Back to Journals » OncoTargets and Therapy » Volume 13
Role of GLUT-1 in the Upregulation of PD-L1 Expression After Radiotherapy and Association of PD-L1 with Favourable Overall Survival in Hypopharyngeal Cancer
Authors Shen LF, Zhou SH , Guo Y
Received 7 July 2020
Accepted for publication 22 September 2020
Published 3 November 2020 Volume 2020:13 Pages 11221—11235
DOI https://doi.org/10.2147/OTT.S269767
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Li-Fang Shen, Shui-Hong Zhou, Yu Guo
Department of Otolaryngology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Shui-Hong Zhou
Department of Otolaryngology, The First Affiliated Hospital, College of Medicine, Zhejiang University, 79# Qingchun Road, Hangzhou City, Zhejiang Province, People’s Republic of China
Tel +86-571-87236894
Fax +86-571-87236895
Email [email protected]
Purpose: The alteration of tumor immunity after radiotherapy (RT) has been widely studied in recent years. However, the mechanism through which RT mediates tumor immunity and the involvement of glycolysis in this mediation in hypopharyngeal cancer remain unclear. This study investigated whether RT regulates programmed cell death ligand 1(PD-L1) partly via glucose transporter 1(GLUT-1) expression and whether PD-L1 expression predicts overall survival (OS) in patients with hypopharyngeal cancer.
Methods: The expression of PD-L1 and Glut-1, and the numbers of CD4+, CD8+ T cells were detected by immunohistochemical analysis on 47 pre-RT and 25 post-RT specimens of hypopharyngeal cancer. Changes in these indicators before and after RT were compared, and their association with the OS of patients was analyzed. Moreover, we used siRNA-GLUT-1 to inhibit GLUT-1 expression and examined whether GLUT-1 was a key factor involved in the mediation of PD-L1 expression by RT in vitro.
Results: In the multivariate analysis, patients with higher PD-L1 expression (p=0.037), higher CD4+ T cell infiltration (p=0.016) and earlier clinical stage (p=0.019) had favourable OS. The expression of PD-L1, and the CD4+ and CD8+ T cells was markedly increased following RT. PD-L1 expression was correlated with Glut-1 pre-RT (p=0.002), but not after RT (p=0.051). The expression of PD-L1 in FaDu cells was upregulated after RT, especially at 96h after RT in vitro. However, the expression of PD-L1 in siRNA-GLUT-1 FaDu cells was markedly decreased at 96h after RT compared with that measured in FaDu cells.
Conclusion: Patients with high PD-L1 expression and CD4+ T cell infiltration may have favourable OS in hypopharyngeal cancer. RT may increase PD-L1 expression and alter tumor immunity. The expression of PD-L1 was correlated with Glut-1, and inhibition of GLUT-1 expression may decrease the expression of PD-L1. GLUT-1 may participate in the alteration of tumor immunity after RT.
Keywords: glucose transporter 1, programmed cell death ligand 1, radiotherapy, prognosis, hypopharyngeal cancer
Introduction
Head and neck squamous cell carcinoma (HNSCC) represents about 6% of all cancer cases, and accounts for approximately 644,000 new cases and 352,000 cancer-related deaths annually worldwide.1 Despite comprehensive treatments such as surgery, RT, and chemotherapy, the 5-year survival rate of patients with HNSCC has not markedly improved. Since 1975, the 5-year survival of patients with laryngeal cancer has decreased from 67% to 61%, while that of patients with oral cavity and pharynx cancer has increased merely from 53% to 63%.2 Therefore, there is an urgent need for more effective treatments. Recently, targeted therapy for programmed cell death 1 (PD-1) and PD-L1 has shown enormous prospects for the treatment of tumors. Even after failure of traditional therapies, immune checkpoints blockade treatments may prolong the progression-free survival of patients with advanced-stage disease by more than 2 years.3 Despite encouraging results, numerous patients with cancer exhibit low response rates to immune checkpoint blockade treatment. For example, the response rate to anti-PD-1/PD-L1 therapy in HNSCC is only 18%-25%.4,5 Combination of other treatment modalities, including RT, chemotherapy, other immunotherapy and targeted therapy, has become a commonly used strategy for HNSCC to overcome the unsatisfactory respond rates.
