spinal location, preoperative Frankel scores, operation blood loss, surgical time, visceral metastasis, and metastasis interval has insignificant impact on the prognosis. Patients who received postoperative chemotherapy survived longer than those who did not receive chemotherapy (15 months vs 10 months, p=0.037). WHO grade 3 was correlated with poorer prognosis (p=0.012) (Figure 3).
 |
Table 4 Log-Rank Analysis of Prognostic Factors Affecting OS of Spinal Metastasis from Urothelial Carcinoma |
 |
Figure 3 Kaplan–Meier curves of overall survival for postoperative chemotherapy (A) and urothelial carcinoma WHO grade (B). |
Discussion
Urothelial carcinoma is one of the most common neoplasms arising from the genitourinary system. The metastasis rate of UC is about 10.1% with poor prognosis. Previously, only a few studies have focused on the treatment and prognostic prediction of UC spinal metastasis.12,18 To the best of our knowledge, this is the first case series regarding the radiological findings, treatments and outcomes of UC spinal metastasis. It was found in our study that the median OS was 14 months, and WHO grade 2 and receiving postoperative chemotherapy were favorable factors for the OS of patients with UC spinal metastasis.
The results of our study demonstrated that UC spinal metastasis had a male predominance (60%), which is consistent with the gender distribution of primary UC reported in the literature.1 The median age of patients with UC spinal metastasis was 66.5 years, which is in line with the previous reports.6,7 With respect to tumor location, the lumbar spine was more likely to be infringed in our series, which maybe was because direct invasion to the adjacent structures is also a progress route as evidenced by soft tissue mass in psoas major muscles. The median metastasis interval of UTUC was significantly shorter than that of LTUC (p=0.044), this is attributed to the advanced clinical course of the disease and the thinner smooth muscle covering of upper tract, which may synergistically contribute to the stronger metastatic potential of UTUC.6 The median metastasis interval of WHO grade 3 was dramatically shorter than that of grade 2 (p=0.002). In addition, our log-rank analysis showed that grade 3 was associated with a lower OS than grade 2 for UC spinal metastasis (p=0.012), which is consistent with the aggressive behavior of WHO classification.19,20
Generally, UC spinal metastasis showed osteolytic lesions in a CT scan in our series, which is dissimilar to a previous report that osteoblastic cases occupied 10.4% of their patients.7 Vertebral pathological fracture was common on X-ray and CT, soft tissue mass around the lesion with a narrowing of the spinal canal and compression of the dura mater were frequently observed on MRI imaging. This explains the most common clinical presentations are pain and neurological deficit which are caused by spine instability and nerve compression.
Since surgical treatment of patients with lung metastasis of UC was first reported by Cowles et al,21 metastasectomy has been applied in UC patients with lymph node, brain, liver and bone metastases.22–24 Given the nearly 15 months of median OS of UC metastasis patients and epidural spinal cord compression observed in almost all of our patients, surgery is conducted in our series.4,25 The main goal of surgery is to relieve pain, restore stability of the spine and improve the neurological function and quality of life. In our study, patients who received palliative surgery seemed to survive for a shorter time than patients who received excisional surgery (12 vs 14 months), however, the difference was statistically insignificant. This may be attributed to the limited number of included patients. However, excisional surgery is usually recommended for single spinal metastatic lesion with no live metastasis,3,5,25–27 as long-term survival was achieved in some bone-predominant metastatic UC patients.28,29
Cisplatin-based chemotherapy is the mainstay of treatment for metastatic UC. Eleven patients who received cisplatin-based adjuvant chemotherapy in our series achieved significantly longer OS than those who did not (p=0.037), which is consistent with the previous report that chemotherapy is a favorable factor for UC bone metastasis.7 Interestingly, one patient in our study who received immune-checkpoint inhibitors after surgery because of cisplatin-ineligibility survived for 16 months. It is reported that immune checkpoint inhibitors will challenge chemotherapy as a frontline treatment option in cisplatin-ineligible patients. However, the usefulness of immune-checkpoint inhibitors in UC spinal metastasis needs further research.30 The role of radiotherapy on local recurrence and OS needs further study.
