Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Role of Janus kinase 1 and signal transducer and activator of transcription 3 in vitiligo
Authors Samaka RM, Basha MA, Menesy D
Received 26 March 2019
Accepted for publication 22 May 2019
Published 28 June 2019 Volume 2019:12 Pages 469—480
DOI https://doi.org/10.2147/CCID.S210106
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Rehab M Samaka,1 Mohammed A Basha,2 Dina Menesy3
1Department of Pathology, Faculty of Medicine, Menoufia University, Shibīn al Kawm, Egypt; 2Department of Dermatology, Andrology and STDs, Faculty of Medicine, Menoufia University, Shibīn al Kawm, Egypt; 3General Organization for Teaching Hospitals and Institutes, Damanhour Medical National Institute, Damanhour, Egypt
Background: Vitiligo is an acquired autoimmune skin disorder. The often-visible lesions of vitiligo have a major impact on patients’ quality of life and the results of the treatment regimens on offer are unsatisfactory, so there is a need for new therapeutic regimens. Recent advances in vitiligo pathogenesis have led to recognition of the importance of the JAK–STAT pathway as an attractive therapeutic option.
Purpose: To evaluate role of JAK1 and STAT3 in vitiligo.
Methods: This prospective case–control study was carried out on 35 patients presenting with vitiligo and 20 apparently healthy age- and sex-matched volunteers. Skin biopsies from controls and cases were taken for histopathological and immunohistochemical JAK1 and STAT3 evaluation.
Results: Epidermal and dermal overexpression of STAT3 was noted in lesional skin compared to the other groups (P=0.02 and P<0.001, respectively). There was a positive correlation between dermal expression of JAK1 and dermal expression of STAT3 (r=0.52, P=0.004).
Conclusion: In conjunction, JAK1 and STAT3 might be involved in the pathogenesis of vitiligo. This could open the gate for the use of JAK1 and STAT3 inhibitors as new targeted therapy for vitiligo.
Keywords: JAK1, STAT3, vitiligo, immunohistochemical
Introduction
Vitiligo is a chronic autoimmune disease that results from the destruction of melanocytes, causing white spots on the skin. Vitiligo affects approximately 1% of population and can affect both adults and children, causing diminished quality of life and marked psychological distress.1
The pathogenesis of vitiligo involves the destruction of melanocytes via cell-mediated immunity, and IFNγ and that CD8 T cells play a key role in this process.2
The JAK–STAT pathway mediates the intracellular signals of more than 60 cytokines, growth factors, and hormones.3 The JAK family includes JAK1, JAK2, JAK3, and TYK2.4 There are seven STAT genes in humans: STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6.5
Treatment with topical corticosteroids, calcineurin inhibitors, and narrowband ultraviolet B phototherapy are unsatisfactory, and poor response to treatment is common.6
Targeting the JAK–STAT pathway has gained huge attraction in various immunological diseases, especially T cell–mediated diseases.7 Although few JAK inhibitors are US Food and Drug Administration–approved, other JAK and possible STAT inhibitors are being developed for vitiligo therapy.8
The aim of this work was to evaluate role of JAK1 and STAT3 in vitiligo.
Methods
This prospective case–control study was carried out on 35 patients presenting with vitiligo and 20 healthy age- and gender-matched individuals as a control group. They were selected from the Outpatient Clinic of Dermatology in Menoufia University Hospital spanning the period between January 2017 and January 2018. This study was approved by the Menoufia University ethics committee and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent after the procedure had been fully explained. Patients included in this study suffered from vitiligo, either segmental or nonsegmental, and had received no topical or systemic treatment for vitiligo within the last month. Patients with leukoderma secondary to other causes were excluded. Each patient was subjected to complete history-taking and general and dermatological examinations. Vitiligo area scoring index (VASI) scores were calculated for all patients to assess the severity of the disease,9 and vitiligo disease activity (VIDA) scores calculated to assess disease activity.10
Skin biopsies
Two skin biopsies were taken with a 3 mm punch from each patient under 2% lignocaine local anesthesia. The first was from the center of the vitiliginous macule and the other from nearby apparently normal skin within a 5 cm radius of any vitiliginous macules.11 They were fixed in 10% neutral buffered formalin and sent to the Pathology Department, Faculty of Medicine, Menofuia University for routine histochemical processing and sectioning. Skin sections were stained with H&E for routine histopathological examination. Other sections were mounted on positive-charged slides and stored at room temperature for JAK1 and STAT3 immunohistochemical staining.