Preclinical studies have shown that RT could upregulate PD-L1 expression in tumor cells, and RT and anti-PD-L1 therapy have a synergistic antitumor effect.6 In clinical studies, patients with lung7,8 and mammary cancer9 who received RT prior to anti-PD-L1 therapy had a better prognosis than those who did not. However, knowledge on the upregulation of PD-L1 following RT in hypopharyngeal cancer is scarce,10 leading us to investigate the expression of PD-L1 after RT in this setting. Moreover, there is little knowledge on the mechanism through which RT-mediated immune responses alter the tumor immunity. The alteration of PD-1/PD-L1 expression following RT is dependent on multiple factors such as signaling cascades, prevalence of somatic mutation, individual genetic background, and tumor environment, therefore such alterations cannot be generalized. Previous studies have found that tumor glycolysis and tumor immune evasion are interdependent. Glycolytic activity was a strong predictor of tumor immunity in a number of cancers, and glycolysis increased the expression of PD-L1 in tumors.11 Li et al assessed the expression of Glut-1 and hexokinase 2(HK-2) in metabolic reprogramming after irradiation. GLUT-1 controls glucose uptake and HK-2 encodes the key kinase involved in glycolysis. They found that after irradiation the expression of these two genes declined whereas that of PD-1 was elevated in both activated CD4+ and CD8+ populations compared with those observed in unirradiated wild-type C57BL/6 male mice.12 Nevertheless, the mechanism through which irradiation mediates tumor immunity in hypopharyngeal cancer and whether glycolysis is involved in this mediation warrant further investigation.
Moreover, the level of PD-L1 expression has been associated with the therapeutic response and prognosis in diverse types of cancers. Jiang et al demonstrated that positive PD-L1 expression was associated with a longer survival in patients with esophageal squamous cell carcinoma (ESCC) who underwent RT. They found that patients with high PD-L1 expression had increased infiltration of tumor-infiltrating lymphocytes (TILs) and highly immunogenic tumors prior to RT. Hence, high PD-L1 expression was an independent predictor of favourable prognosis for patients with ESCC.13 However, the data on the prognostic value of PD-L1 expression in hypopharyngeal cancer remain limited. Thus, it is important to demonstrate the clinical significance of PD-L1 expression in patients with hypopharyngeal cancer.
The present study focused on the immune-related changes of the tumor microenvironment following RT in patients with hypopharyngeal cancer. In addition, the study examined whether RT regulates PD-L1 expression partly via expression of GLUT-1 and assessed the association of PD-L1 expression with OS in patients with hypopharyngeal cancer.
Materials and Methods
Ethics Statement
The study was approved by the appropriate institutional committee and performed in accordance with the ethical standards established by the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. The institutional review board of The First Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, Zhejiang, China) approved the present study (reference number 2020–1020). Written informed consent was provided by all participants included in the study.
Clinical Data
We collected formalin-fixed paraffin-embedded tissues from 61 patients with locally advanced hypopharyngeal cancer treated with (chemo)radiotherapy and surgery at the First Affiliated Hospital, College of Medicine, Zhejiang University between 2008 and 2017. Of those, 14 patients were subsequently excluded due to the unavailability of tumor samples or missing clinical data. The exclusion criteria were: patients with distant metastasis at diagnosis, patients who received preoperative targeted therapy, immune therapy or chemotherapy and those with more than one malignancy.
All patient data were reviewed for the following baseline characteristics: sex, age, primary tumor histologic subtype, tumor location, and clinical stages. RT parameters were also recorded, including total dose, dose per fraction, and time between RT and sample resection.
Follow-Up
OS data for patients were obtained by outpatient service or telephone. OS was determined from the date of diagnosis to that of patient death. Follow-up examination was performed on a monthly basis during year 1, every 3 months during year 2, and every 6 months thereafter. In addition to routine physical examinations, patients underwent laryngoscopy, cervical computed tomography or magnetic resonance imaging (MRI), or whole body positron emission tomography/computed tomography (PET/CT).
