Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Role of insulin-like growth factor-1 in skin tags: a clinical, genetic and immunohistochemical study in a sample of Egyptian patients
Authors Farag AGA , Abdu Allah AMK, El-Rebey HS, Mohamed Ibraheem KI, Mohamed ASED, Labeeb AZ , Elgazzar AE , Haggag MM
Received 31 October 2018
Accepted for publication 5 March 2019
Published 26 April 2019 Volume 2019:12 Pages 255—266
DOI https://doi.org/10.2147/CCID.S192964
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Azza Gaber Antar Farag,1 Azza Mohamed Kamel Abdu Allah,2 Hala Said El-Rebey,3 Kawthar Ibraheem Mohamed Ibraheem,4,5 Asmaa Shams El Dein Mohamed,3 Azza Zaghlol Labeeb,6 Ayman Elhussien Elgazzar,1 Magda Mostafa Haggag1
1Department of Dermatology, Andrology and STDs, Faculty of Medicine, Menoufia University, Shibīn al Kawm, Egypt; 2Department of Biochemistry and Molecular biology, Faculty of Medicine, Menoufia University, Shibīn al Kawm, Egypt; 3Department of Pathology, Faculty of Medicine, Menoufia University, Shibīn al Kawm, Egypt; 4Department of Medical Microbiology and Immunology, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 5Microbiology Department, Taibah University, Medina, Kingdom of Saudi Arabia; 6Department of Microbiology, Faculty of Medicine, Menoufia University, Shibīn al Kawm, Egypt
Background: Skin tags (STs) are benign connective tissue neoplasms, in which insulin-like growth factor −1 (IGF-1) has a mitogenic and antiapoptotic activity.
Purpose: We aimed to study for the first time, the possible role of IGF-1 (CA) 19 and rs6214 gene polymorphisms, and its tissue immunoreactivity in the pathogenesis of STs.
Patients and methods: This case–control study included 40 ST patients and 20 controls. We searched for (CA) 19 single-nucleotide polymorphism (SNP) using conversional PCR and for rs6214 gene polymorphism using real-time PCR. IGF-1 tissue immunoreactivity was investigated using polyclonal IGF-1 antibody.
Results: IGF-1 immunoreactivity showed significantly strong upregulation in epidermis (p=0.002) and dermal components (endothelial cells [p=0.038] and fibroblasts [p=0.004]) of excised STs than control skin. TT and CT rs6214 genotypes and its T allele were significantly associated with STs (p=0.006 and P=0.002, respectively). Also (p=0.013). These 4 genotypes were significantly associated with development of multiple STs and epidermal IGF-1 tissue immunoreactivity in studied patients.
Conclusions: IGF-1 (CA) 19 and rs6214 gene polymorphisms may contribute to a predisposition of STs in Egyptian patients, the role of which could be mediated through local upregulation of IGF-1 in cutaneous tissues.
Keywords: skin tags, insulin growth factor-1, gene polymorphism, immunohistochemistry
Introduction
Skin tags (STs) or acrochordons are common benign connective tissue neoplasms of the dermis that are mainly composed of loose fibrous tissue.1 They occur mainly on the neck and major flexures as a small, soft, pedunculated protrusions.2 STs are associated with several conditions, including type 2 diabetes mellitus,3 obesity and acromegaly.4 Disturbance in insulin action presents with many conditions including STs.5
Insulin-like growth factor –I (IGF-1) is a hormone with a structure like insulin. It has a significant role in childhood growth and anabolic effects in adults.6 It is one of the most important activators of serine/threonine-specific protein kinase (AKT) signaling pathway that promotes cell growth and proliferation and inhibits programmed cell death.7 The gene encoding for IGF-1 is located in the long arm of chromosome 12 (12q22-24.1).8 IGF-1 (CA) 19 and rs6214 gene polymorphisms were investigated in some dermatological9,10 and nondermatological disorders.11,12
In the skin, IGF-1 is produced by dermal fibroblasts, where it stimulates these fibroblasts to proliferate and provide loose connective tissue. Also, it acts on epidermal keratinocytes stimulating their proliferation resulting in acanthosis, hyperkeratosis and sometimes papillomatosis, hence forming ST lesions.13
The relationship between STs and IGF-1 was previously investigated through assessment of IGF-1 serum levels14 and its tissue expression,13 and till now no one analyzed IGF-1 gene polymorphism in ST patients.