Immunohistochemical staining
Sections were stained. They were dewaxed and rehydrated in graded alcohol solutions. For antigen retrieval, sections were placed in target-retrieval solution (pH 6.1 or 9, 97°C) for 10–30 minutes. Slides were blocked using a peroxidase-blocking reagent and incubated for 1 hour at room temperature with the primary diluted antibodies JAK1 (mouse monoclonal antibody, 0.1 mL, each vial containing 200 μg IgG2b in 1 mL PBS, A9 sc1677; Santa Cruz Biotechnology) and STAT3 (mouse monoclonal antibody, 0.1 mL, each vial containing 200 μg IgG1in 1 ml PBS, F2 sc-8019; Santa Cruz Biotechnology). They were diluted 1:200 and 1:100 for JAK1 and STAT3, respectively. Deparaffinized sections were stained using immunohistochemistry products in Dako automatic immunohistochemistry apparatus. Detection was performed using the detection reagent and DAB. Slides were counterstained using Mayer’s hematoxylin. Finally, sections were mounted and covered using distyrene, plasticizer and xylene. Positive-control slides were mouse-kidney tissue for JAK1 and human fallopian-tube tissue for STAT3. Negative-control slides were prepared by omitting primary antibodies from the staining procedure.
Interpretation of JAK1 and STAT3 expression
Stained JAK1 and STAT3 sections were scored: staining status positive or negative, localization:nucleocytoplasmic, cytoplasmic, or nuclear,12 and histochemical score (H-score) applied to evaluate positive cases, where both the intensity and the percentage of positivity were considered using formula: H-score = + 3 (strong intensity) × % + 2 (moderate intensity) × % + 1 (mild intensity) × %. The score ranges between 0 and 300.13
Statistical analysis
Statistical analysis was conducted using SPSS version 20 on an IBM computer. Qualitative data are expressed as numbers and percentage. Quantitative data are expressed as mean ± SD, percentages, and medians. Fisher’s exact test and χ2were used for comparing qualitative variables. Mann–Whitney U test was used in comparing two nonparametric quantitative variables. The Kruskal–Wallis test was used for testing equality of population medians among groups, as it is an extension of the Mann–Whitney test. ANOVA was used for comparison between three or more quantitative variables. The results were considered statistically significant where P≤0.05.
Results
Clinical criteria of vitiligo patients and demographic and histopathological data of lesional and perilesional vitiligo skin are given in Table 1.
 |
Table 1 Clinical and demographic data and histopathological characteristics of the vitiligo patients |
Comparison between controls and vitiligo skin (lesional and perilesional) regarding JAK1 and STAT3 tissue immunoreactivity
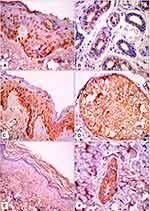
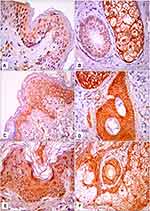
There were no significant differences between the studied groups regarding JAK1 expression. There were significant differences between the groups regarding epidermal and dermal STAT3 expression (P=0.03 and P=0.04, respectively). Epidermal and dermal H-scores for STAT3 expression were significantly higher in lesional skin than the other groups (P=0.02 and P<0.001, respectively; Table 2, Figures 1 and 2).
 |
Table 2 Comparison between controls and vitiligo skin (lesional and perilesional) regarding JAK1 and STAT3 tissue immunoreactivity |
Relationships among lesional H-scores of JAK1 and STAT3 and demographic and clinical parameters (sex, occupation, family history, distribution, and VIDA score)
There was a significant relationship between epidermal H-score for JAK1 expression and the occupation of patients (P=0.05). There were significant relationships between high epidermal and dermal H-scores for JAK1 expression and family history of patients (P=0.02 and P=0.04, respectively). There was no significant relationship between epidermal and dermal H-scores for STAT3 expression and demographic and clinical parameters (Table 3).
 |
Table 3 Relationship between lesional H-scores of JAK1 and STAT3 with demographic and clinical parameters |
Relationships among lesional H-scores for JAK1 and STAT3 and histopathological parameters
There was a significant association between high epidermal H -scores for JAK1 expression and epidermal atrophy (P=0.03). Also, dermal H- scores for JAK1 expression had significant relationships with focal DEJ disruption and vacuolar alteration (P=0.008 and P=0.01, respectively). There were significant associations between epidermal H- scores for STAT3 expression and focal DEJ disruption and vacuolar alteration (P=0.008 and P=0.01, respectively; Table 4).
 |
Table 4 Relationship between lesional H-scores of JAK1 and STAT3 with histopathological parameters |
Correlation between JAK1 and STAT3 (lesional and perilesional)
There was a positive correlation between lesional expression of JAK1 in dermis and lesional expression of STAT3 in dermis (r=0.52, P=0.004; Figure 3).