Immunohistochemical Analysis and Evaluation
We analyzed the pre- and post-RT specimens. The expression of PD-L1 and Glut-1, as well as the numbers of CD4+ and CD8+ T cells in the tumor tissues were detected by immunohistochemistry in 47 cases of hypopharyngeal cancer. Formalin-fixed paraffin-embedded specimens were obtained from the predominant lesions of each participant. Immunohistochemical staining was performed to detect the expression of PD-L1 (1:100 dilution; catalog number: 66248-1-Ig, Proteintech, Chicago, IL, USA) and Glut-1 (dilution, 1:100; catalog number: ab14683; Abcam, Cambridge, UK) in tumor cells, as well as the number of CD4+ T cells (1:50 dilution; catalog number: DF6451, Affinity) and CD8+ T cells (1:200 dilution; catalog number: 66868-1-1, Proteintech) in the tumor specimens. Serial sections (4µm) subjected to immunohistological staining were fixed with 3% hydrogen peroxide, and treated with antigen retrieval solution for 15 min. The sections were incubated with a monoclonal antibody for 30 min at 36‒38°C, followed by incubation with a secondary antibody (K5007, Dako) incubation for 15 min at 20‒25°C. The final reaction product was developed by exposure to 0.03% diaminobenzidine, and the nuclei were counterstained with hematoxylin.
We scored the percentage of tumor cells that were positively stained for PD-L1, in 5% increments, and used a semiquantitative scoring method to evaluate the expression of PD-L1, high PD-L1 expression was defined at as minimum of 10% stained cells.
The staining intensity of Glut-1 was classified as no staining, weak, moderate, and strong (scores 0, 1, 2, or 3, respectively). The percentage of stained cells was classified as follows: 0‒25% stained cells (score 1), 26‒50% stained cells (score 2), 51‒75% stained cells (score 3), and >75% stained cells (score 4). The expression of Glut-1 was assessed semi-quantitatively using the product of these scores (intensity × percentage of stained cells): 0‒5 points denoted negative expression, whereas 6‒12 points denoted positive expression.
We counted the numbers of CD4+ and CD8+ T cells in selected hotspot under 400×magnification to evaluate their infiltration in tumors. We selected the median number of CD4+ and CD8+ T cells as the cut-off point for their cell density. Two experienced pathologists independently calculated the staining intensity and the percentage of stained cells.
Cell Culture and Reagents
The FaDu cell line was purchased from the Cell Research Institute of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (Sigma, USA), supplemented with 100 µg/mL streptomycin, 100 U/mL penicillin (Gibco, USA) and 10% heat-inactivated fetal calf serum, at 37°C in a 5% CO2 atmosphere.
Sequences of the GLUT-1 (Gene ID 6513) and PD-L1 (Gene ID 533834) entire coding regions were obtained from GenBank, and primers were designed using the ClustalX and the Omega 2.0 software. The high-purity total RNA rapid extraction kit was purchased from Generay (catalog number: GK3016, Batch: 1703G01), the reverse transcription kit HiScript-II Q RT SuperMix for quantitative polymerase chain reaction (qPCR) was purchased from Vazyme (catalog number: R222-01, Batch: 7E092G6), PrimeScriptTM RT reagent Kit was purchased from TaKaRa (catalog number: RR037A, Batch: AK5302-1), and the qPCR reagent ChamQ SYBR Color qPCR Master Mix was purchased from Vazyme (catalog number: Q411-02, Batch: 7E092H6). The qPCR instrument was the CFX connect Real-Time PCR System. Polyvinylidene difluoride membrane was purchased from Millipore (catalog number: IPVH00010, Batch: K5JA5013L).
Tumor Cell Line Irradiation
To determine whether GLUT-1 is a key factor involved in the mediation of the tumor immune microenvironment by RT, we inhibited its expression using GLUT-1 siRNA. GLUT-1 siRNA was purchased from GenePharma Co. Ltd. (Shanghai, China). The sequences were sense, 5ʹ-GGAAUUCAAUGCUGAUGAUTT-3ʹ; antisense, 5ʹ-AUCAUCAGCAUUGAAUUCCTT-3ʹ. We performed the GLUT-1-siRNA transfection when the cells reached 50% confluency. The FaDu cells and siRNA-GLUT-1 FaDu cells were both seeded at a density of 10,000–20,000 per 25 cm2 and were divided into four groups respectively: control group, and 24-h, 48-h, 96-h groups. Tumor cells were subjected to irradiation after resting overnight, except for the control group. RT was performed using an X-ray generator (22.7 mA, 120 kV, variable time; GE Inspection Technologies, Germany) with a single dose of 10 Gray on day 1. The tumor cells of the 24-h, 48-h, 96-h groups were harvested at 24 h, 48 h and 96 h after RT, respectively. The expression of PD-L1 and GLUT-1 in these tumor cells was subsequently analyzed. The control group tumor cells were harvested and analyzed on day2.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
The PCR primers used were as follows: GLUT-1 sense, 5′-GTCAACACGGCC TTCACTG-3′, GLUT-1 antisense, 5′-GGTCATGAGTATGGCACAACC-3′ (111 bp), PD-L1 sense, 5′-TTACAGCAGCCAGACGATCA-3′, PD-L1 antisense, 5′-CCCTGC AGTAGGTTTCTGCT-3′(233 bp). GAPDH sense, 5′-TGTTGCCATCAATGACCCCTT-3′, GAPDH antisense, 5′-CTCCACGACGTACTCAGCG-3′ (202bp). The specific steps were previously described.14 The 2−ΔΔCt formula was used for the calculation of differential gene expression.