The aim of this study was to shed light on the possible role of IGF-1 in the pathogenesis of skin tags through investigating its (CA) 19 and rs6214 gene polymorphisms as well as its immunohistochemical tissue expression in patients having skin tags. Also, our aim was extended to correlate the analyzed IGF-1genotypes with the clinico-pathologic parameters of STs in those patients.
Material and methods
This retrospective case–control study was carried out on 40 patients having STs in addition to 20 age- and sex-matched apparently healthy volunteers as a control group. They were selected during the period from March to June 2017.
The study protocol was approved by the ethical committee of Faculty of Medicine, Menoufia University, that was in accordance with Helsinki Declaration in 1975 (revised in 2000). Written informed consent forms were obtained from all participants prior to study initiation.
ST patients from both sexes were included in this study. Subjects having diabetes mellitus, thyroid disorders, polycystic ovarian syndrome, and those who are either on treatment or had history of treatment with drugs that can influence IGF-1 level (glucocorticoids, insulin, oral hypoglycemic agents, oral contraceptive pills and isotretinoin) within the last month were excluded. Also, we excluded pregnant and lactating women.
Methods
Every participant was subjected to full history, clinical examinations, weight and height measuring to calculate body mass index (BMI)15 and dermatological examination including assessment of site, size and the total number of STs. Additionally, analysis of IGF-I (CA) 19 gene polymorphism using conventional PCR and IGF-I rs6214 gene polymorphism using real-time PCR were performed. Moreover, immunohistochemical tissue reactivity of IGF-1 in excised ST lesions and control skin was done.
IGF-1 genotyping
Three milliliters of venous blood was taken under complete aseptic conditions in sterile EDTA vacutainer tubes. Samples were collected during daytime from 9 am to 12 am and stored at −20°C for DNA extraction.
DNA extraction was done using Zymo Research Quick-g DNA mini prepGenomic DNA purification kit (Tustin, CA, USA). DNA was eluted and stored at −20°C for further PCR procedure.
REFLP analysis of IGF-1 CA19 gene polymorphism
Restriction fragment length polymorphism (PCR-RFLP) analysis was performed in the genomic DNAs isolated by using proper primers for the IGF-I (CA19) polymorphic area. Forward primer 5ʹ-GCTAGCCAGCTGGTGTTATT-3ʹ, reverse primer 5ʹ-ACCACTCTGGGAGAAG GGTA-3ʹ.16 A total volume of 0.5 ng⁄L of genomic DNA sample was added into the reaction mixture. The mixture also included 0.5 nmol⁄L forward primer, 0.5 nmol⁄L reverse primer, 0.2 mmol⁄L dNTP, 1.5 mmol⁄L MgCl2, 10 PCR buffer and 0.025 units⁄lL taq DNA polymerase. Totally, 50 lL of PCR volume was used in the study. The procedure was denaturation at 95°C for 3 mins and 94°C for 45 s, annealing at 57°C for 45 s, extension at 72°C for 1 min in a total of 30 cycles with a waiting period for 10 mins at 72°C in stepwise manner. Samples were kept at 4°C until the analysis time. Visualization of the amplified products by 2% agarose gel electrophoresis was done. The resulting bands were evaluated with UV gel documentation system. The IGF-1 polymorphism represents CA repeats. Three genotypes were categorized: <192 bp (<19 CA repeat), 192–194 bp (=19 repeat) and >194 bp (>20 CA repeat).