The median OS for UC metastasis varied from 5.4 to 15 months,25,26,31 10.8 months for bone as the only metastatic site9 and 6.2 months for bone as a related metastatic site.7 In our study, the median OS for UC spinal metastasis is 14 months, a little longer than that reported in previous studies. Several reasons may contribute to this: 1) surgery is a useful method to manage skeletal adverse events, while skeletal adverse events are significantly correlated with immobilization, loss of independence, poor quality of life and reduced survival.32 2) The rate of receiving first-line chemotherapy in our series is higher than that reported in the literature (73.3% vs 57%), while chemotherapy is a significant favorable prognostic factor for UC bone metastasis.9 Traditionally, UC metastasis patients with ECOG>2 are not suitable for cisplatin-based chemotherapy. However, three of the six patients with preoperative ECOG>2 in our series received postoperative chemotherapy. This is because surgery improves patients’ performance status, as evidenced by ECOG, VAS and Frankel scores after surgery, which provides an opportunity for further systematic therapies. 3) In our study, bisphosphonates were applied to all patients. Its usefulness in patients with UC bone metastasis has been proved by Tsuda et al,7 who reported that the median survival duration was significantly improved from 5.2 months to 15.8 months by using bone-modifying agents.
Although this is the largest series reporting the outcomes and prognosis of UC spinal metastasis, limitations do exist. First, this is a retrospective study and the number of patients included is relatively small, which precludes conducting multivariate statistics. In addition, we only included patients undergoing surgical treatment and excluded those who received chemotherapy only. We are looking forward to conducting larger-sample research on this challenging disease.
Conclusions
Pathological fracture and soft tissue mass with dura mater compression is frequently observed on radiological images of patients with UC spinal metastasis. Surgery is useful to prevent the deterioration of performance status and improve the quality of life. A promising outcome could be achieved by surgical intervention followed by postoperative chemotherapy and bisphosphonates. WHO grade 2 and receiving postoperative chemotherapy were favorable prognostic factors for the OS of patients with UC spinal metastasis.
This work was sponsored by Shanghai Sailing Program (20YF1449100), Sailing Talent Program of Navy Medical University (2019-QH-30) and Shanghai Science and Technology Commission (17140900202).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi:10.3322/caac.21442
2. Eble J, Sauter G, Epstein J, Sesterhenn I. Pathology and Genetics of Tumors of Urinary System and Male Genital Organs. World Health Organizatoion Classification of Tumors. Lion: International Agency for Research on Cancer press; 2004:93–109.
3. Hsieh MC, Chiang PH, Rau KM, Chen YY, Su YL, Huang CH. The comparison of oncologic outcomes between metastatic upper tract urothelial carcinoma and urothelial carcinoma of the bladder after cisplatin-based chemotherapy. Urol Oncol. 2015;33:
4. Inokuchi J, Naito S, Fujimoto H, et al. Impact of multimodal treatment on prognosis for patients with metastatic upper urinary tract urothelial cancer: subanalysis of the multi-institutional nationwide case series study of the Japanese urological association. Int J Urol. 2016;23:224–230. doi:10.1111/iju.13031
5. Dong F, Fu H, Shi X, et al. How do organ-specific metastases affect prognosis and surgical treatment for patients with metastatic upper tract urothelial carcinoma: first evidence from population based data. Clin Exp Metastasis. 2017;34:467–477. doi:10.1007/s10585-018-9884-z
6. Xie J, Zhang XB, Wen J, Zhang YS, Li HZ. Comparison of clinicopathological features in metastatic upper tract urothelial carcinoma and urothelial bladder cancer. Int Urol Nephrol. 2016;48:481–487. doi:10.1007/s11255-016-1214-2
7. Tsuda Y, Nakagawa T, Shinoda Y, et al. Skeletal-related events and prognosis in urothelial cancer patients with bone metastasis. Int J Clin Oncol. 2017;22:548–553. doi:10.1007/s10147-016-1075-9
8. Kimura T. Multidisciplinary approach for bone metastasis: a review. Cancers. 2018;10:156. doi:10.3390/cancers10060156