Western Blotting
Tumor cells were lysed in radio immunoprecipitation assay lysis solution separated by gel electrophoresis and transferred onto membranes. The membranes were blocked with 5% non-fat dry milk in tris-buffered saline with Tween and incubated with the primary antibody at 4°C overnight (PD-L1 1:800 dilution, Art No: 66248-1-1g, Proteintech, Chicago, IL, USA), (GLUT-1 1:800 dilution, Art no: 20960-1-AP, Proteintech, Chicago, IL, USA). Subsequently, the membranes were incubated with the secondary antibody at room temperature for 2 h. Enhanced chemiluminescence was used to visualize the proteins through exposure to X-ray film. Protein expression was analyzed semi-quantitatively using the ChemiDoc XRS+ System (Bio-RAD, USA).
Statistical Analysis
The SPSS software (version. 22.0; IBM Corp., Armonk, NY, USA) was used for statistical analyses. Categorical variables were assessed using the χ2 or Fisher’s exact tests. Correlation analyses were performed using Spearman’s rank analysis. Changes in PD-L1and Glut-1 expression, as well as CD4+ and CD8+ T cells prior to and after RT, were tested using Student’s t-test. The Kaplan–Meier method and Log rank test were used to calculate survival curves and compare the results. The Cox proportional hazards regression model was used for multivariate analysis. P values <0.05 indicated statistical significance.
Results
Expression of PD‑L1 and Glut-1, and Infiltration of CD4+ and CD8+ T Cells in Hypopharyngeal Cancer at Baseline (Pre-RT)
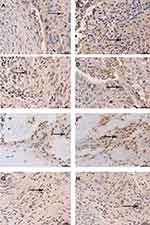
PD-L1 was mainly expressed on the membranes (cell surface) and partially in the cytoplasm of tumor cells, and its expression exhibited heterogeneity in the specimens, a cutoff of 10% was used to define “low” vs “high” expression. Prior to RT, the percentage of stained tumor cells in the patient population ranged 3.7s–23.1%. Of the patients, 53.2% (25/47) had high PD-L1 expression. Glut-1 was expressed on the membranes of tumor cells, and its expression was positive in 51.1% (24/47) of the patients. Based on the median number of CD4+ and CD8+ cells, respectively, cutoff values of 8% and 25% were used to define “low” versus “high” cells infiltration, respectively. Prior to RT, the percentage of CD4+ T cells in the tumor tissues ranged 2.18–28.0%. Of the patients, 46.8% (22/47) had high CD4+ T cell infiltration, while the percentage of CD8+ T cells in the tumor tissues prior to RT ranged 11.9–48.9%, 40.4% (19/47) of the patients had high CD8+ T cell infiltration (Figure 1).
Associations of PD‑L1 and Glut-1 Expression, and Infiltration of CD4+ and CD8+ T Cells with Clinicopathological Factors in Hypopharyngeal Cancer
All 47 patients with hypopharyngeal cancer were males (median age 64 years, range, 44–88 years). The clinical stage was classified according to the National Comprehensive Cancer Network (NCCN) guidelines, 23 and 24 patients had clinical stage III and IV disease, respectively. Seventeen patients underwent surgery and postoperative (chemo)radiotherapy (50–70Gy), and 11 of 17 patients received chemotherapy with cis-platinum or nedaplatin. Only five patients received RT following the biopsy, and the time interval between the surgery and RT was 30.6 days. Twenty-five patients underwent biopsy and adjuvant RT (30–50Gy), followed by surgery, the time interval between the end of RT and cancer resection was 62.8 days. The follow-up time ranged 6‒59 months (median: 27.5 months). During the follow-up period, 25 patients expired (53.2%). The median OS time was 35.3 months (95% confidence interval (CI), 29.5–41.0 months). Other clinicopathological characteristics of patients are summarized in Table 1.
 |
Table 1 Patient Characteristics |
The expression of PD-L1 was not associated with the clinicopathological factors, including age, clinical stages, histologic grade and the tumor location. Furthermore, the expression of Glut-1 and numbers of CD4+ and CD8+ T cells in the tumor tissue did not show a significant association with these clinicopathological factors.