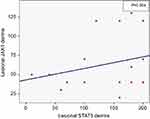 |
Figure 3 Positive correlation between lesional expression of JAK1 in dermis and lesional expression of STAT3 in dermis (r=0.52, P=0.004). |
Discussion
Vitiligo is a common skin and mucous-membrane depigmenation disorder14 that is characterized by well-circumscribed depigmented macules and patches.15
The JAK–STAT) pathway is utilized by such cytokines as interleukins and interferons to transmit signals from the cell membrane to the nucleus. Upon engagement of extracellular ligands, intracellular JAK proteins become activated and phosphorylate STAT proteins, which dimerize, and then phospo-STAT (pSTAT), which is the active form, translocates to the nucleus to directly regulate gene expression.16
JAK1 is involved in IFNγ signals through binding of IFNγ to the IFNγ receptor, which initiates phosphorylation of STAT1 and translocates it to the nucleus, where IFNγ–dependent genes, including CXCL9 and CXCL10, are transcribed. Then, CXCL9 and CXCL10 recruit CD8 T cells to the skin, where they attack melanocytes.17
STAT3 is involved in the pathogenesis of vitiligo through its activation, possibly in response to Langerhans cell activation, which induces the recruitment and differentiation of TH17 cells in vitiligo and may downregulate melanogenic activity.18
IFNγ signaling, which has a role in the pathogenesis of vitiligo through targeted destruction of melanocytes by CD8 T cells, utilizes the JAK–-STAT pathway;19 therefore, vitiligo may be susceptible to treatment with JAK and STAT inhibitors.
Significant repigmentation is reported in patients after oral administration of tofacitinib (JAK1/3 inhibitor),20 ruxolitinib (JAK1/2 inhibitor),21 and topical ruxolitinib, particularly on the face.22
To the best of our knowledge, there have been few studies to investigate immunohistochemical expression of JAK1 and STAT3 in vitiligo and correlate this with clinical and histopathological parameters.
In the present study, the immunohistochemical expression of JAK1 in the epidermis and dermal adnexa showed no significant differences between patients and controls, although Nada et al23 found that that JAK1 levels on Western blot assay were significantly higher in vitiligo patients than controls. This discrepancy in results could be attributed to different techniques used and fewercontrols.
There were significant relationships between epidermal and dermal H- scores for JAK1 expression and family history of patients. Hu et al24 found that three single-nucleotide polymorphisms (rs310230, rs310236, and rs310241) in JAK1 were associated with susceptibility to Vogt–Koyanagi–Harada syndrome, which is a rare presentation of vitiligo.
In the current study, there was a significant relationship between epidermal H-scores for JAK1 expression and the occupation of patients. This could be explained by exposure to such chemicals as para-tert-butylphenol, which can be found in adhesive resins and other products that were presumed to cause vitiligo in genetically susceptible patients.25
There were significant associations between overexpression of JAK1 and epidermal atrophy, degree of DEJ disruption, and degree of DEJ vacuolar alteration. This could be explained by oxidative damage and autoimmune mechanisms that cause damage to skin lipids, DNA, and proteins, leading to pathological alterations and separation at the DEJ.26
There were significant differences between studied groups regarding epidermal and dermal STAT3 expression. Overexpression of STAT3 was noted more in lesional skin than the other groups. This is in agreement with Tanemura et al,27 who reported that there was much more pSTAT3 in lesional skin than perilesional skin, as pSTAT3 is located in the nuclei of keratinocytes and/or dermal inflammatory cells, suggesting the significance of STAT3 activation.12
There were significant associations between overexpression of STAT3 and focal DEJ disruption and vacuolar alteration. These relationships have not previously been reported, and further studies are recommended to investigate these correlations.
In the current study, there was a positive correlation between dermal expression of JAK1 and dermal expression of STAT3, which suggests arole of JAK1 and STAT3 in the pathogenesis of vitiligo upon activation of the JAK–STAT pathway. Further studies are recommended to assess this correlation.
Limitation
There were fewer controls than patients.