Real-time PCR analysis of IGF-1 rs6214 gene polymorphism
For genotyping, we used the universal taqMan Master Mix from Thermo Scientific (Waltham, MA, USA), while the primers and Taqman probes were designed by Applied Biosystems (Foster City, CA, USA) Life Technologies. Forward primer: 5´-AAT TAT TCC CTC TCA ACA AAA CTT TAT AGG-3ʹ and reverse primer: 5´-TGA AGG AAA TAA GTC ATA GAC ACT CTT AGA A-3ʹ was used for rs6214. Probes VIC: CTG CAG ACT TAA CGT GT, FAM: CTG CAG ACT TAA CAT GT were used. Primers and probes were purchased from Bioneer (Bioneer, Inc., Oakland, CA, USA). The reaction mixture was prepared by mixing 10 μL of the master mix, 1.25 μL of a 20x single-nucleotide polymorphism (SNP) assay kit containing primers and probes and 3.75 μL of DNAse-free water. For each unknown reaction, 5 μL of genomic DNA template was added and for the negative control reaction, 5 μL of DNAse-free water was added. The cycling conditions were set as follows: initial denaturation at 95°C for 5 mins followed by 35 cycles of; 95°C for 45 s, for 45 s, and 72°C for 30 s, with final extension for 7 mins at 72°C. This was done using the 7500 real-time PCR system (Applied Biosystems). Figure 2 shows the allelic discrimination plot.
IGF-1 immunohistochemistry
Under local anesthesia (xylocaine 2%), one ST from each patient was held gently with a nontoothed forceps and cut with a scalpel from its base, then the base was shortly cauterized with diathermy. The control skin biopsies were obtained from matched sites of the healthy volunteers. Biopsies were fixed in 10% neutral buffered formalin, dehydrated in ascending grades of ethanol followed by immersion in xylene and then impregnation in paraffin. Two 5 μm-thick sections from each block were prepared. One was to be stained with H&E for routine histopathological examination, the other was mounted on super frost plus slide for immunohistochemistry. Streptavidin-biotin amplified system was used. The primary antibody for IGF-1 was rabbit polyclonal antibody raised against IGF-1 provided from Chongqing Biops Co., Ltd, Chongqing, China (Cat No. YPA1106). It was received as 100 mcg concentrated with dilution of 1:200, sections of prostatic carcinoma were used as positive control. The detection Kit was the ultravision detection system anti-polyvalent horseradish peroxidase/diaminobenzidine (HRP/DAB) (Ready to use; Cat. No.TP-015-HD; Lab Vision, CA).
Positive IGF-1 expression was assigned when ≥10% of cells showed brown cytoplasmic staining. The staining intensity of IGF-1 was assessed subjectively as mild, moderate and strong. The expression of IGF-1was examined in epidermis and dermis (inflammatory cells [lymphocytes], endothelial cells and fibroblasts).
Statistical analysis
Results were collected, tabulated and statistically analyzed by IBM personal computer and statistical package SPSS version 20 (SPSS Inc., Chicago, IL, US). Hardy–Weinberg, equilibrium was computed to exclude any bias of results. Qualitative data were described using number and percent, while quantitative data were expressed in mean ± SD. Student t-test was used for comparison between two groups having quantitative variables. Mann–Whitney and Kruskal–Wallis tests were used for comparison of two and three groups of nonparametric variables, respectively. P-value <0.05 was considered statistically significant.
Results
Demographic and clinical data of studied subjects
Personal data as well as clinical characteristics of investigated subjects are demonstrated in Table 1. Patient and control groups were matched as regards demographic characteristics except that BMI that was significantly higher in ST patients (p=0.001) (Table 1).
 | Table 1 Demographic and clinical characteristics of patients with skin tags and controls |
IGF-1 immunohistochemical expression
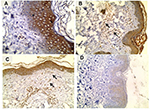
IGF-1 immunoreactivity (Figure 1) in ST lesions was significantly higher and strongly expressed than in control skin sections in both epidermis (p=0.012; p=0.002) and dermal components (endothelial cells [p=0.0380] and fibroblasts [p=0.004]) (Table 2).
 | Table 2 Tissue expression of IGF‐1 in excised ST lesions of patients and controls |
IGF-1 rs6214 and (CA) 19 gene polymorphisms
Analyzing rs6214 SNP (Figure 2) revealed that TT and CT rs6214 genotypes and T allele were significantly predominant in patients than controls (p=0.006; p=0.002, respectively) (Table 3).
 | Table 3 Distribution of IGF-1 rs6214 and (CA) 19 gene polymorphisms in studied groups |
Regarding IGF-1 (CA) 19 gene polymorphism (Figure 3), we categorized the IGF-1 (CA) 19 SNP area into three groups: lower than 192 bp (<192 bp), from 192 to 194 bp (192–194 bp) and higher than 194 bp (>194 bp). We observed that (<192 bp) and 192–194 bp genotypes as well as its (<192 bp) allele were significantly recorded in individuals having STs compared to controls (P=0.013; P=0.009, respectively) (Table 3).