PD-L1 Expression, CD4+ T Cells Infiltration and Clinical Stage Were Associated with OS of Patients with Hypopharyngeal Cancer
The OS of patients with hypopharyngeal cancer was assessed based on the expression of PD-L1 and Glut-1, as well as CD4+ and CD8+ T cells infiltration at baseline (Pre-RT).
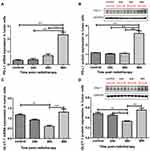
The OS of patients with high and low PD-L1 expression was 41.9 months (95% CI, 33.6–50.2 months) and 25.5 months (95% CI, 21.1–29.9 months), respectively. The OS was significantly different between patients with high and low PD-L1 expression, as presented by the Kaplan-Meier analysis (p=0.0025, Figure 2A).
The OS of patients with high and low Glut-1 expression was 37.9 months (95% CI, 35.7–40.6 months) and 36.2 months (95% CI, 31.4.2–41.2 months), respectively. The two groups of patients did not exhibit significant differences in OS, as determined by Kaplan-Meier analysis (p=0.45).
The OS of patients with high and low numbers of CD4+ T cells was 45.8 months (95% CI, 36.6–55.0 months) and 26.0 months (95% CI, 21.6–30.4 months), respectively. The OS of patients with high CD4+ T cell infiltration was significantly longer than that of patients with low CD4+ T cells infiltration (p<0.001, Figure 2B).
The OS of patients with high and low numbers of CD8+ T cells was 39.2 months (95% CI, 30.4–48.1 months) and 35.7 months (95% CI, 28.1–43.3 months), respectively. There was no significant difference between the two groups (p=0.07, Figure 2C). However, there was a tendency for patients with high numbers of CD8+ T cells to have a longer OS versus those with low numbers of CD8+ T cells.
The OS of patients with clinical stages III and IV hypopharyngeal cancer was 43.0 months (95% CI, 34.0–51.9 months) and 29.7 months (95% CI, 22.5–36.9 months), respectively. Kaplan–Meier curve analysis showed that patients with clinical stage III disease had a longer OS than those with clinical stage IV disease (p=0.038). However, age (p=0.929), histologic grade (p=0.249) and tumor location (p=0.334) did not show a correlation with the OS of patients with HNSCC.
Multivariate Analyses of Factors Affecting OS in Patients with Hypopharyngeal Cancer Patients
Multivariate Cox regression hazards analysis demonstrated that low PD-L1 expression (hazard ratio [HR]: 3.48; 95% CI: 1.1–11.2; p=0.037), low CD4+ T cell infiltration (HR: 4.30; 95% CI: 1.31–14.7; p=0.016), and advanced clinical stage (HR: 3.33; 95% CI: 1.22–9.09; p=0.019) were independent prognostic factors in patients with hypopharyngeal cancer (Table 2). Patients with low PD-L1 expression, low CD4+ T cell infiltration, and advanced clinical stage (stage IV) had shorter survival than those with high PD-L1 expression, high CD4+ T cell infiltration, and early clinical stage (stage III). However, GLUT1 expression (HR: 0.95; 95% CI: 0.35–2.57; p=0.927) and CD8+ T cell infiltration status (HR: 2.48; 95% CI: 0.72–8.55; p=0.149) were not significant independent prognostic factors.
 |
Table 2 Multivariate Analysis of Overall Survival in Patients with Hypopharyngeal Cancer |
PD-L1 Expression and the Numbers of CD4+ and CD8+ T Cells Increased After RT, and RT Influenced Tumor Immunity
The expression of PD-L1 in hypopharyngeal cancer was evaluated in each patient pre-RT and/or post-RT. A total of 47 pre-RT biopsies or surgical specimens and 25 post-RT specimens were available. The expression of PD-L1 in tumor cells was obviously increased after RT with statistical significance. In the pre-RT specimens, the proportion of tumor cells with high PD-L1 expression was only 11.4%. This proportion was obviously increased after RT, reaching 19.8% (p=0.003) (Figures 1B and 3A).