9. Necchi A, Pond GR, Pal SK, et al. Bone metastases as the only metastatic site in patients with urothelial carcinoma: focus on a special patient population. Clin Genitourin Cancer. 2018;16:e483–e490. doi:10.1016/j.clgc.2017.10.012
10. Abe T, Matsumoto R, Shinohara N. Role of surgical consolidation in metastatic urothelial carcinoma. Curr Opin Urol. 2016;26:573–580. doi:10.1097/MOU.0000000000000329
11. Abe T, Kitamura H, Obara W, et al. Outcome of metastasectomy for urothelial carcinoma: a multi-institutional retrospective study in Japan. J Urol. 2014;191:932–936. doi:10.1016/j.juro.2013.11.004
12. Larkin JO, Cullen IM, Kelleher MO, et al. Transitional cell carcinoma of the upper ureter metastatic to the thoracic spine presenting as a spinal cord compression. Sci World J. 2008;8:223–227. doi:10.1100/tsw.2008.43
13. Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179–192. doi:10.1038/sc.1969.30
14. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. doi:10.1097/00000421-198212000-00014
15. Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine: terminology and surgical staging. Spine. 1997;22:1036–1044. doi:10.1097/00007632-199705010-00020
16. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30:2186–2191. doi:10.1097/01.brs.0000180401.06919.a5
17. Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29:3072–3077. doi:10.1200/JCO.2010.34.3897
18. Daniels CJ, Wakefield PJ, Bub GA. Bladder metastasis presenting as neck, arm and thorax pain: a case report. Chiropr Man Therap. 2016;24:14. doi:10.1186/s12998-016-0097-8
19. Akdogan B, Dogan HS, Eskicorapci SY, Sahin A, Erkan I, Ozen H. Prognostic significance of bladder tumor history and tumor location in upper tract transitional cell carcinoma. J Urol. 2006;176:48–52. doi:10.1016/S0022-5347(06)00511-8
20. Novara G, De Marco V, Gottardo F, et al. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: multi-institutional dataset from 3 European centers. Cancer. 2007;110:1715–1722. doi:10.1002/cncr.22970
21. Cowles RS, Johnson DE, McMurtrey MJ. Long-term results following thoracotomy for metastatic bladder cancer. Urology. 1982;20:390–392. doi:10.1016/0090-4295(82)90462-9
22. Dodd PM, McCaffrey JA, Herr H, et al. Outcome of postchemotherapy surgery after treatment with methotrexate, vinblastine, doxorubicin, and cisplatin in patients with unresectable or metastatic transitional cell carcinoma. J Clin Oncol. 1999;17:2546–2552. doi:10.1200/JCO.1999.17.8.2546
23. Lehmann J, Suttmann H, Albers P, et al. Surgery for metastatic urothelial carcinoma with curative intent: the German experience (AUO AB 30/05). Eur Urol. 2009;55:1293–1299. doi:10.1016/j.eururo.2008.11.039
24. Kim T, Ahn JH, You D, et al. Pulmonary metastasectomy could prolong overall survival in select cases of metastatic urinary tract cancer. Clin Genitourin Cancer. 2015;13:e297–e304. doi:10.1016/j.clgc.2015.04.013
25. Taguchi S, Nakagawa T, Hattori M, et al. Prognostic factors for metastatic urothelial carcinoma undergoing cisplatin-based salvage chemotherapy. Jpn J Clin Oncol. 2013;43:923–928. doi:10.1093/jjco/hyt096
26. Sengeløv L, Kamby C, von der Maase H. Metastatic urothelial cancer: evaluation of prognostic factors and change in prognosis during the last twenty year. Eur Urol. 2001;39:634–642. doi:10.1159/000052520
27. Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum containing regimens. J Clin Oncol. 2010;28:1850–1855. doi:10.1200/JCO.2009.25.4599
28. Ramos JD, Cheng HH, Yu EY. Long-term survival in bone-predominant metastatic urothelial carcinoma. Clin Genitourin Cancer. 2014;12:e241–244. doi:10.1016/j.clgc.2014.07.005
29. Joudi FN, Dahmoush L, Spector DM, et al. Complete response of bony metastatic bladder urothelial cancer to neoadjuvant chemotherapy and cystectomy. Urol Oncol. 2006;24:403–406. doi:10.1016/j.urolonc.2005.12.002
30. Niglio SA, Jia R, Ji J, et al. Programmed death-1 or programmed death ligand-1 blockade in patients with platinum-resistant metastatic urothelial cancer: a systematic review and meta-analysis. Eur Urol. 2019;76(6):782–789. doi:10.1016/j.eururo.2019.05.037
31. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602. doi:10.1200/JCO.2005.07.757
32. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243–6249. doi:10.1158/1078-0432.CCR-06-0931
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