Relationship between IGF-1 rs6214 and (CA) 19 genotypes and assessed parameters in ST patients
TT and CT genotypes were significantly demonstrated in male patients (P=0.022) and were significantly associated with multiple (high number) STs (P=0.005) and strong epidermal IGF-1 tissue expression (p=0.001), but nearly significant association was noticed regarding increased BMI (p=0.059) (Table 4).
 | Table 4 Relation between IGF-1 rs6214 gene polymorphism and studied data in ST patients |
Additionally, there was a significant difference between obese patients and obese controls as regards IGF-1 gene polymorphism where CC and CT genotypes were predominated in patients than controls while TT was the predominant genotype in controls, indicating that the predominance of CT and TT in patients is not attributed to obesity. On the other hand, there was no significant difference between both groups as regards C or T alleles (Table 5).
 | Table 5 IGF-1 gene polymorphism in obese skin tag patients and obese control group |
Regarding IGF-1 (CA) 19 genotypes, (<192 bp) genotype was significantly associated with increased BMI (p=0.031), multiple ST lesions (p=0.004) and strong epidermal tissue expression of IGF-1 (P<0.001) (Table 6).
 | Table 6 Relation between IGF-1 (CA) 19 gene polymorphism and studied data in ST patients |
Discussion
The present study was designed to shed light on the possible role of IGF-1 tissue expression and two different IGF-1 gene polymorphisms (rs6214 and CA 19) in the pathogenesis of STs.
Our patients had a significantly higher BMI than that of controls. These results were in accordance with that of many investigators.17–19 Jusuf et al17 found that there is a significant correlation between increased BMI and the occurrence of STs. Plausible mechanisms that explain the pathogenesis of STs in obesity are hyperinsulinemia and increased leptin levels.
In obese individuals, the increased body fat can affect the sensitivity of insulin by decreasing the expression of insulin receptor substrate-1.20 Consequently, the pancreas will compensate by producing greater quantities of insulin.21 This hyperinsulinemia promotes an increase in IGF-1 that can stimulate the proliferation of keratinocytes and produce epidermal hyperplasia.20 Additionally, IGF-1 stimulates dermal fibroblasts to proliferate and form connective tissue.13 Moreover, the elevated level of IGF-1 reduces insulin-like growth factor-binding protein-3 (IGFBP-3) concentration. IGFBP-3 is the hormone responsible for regulating antiproliferation gene transcription in the epidermis through inhibition of the binding of IGF-1 to its receptor. A decreased level of IGFBP-3 would lead to increased proliferation and excessive cell growth, which can manifest as ST lesions.14,22
Leptin is a growth hormone that has a role in the process of proliferation, differentiation and apoptosis. It has an ability to induce proliferation of various cell types including human keratinocyte.20,23 In obese subjects, leptin can affect insulin resistance process by inhibition of receptor kinase activity and phosphorylation of insulin receptor substrate-1, resulting in impairment of metabolic action of insulin in the adipocytes,20 leading to insulin resistance and hyperinsulinemia.This hyperinsulinemia stimulates an increase in IGF-1 circulatory levels that can promote proliferation of both keratinocytes20 and fibroblasts13 which manifest as acrochordons.17
In the current study, there was a significant higher and strong expression of IGF-1 in both epidermis and dermal elements especially the endothelium of excised ST lesions than in control skin specimens. Confirming this result, Bosseila and Shaker13 using two-step sandwich type immunoassay (ELISA) demonstrated a significant tissue expression of IGF-1 in STs which was almost sevenfolds that of normal skin, denoting the active role of local IGF-1 in promoting the proliferative process seen in epidermis as well as dermis of acrochordons.