GLUT1 expression, as well as CD4+ and CD8+ T cell infiltration, were also investigated in pre-RT and post-RT specimens. CD4+ T cell infiltration in tumor tissues was markedly increased after RT with statistical significance. For the pre-RT specimens, the proportion of CD4+ T cells was only 8.6%; this proportion was markedly increased after RT, reaching 21.0% (p<0.001) (Figure 3B). Meanwhile, CD8+ T cell infiltration in tumor tissues was also markedly increased after RT with statistical significance. The proportion of CD8+ T cells in pre-RT specimens was only 24.9% and was increased after RT to 35.7% (p=0.006) (Figure 3C). However, only GLUT1 expression showed a tendency of increase in post-RT specimens compared with pre-RT specimens (p=0.097).
Furthermore, PD-L1 expression was correlated with GLUT1 expression in pre-RT specimens and showed a tendency towards correlation with the expression of GLUT1 in post-RT specimens (p=0.002 and 0.051, respectively) (Figure 3D and E). However, the CD4+ and CD8+ T cell infiltration status was not correlated with the expression of GLUT1 in pre-RT or post-RT specimens.
RT Induced Upregulation of PD-L1 in FaDu Cells in vitro, and GLUT-1 May Be a Key Factor of This Mechanism
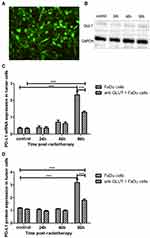
FaDu cells were treated with a single fraction of 10 Gy in vitro to study the influence of RT on the expression of PD-L1. The mRNA expression of PD-L1 in FaDu cells was upregulated after 10 Gy of RT compared with the control group; this upregulation was most significant at 96 h after RT (p<0.001) (Figure 4A). The protein expression of PD-L1 in FaDu cells was also upregulated following RT and was significantly higher than that observed in the control group at 96 h after RT (p<0.001) (Figure 4B).
Following RT, the mRNA expression of GLUT1 in FaDu cells was initially decreased and subsequently increased. It was decreased at 48 h after RT compared with the control group (p<0.01); it was significantly increased at 96 h after RT compared with 48 h after RT (p<0.001) (Figure 4C). The alteration in the protein expression of GLUT1 reflected the change observed in the mRNA expression of GLUT1. The protein expression of GLUT1 in FaDu cells was decreased at 48 h after RT compared with the control group and the 24-h post-RT group (p<0.01 and p<0.05, respectively). The level of protein expression was significantly increased at 96 h after RT compared with 48 h after RT (p<0.001) (Figure 4D).
We used GLUT1 siRNA to inhibit the expression of GLUT1 (Figure 5A and B). In the siRNA-GLUT1 FaDu cells, the mRNA and protein expression levels of PD-L1 were also significantly upregulated at 96 h after RT compared with those measured in the control group (p<0.01 and p<0.001, respectively). More importantly, at 96 h after RT, the mRNA and protein expression levels of PD-L1 in siRNA-GLUT1 FaDu cells was significantly decreased compared with those recorded in FaDu cells, as presented by the two-way analysis of variance (p<0.001 and p<0.001, respectively) (Figure 5C and D). This result demonstrated that inhibiting the expression of GLUT1 could interfere with the increase in PD-L1 expression after RT.
Discussion
In 2016, the Food and Drug Administration approved the use of anti-PD-1 monoclonal antibodies pembrolizumab and nivolumab for the treatment of HNSCC, turning a new page in the treatment of HNSCC. More recently, the Food and Drug Administration approved the use of anti-PD-1/PD-L1 drugs for head and neck cancers, and there are numerous clinical trials conducted worldwide. However, the limitations of this therapy have also emerged, including the development of drug resistance. Therefore, combination therapy has been adopted to improve the efficacy of treatment.15
Several studies have examined the expression of PD-L1 in HNSCC and found that its expression levels ranged 46–100%, depending on the fixation, staining method and site.16–18 However, there is limited research on hypopharyngeal cancer. In our study, we found that PD-L1 was expressed in 100% of patients with hypopharyngeal cancer. The percentage of stained tumor cells in this patient population prior to RT ranged 3.7–23.1%.