Conclusion
In conjunction, JAK1 and STAT3 might be involved in the pathogenesis of vitiligo. This could open the gate for the use of JAK1 and STAT3 inhibitors as new targeted therapy for vitiligo.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Linthorst Homan MW, Spuls PI, de Korte J, et al. The burden of vitiligo: patient characteristics associated with quality of life. J Am Acad Dermatol. 2009;61(3):411–420. doi:10.1016/j.jaad.2009.03.022
2. Guerra L, Dellambra E, Brescia S, Raskovic D. Vitiligo: pathogenetic hypotheses and targets for current therapies. Curr Drug Metab. 2010;11:451–467. doi:10.2174/138920010791526105
3. Mok CC. The Jakinibs in systemic lupus erythematosus: progress and prospects. Expert Opin Investig Drugs. 2019;28:85–92. doi:10.1080/13543784.2019.1551358
4. Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662–5679. doi:10.1038/sj.onc.1203925
5. Cheh X, Vinkemeier U, Zhao Y, et al. Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell. 1998;93:827–839.
6. Grimes PE. New insights and new therapies in vitiligo. JAMA. 2005;293:730–735. doi:10.1001/jama.293.6.730
7. O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi:10.1146/annurev-med-051113-024537
8. Ghoreschi K1, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib. J Immunol. 2011;186:4234–4243. doi:10.4049/jimmunol.1003668
9. Hamzavi I, Jain H, McLean D, Shapiro J, Zeng H, Lui H. Parametric modeling of narrowband UV-B phototherapy for vitiligo using a novel quantitative tool: the Vitiligo Area Scoring Index. Arch Dermatol. 2004;140(6):677–683. doi:10.1001/archderm.140.6.677
10. Bhor U, Pande S. Scoring systems in dermatology. Indian J Dermatol Venereol Leprol. 2006;72(4):315–321.
11. Aslanian F, Noe R, Antelo D, et al. Immunohistochemical findings in active vitiligo including depigmenting lesions and non-lesional skin. Open Dermatol J. 2008;2:105–110. doi:10.2174/1874372200802010105
12. Alves De Medeiros AK, Speeckaert R, Desmet E, et al. JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One. 2016;16:1–16.
13. Bilalovic N. Sandstad B and Torlakovic E. CD10 protein expression in tumor and stromal cells of malignant melanoma is associated with tumor progression. Mod Pathol. 2004;17:1251–1258. doi:10.1038/modpathol.3800174
14. Wen X, Hamblin MR, Xian Y, et al. A preliminary study of fractional CO2 laser added to topical tacrolimus combined with 308 nm excimer lamp for refractory vitiligo. Dermatol Ther. 2018;32:e12747.
15. Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473–491. doi:10.1016/j.jaad.2010.11.061
16. Schwartz DM, Bonelli M, Gadina M, et al. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12:25–36. doi:10.1038/nrrheum.2015.167
17. Rashighi M, Harris JE. Interfering with the IFN-gamma/CXCL10 pathway to develop new targeted treatments for vitiligo. Ann Transl Med. 2015;3:343.
18. Moretti S, Fabbri O, Baroni G, et al. Keratinocyte dysfunction in vitiligo epidermis: cytokine microenviroment and correlation to keratinocyte apoptosis. Histol Histopathol. 2009;24:849–857. doi:10.14670/HH-24.849
19. Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76:736–744. doi:10.1016/j.jaad.2016.12.005
20. King BA, Craiglow BG. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015;151:1110–1112. doi:10.1001/jamadermatol.2015.1520
21. Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370–371. doi:10.1016/j.jaad.2015.09.073
22. Rothstein B, Joshipura D, Saraiya A, et al. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib. J Am Acad Dermatol. 2017;76:1054–1060. doi:10.1016/j.jaad.2017.02.049
23. Nada HR, El Sharkawy DA, Elmasry MF, Rashed LA, Mamdouh S. Expression of Janus Kinase 1 in vitiligo & psoriasis before and after narrow band UVB: a case-control study. Arch Dermatol Res. 2018;310:39–46. doi:10.1007/s00403-017-1792-6
24. Hu K, Hou S, Li F, Xiang Q, Kijlstra A, Yang P. JAK1, but not JAK2 and STAT3, confers susceptibility to Vogt-Koyanagi-Harada (VKH) syndrome in a Han Chinese population. Invest Ophthalmol Vis Sci. 2013;54:3360–3365. doi:10.1167/iovs.13-11615
25. Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1–13. doi:10.1016/j.jaad.2016.10.048
26. Sander CS, Cooper SM, Ali I, Dean D, Thiele JJ, Wojnarowska F. Decreased antioxidant enzyme expression and increased oxidative damage in erosive lichen planus of the vulva. Bjog. 2005;112:1572–1575. doi:10.1111/j.1471-0528.2005.00743.x
27. Tanemura A, Kotobuki Y, Itoi S, et al. Positive link between STAT3 activation and Th17 cell infiltration in vitiligo vulgaris. J Dermatol Sci. 2012;67:190–212. doi:10.1016/j.jdermsci.2012.05.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.