IGF-1 and IGF-2 through activation of the insulin-like growth factor-1 receptor (IGF1R) are potent mitogens and inhibitors of apoptosis.11 The IGF-1/IGF1R pathway has been implicated in a number of hyperplastic epidermal disorders such as psoriasis,24 wound healing and plaques of mycosis fungoides,25 and in hyperplastic dermal conditions including keloid and hypertrophic scar.26 Although all these disorders are histologically distinctive from STs, all share local cellular proliferation. Thus, IGF-1 is secreted by dermal fibroblasts and acts in an autocrine fashion motivating them to proliferate and produce extra loose connective tissue. Also, it stimulates in a paracrine fashion epidermal keratinocytes, yielding acanthosis and hyperkeratosis with or without papillomatosis, which leads to the formation of acrochordon.27
Herein, we analyzed for the presence of two different polymorphisms of IGF-1 gene in patients having STs: rs6214 and (CA) 19 gene polymorphisms.
To our knowledge, this work may be the first to study rs6214 and (CA) 19 SNP in patients having STs, in which we found a significant difference between STs patients and their matched peers. TT and CT genotypes were predominant in patients than controls. Also, T allele was the main allele in the patient group.
Parallel to our result, Yosry et al12 studied the same polymorphism in colorectal cancer, a locally cellular proliferative disease. They found that rs6214 CT and TT genotypes were significantly associated with colorectal cancer. Also, Feik et al27 stated that carriers of polymorphic genotype of the SNP rs6214CT and TT were associated with an increased risk for colorectal cancer compared to the colonoscopy-negative controls with an odds ratio of 1.79 (95% CI 1.04–3.08).
In the current study, we observed that TT and CT IGF-1 rs6214 genotypes carriers had a higher number of STs lesions. Moreover, these two genotypes (TT and CT) were significantly associated with epidermal IGF-1 tissue expressions. Therefore, we may suggest that the mitogenic effect of increased tissue IGF-1 in rs6214 TT and CT genotypes was involved in enhancement of cell proliferation and differentiation, and decreased apoptosis resulting in multiple STs development.
Based on this result, we can conclude the direct relation between TT and CT genotypes of rs6214 IGF-1 SNP and occurrence of STs. However, due to the novelty of our study on this specific polymorphism, more studies on large number of patients are required to clarify this issue.
In the same context, we observed a significant difference between patients and controls regarding IGF-1 (CA) 19 gene polymorphism. (<192 bp) and 192–194 bp genotypes were significantly predominant in patients than controls. Additionally, IGF-1 (CA 19) 192–194 bp and (<192 bp) genotypes were significantly associated with high BMI, higher number of STs lesions and strong epidermal IGF-1 tissue expression.
Likewise, in acne vulgaris that shares ST pathogenicity regarding insulin resistance28 and hyperandrogenic state,29 Tasli et al9 reported that (<192 bp) and 192–194 bp carriers were significantly higher in acne vulgaris patients than controls. Also, they found a high frequency of 192–194 bp genotype in patients with severe acne compared to those having mild-to-moderate forms.
There is a functional relationship between IGF-I (CA) 19 polymorphism and circulating IGF-1 levels. Carriers of the (<192 bp) and ⁄ or (>194 bp) alleles of the IGF-1 promoter have higher circulating IGF-1 levels than noncarriers.16 Moreover, Yaylali et al11 reported that these polymorphisms may directly influence tissue expression of IGF-I.
Accordingly, the presence of multiple STs in IGF-1 (CA 19) 192–194 bp and (<192 bp) genotype carriers can be explained by the demonstrated high tissue levels of IGF-1. This IGF-1, in addition to its direct effect on fibroblasts and keratinocyte,13 may reduce the levels of IGFBP-3, which regulate antiproliferation gene transcription in the epidermis. Consequently, this causes an increase in proliferation and excessive cell growth, which can manifest as ST lesions.22 Moreover, the significant high BMI in those genotypes (192-194 bp and [<192 bp]) can explain development of STs as a result of insulin resistance and/or high leptin levels in obese subjects.