In our study, PD-L1 expression and CD4+ T cell infiltration in tumor tissue were linked to the OS of patients with hypopharyngeal cancer. Multivariate analysis revealed that high PD-L1 expression, high CD4+ T cell infiltration, and early clinical stage were independent prognostic factors of favorable OS in patients with hypopharyngeal cancer. According to our findings, Fukushima et al demonstrated that in oropharyngeal squamous cell carcinoma, patients with high PD-L1 expression in tumor cells had a favourable outcome than those with low PD-L1 expression in terms of progression-free survival (PFS) and OS.19 Vassilakopoulou et al showed that TILs density was associated with PD-L1 protein expression in tumor cells of patients with laryngeal squamous cell carcinoma. High TILs and high PD-L1 expression were independently associated with better OS and disease-free survival in the multivariate analysis.20 Liu et al showed that low PD-L1 expression in tumor cells is significantly correlated with local recurrence in Epstein–Barr virus positive nasopharyngeal carcinoma patients after RT.21 Combined low PD-L1 expression in inflammatory cells and tumor cells is an independent negative prognostic factor for OS in patients with rectal adenocarcinoma.22 Adversely, Lin et al found that high PD-L1 expression was associated with poor outcome and metastasis in oral squamous cell carcinoma.23 Furthermore, several clinical reports suggested that a high density of TILs was associated with favorable prognosis in patients with locally advanced non-small cell lung cancer24 and breast cancer.25
As mentioned above, high PD-L1 expression was correlated with favorable and unfavorable prognosis in different tumors and different studies. This may be attributed to the use of different cutoffs to determine PD-L1 positivity, as well as the heterogeneity of the clinical and pathological features in diverse tumors. Future studies should develop a standardized protocol for evaluating PD-L1 expression and include larger numbers of patients.
In addition to its cytotoxic effect, RT exerts an anti-tumor immune effect.26 The effects of RT on the tumor microenvironment and its interaction with tumor immunity is a complex balance of suppressing and activating signals.27 Evidence has shown that RT may enhance the therapeutic effects of anti-PD-1/PD-L1 agents in HNSCC.28 Nevertheless, many questions remain regarding the mechanism through which RT affects tumor immunity in hypopharyngeal cancer.
In fact, RT plays a role in the recruitment of T cells in the tumor microenvironment29 and increases PD-L1 expression in tumors.30 However, knowledge on the upregulation of PD-L1 expression and TILs recruitment in tumor cells following exposure to RT in HNSCC, especially in hypopharyngeal cancer, is scarce. Our study demonstrated that PD-L1 expression was increased after RT in patients with hypopharyngeal cancer. Moreover, in vitro experiments revealed that PD-L1 expression was increased after RT at 24, 48 and 96 h, the expression of PD-L1 reached its highest value at 96 h after RT. For cytotoxic T lymphocytes, we found that the CD4+, CD8+ TILs increased after RT in patients with hypopharyngeal cancer, RT could transform the tumor microenvironment. The increase in TILs following RT demonstrated that RT can activate the immune system. Thus, our data suggest that RT induces favorable changes within the tumor microenvironment in hypopharyngeal cancer for additional blockade of the PD-1/PD-L1 axis.
Similar to our study, other preclinical studies have demonstrated that RT could upregulate PD-L1 expression in tumor cells, and RT and PD-L1 blockade had a synergistic antitumor effect on MC38 colon adenocarcinoma and TUBO mammary carcinoma.30,31 Herter-Sprie et al observed that the CD8+/regulatory T ratio increased 96 h after RT in Kras-mutant lung cancer.32 Clinical data also demonstrated that RT is correlated with an increased PD-L1 expression in rectal adenocarcinoma22 and locally advanced esophageal adenocarcinoma.33 Similar to our results, Keung et al found that the PD-L1 expression in undifferentiated pleomorphic sarcoma of the extremity and trunk increased after irradiation and also recognized an increase in the median number of CD4+, CD8+ T cells after RT. Moreover, although PD-L1 was not expressed at baseline, positive PD-L1 expression was observed in 21% (3/14) of undifferentiated pleomorphic sarcoma of the extremity and trunk tumor cells after RT.34
Oweida et al demonstrated that RT could transform the immune landscape of tumors and render poorly immunogenic murine orthotopic HNSCC sensitive to anti-PD-L1 drugs.35 Indeed, a number of preclinical and clinical studies showed that RT and immunotherapy exert a synergistic anti-tumor effect in other tumors.6,30,36
Therefore, changes in the immune microenvironment after RT render the tumor more sensitive to immunotherapy. However, the mechanism through which RT interferes with the tumor immune microenvironment remains unclear and warrants further investigation.