Patients with STs have a higher risk of impaired carbohydrate metabolism or diabetes. This finding has important clinical implications, as many cases of type 2 diabetes mellitus remain undiagnosed until the patients develop end-organ damage such as cardiovascular diseases, impairment of renal function or loss of visual acuity. Recognition of possible risk factors of impaired carbohydrate metabolism such as STs helps in earlier diagnosis of at-risk patients and prevention of the above-mentioned complications .30
The main limitation of the current work was the small sample size. Therefore, we recommended further large-scale studies to validate our results.
Conclusion
From the result of the current study, we may suggest that IGF-1 gene polymorphisms in (CA) 19 and rs6214 may contribute to a predisposition to STs in Egyptian patients. Also, we may conclude and confirm that IGF-1, expressed by epidermal and dermal elements, has an active role in STs development that may be mediated through its action in dermal fibroblasts stimulating their growth and excess loose connective tissue production and epidermal keratinocytes forming hyperkeratosis.
Acknowledgments
The authors are grateful to administrative and technical staffs at Dermatology Clinic, Biochemistry and Pathology Departments, Faculty of Medicine, Menoufia University, Egypt, who kindly helped throughout this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Abdou AG, Maraee AH, Antar AG, Fareed S. Role of mast cells in skin tag development: an immunohistochemical study. Anal Quant Cytopathol Histpathol. 2014;36(4):222–230.
2. Allegue F, Fachal C, Perez-Perez L. Friction induced skin tags. Dermatol Online J. 2008;14(3):18.
3. Kahana M, Grossman E, Feinstein A, Ronnen M, Cohen M, Millet MS. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol. 1987;67(2):175–177.
4. Ben-Shlomo A, Melmed S. Skin manifestations in acromegaly. Clin Dermatol. 2006;24(4):256–259. doi:10.1016/j.clindermatol.2006.04.011
5. Savage DB, Semple RK, Chatterjee VK, Wales JK, Ross RJ, O’Rahilly S. A clinical approach to severe insulin resistance. Endocr Dev. 2007;11:122–132. doi:10.1159/000111067
6. Keating GM. Mecasermin. BioDrugs. 2008;22(3):177–188. doi:10.2165/00063030-200822030-00004
7. Yilmaz A, Davis ME, Simmen RC. Reproductive performance of bulls divergently selected on the basis of blood serum insulin-like growth factor I concentration. J Anim Sci. 1999;77(4):835–839. doi:10.2527/1999.774835x
8. Kwasniewski W, Gozdzicka-Jozefiak A, Kotarska M, et al. Analysis of cytosine‑adenine repeats in P1 promoter region of IGF‑1 gene in peripheral blood cells and cervical tissue samples of females with cervical intraepithelial lesions and squamous cervical cancer. Mol Med Rep. 2015;11:766–774. doi:10.3892/mmr.2014.2916
9. Tasli L, Turgut S, Kacar N, et al. Insulin-like growth factor-I gene polymorphism in acne vulgaris. J Eur Acad Dermatol Venereol. 2011;27:254–257. doi:10.1111/j.1468-3083.2011.04299.x
10. Rahaman SMA, Dipankar D, Handa S, et al. Association of insulin-like growth factor (IGF)-1 gene polymorphisms with plasma levels of IGF-1 and acne severity. J Am Acad Dermatol. 2016;75(4):768–773. doi:10.1016/j.jaad.2016.05.019
11. Yaylali GF, Akin F, Turgut S, Kursunluoglu R. IGF-1 gene polymorphism in obese patients with insulin resistance. Mol Biol Rep. 2010;37(1):529–533. doi:10.1007/s11033-009-9729-6
12. Yosry A, Omran D, Yousef M, et al. SNPs in the insulin-like growth factor gene and obesity impact on colorectal cancer in Egyptians. Asian Pac J Cancer Prev. 2017;18(11):2959–2964. doi:10.22034/APJCP.2017.18.11.2959
13. Bosseila M, Shaker O. The tissue expression of Insulin-Like Growth Factor (IGF-1) in acrochordons. J Egypt wom Dermatol Soc. 2007;4(2):557–562.
14. Jowkar F, Fallahi A, Namazi MR. Is there any relation between serum insulin and insulin-like growth factor-I in non-diabetic patients with skin tag? J Eur Acad Dermatol Venereol. 2010;24:73–74. doi:10.1111/j.1468-3083.2009.03268.x
15. Garrow JS, Webster J. Quetelet’s index (W/H2) as a measure of fatness. Int J Obes. 1985;9(2):147–153.
16. Kursunluoglu R, Turgut S, Akin F. Insulin-like growth factor-I gene and insulin-like growth factor binding protein-3 polymorphism in patients with thyroid dysfunction. J Archmed. 2009;40(1):42–47.
17. Jusuf NK, Putra IB, Kartayana J. The correlation between body mass index with the occurrence of skin tag. Open Access Maced J Med Sci. 2017;5(3):271–274.
18. Platsidaki E, Vasalou V, Gerodimou M, et al. The association of various metabolic parameters with multiple skin tags. J Clin Aesthet Dermatol. 2018;11(10):40–43.
19. Farag AGA, Badr EAE, Eltorgoman AA, et al. Role of 11HSD 1, rs12086634, and rs846910 single-nucleotide polymorphisms in metabolic-related skin diseases: a clinical, biochemical, and genetic study. Clin Cosmet Investig Dermatol. 2019;12:91–102. doi:10.2147/CCID.S193156
20. Agamia NF, Gomaa SH. Assessment of serum leptin, atherogenic lipids, glucose level, insulin resistance and metabolic syndrome in patients with skin tags. Egypt J Dermatol Venerol. 2014;34:58–64. doi:10.4103/1110-6530.137314
21. Tamega AA, Aranha AM, Guiotoku MM, et al. Association between skin tags and insulin resistance. An Bras Dermatol. 2010;85(1):25–31.
22. Shahjee HM, Bhattacharyya N. Activation of various downstream signaling molecules by IGFBP-3. J Cancer Ther. 2014;5(9):830–835. doi:10.4236/jct.2014.59091
23. Gorpelioglu C, Erdal E, Ardicoglu Y. Adam B and Sarifakioglu E. Serum leptin atherogenic lipids and glucose levels in patients with skin tag. Indian J Dermatol. 2009;54(1):20–22. doi:10.4103/0019-5154.48980
24. Miura H, Sano S, Higashiyama M, Yoshikawa K, Itami S. Involvement of insulin like growth factor-1 in psoriasis as a paracrine growth factor: dermal fibroblasts play a regulatory role in developing psoriatic lesions. Arch Dermatol Res. 2000;292(12):590–597.
25. Hodak E, Gottlieb AB, Anzilotti M, Krueger JG. The insulin-like growth factor 1 receptor is expressed by epithelial cells with proliferative potential in human epidermis and skin appendages: correlation of increased expression with epidermal hyperplasia. J Invest Dermatol. 1996;106(3):564–570.
26. Hu ZC, Tang B, Guo D, et al. Expression of insulin-like growth factor-1 receptor in keloid and hypertrophic scar. Clin Exp Dermatol. 2014;39(7):822–828. doi:10.1111/ced.12407
27. Feik E, Baierl A, Hieger B, et al. Association of IGF1 and IGFBP3 polymorphisms with colorectal polyps and colorectal cancer risk. Cancer Causes Control. 2010;21:91–97. doi:10.1007/s10552-009-9438-4
28. Napolitano M, Megna M, Monfrecola G. Insulin resistance and skin diseases. Sci World J. 2015;2015:479354. doi:10.1155/2015/479354
29. Clark CM, Rudolph J, Gerber DA, Glick S, Shalita AR, Lowenstein EJ. Dermatologic manifestations of hyperandrogenism: a retrospective chart review. Skin med. 2014;12(2):84–88.
30. Rasi A, Soltani-Arabshashi R, Shahbazi N. Skin tag as a cutaneous marker for impaired carbohydrate metabolism: a case control study. Int J Dermatol. 2007;46(11):1155–1159. doi:10.1111/j.1365-4632.2007.03287.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.