The alteration of PD-1/PD-L1 expression after RT is dependent on multiple factors, such as the signaling cascades, individual genetic background, prevalence of somatic mutation, and tumor microenvironment, thus, it cannot be generalized. RT kills tumor cells through free radical-induced DNA damage and also promotes metabolic changes in tumor cells (metabolic reprogramming). In metabolic reprogramming, the activity and expression of enzymes and their regulators involved in the metabolic activities of tumor cells are altered, involving multiple metabolic pathways, with the most important being the glycolysis pathway. Aerobic glycolysis and immune escape are two major features of tumors.37 The dependence of immune cell proliferation and activity on cell metabolism has received increasing attention.38
GLUT is an important energy transporter that mediates the Warburg effect. The glucose transporter is a protein that mediates the transmembrane transport of glucose, which is a major reason for the increased glucose metabolism observed in malignant tumor cells. GLUT-1 is a representative protein of the GLUT family and widely expressed in cells of many body tissues. Our study demonstrated that GLUT-1 expression increased in post-RT specimens compared with pre-RT, but not significantly. Moreover, the expression of GLUT-1 was correlated with the expression of PD-L1 in pre-RT specimens; after RT, GLUT-1 expression tended to correlation with PD-L1 expression. Furthermore, in vitro experiments detected a significant decline in GLUT-1 expression 48 h after RT; the expression levels began to increase significantly at 96 h after RT. PD-L1 expression was increased at 96 h after RT. However, following the inhibition of GLUT-1 expression, the expression of PD-L1 in siRNA-GLUT-1 FaDu cells was significantly decreased at 96 h after RT compared with that measured in FaDu cells. This result demonstrated that inhibiting the expression of GLUT-1 could interfere with the increase of PD-L1 expression after RT. Therefore, we hypothesized that changes in PD-L1 expression after RT are related to the tumor glucose metabolism, ie, metabolic reprogramming after RT.
There are currently few research studies on the interaction between glucose metabolism and tumor immunity following RT against tumors. Li et al analyzed the expression levels of checkpoint receptors on T cell populations at multiple post-RT time points ranging 1–4 weeks in mice receiving a single fraction of 1 or 4 Gy. Their results showed that RT resulted in significantly increased expression of PD-1 in both CD8+ and CD4+ populations. They also studied the metabolic reprogramming parameters and found that the expression of Glut1 and HK-2 decreased in activated T cells after RT compared with unirradiated controls.12 In addition, Li et al found that RT influences T cell activation via metabolic reprogramming.39
Previous studies showed that tumor immune evasion and tumor glycolysis are interdependent.40,41 Metabolic reprogramming of tumor cells is an important biochemical basis for tumor immune escape. Enhanced tumor glycolysis attenuates the clearance of tumor cells by immune cells.41 It is increasingly appreciated that the immune cell proliferation and function are dependent on cellular metabolism.38 Recent studies reported that the expression of PD-L1 is regulated by GLUT-1 in clear cell renal cell carcinoma42 and pulmonary pleomorphic carcinoma.43 Chang et al reported that the metabolic competition between tumor cells and immune cells may induce tumor immunosuppression, the competition for glucose in tumor microenvironment could drive cancer progression, it would occur when tumors surpass T cells for glucose supply, and impeded their production of IFN-γ, which is critical for anti-tumor activity.40
Glycolytic activity is a more consistent and stronger predictor of immune signatures in a number of cancers. It increases the effects of anti-PD-1/PD-L1 immunotherapy by enhancing the expression of PD-L1 in tumor cells. Thus, the glycolytic activity of tumor cells could be a predictive factor for response to immunotherapy in diverse types of cancers.11 Many metabolic mechanisms are thought to be related to tumor immune escape and could serve as co-targets in immunotherapy.44
Therefore, we hypothesized that RT can change the tumor immunity, and increase PD-L1 expression and the content of CD4+, CD8+ T cells in hypopharyngeal cancer. The mechanism involved in this process may be that RT interferes with cell glycolysis and alleviates competition for glucose with T cells, metabolic reprogramming after RT is one of the causes of tumor immune changes. Nonetheless, this still warrants further investigation.
The present study had some limitations. Firstly, this was a single-institution retrospective study with a small sample size, rather than a trial-based correlative study. Hence, there was bias due to the small sample size and its retrospective design. Secondly, PD-L1 immunohistochemistry was conducted using only one cutoff value and one antibody, the cutoff values used for high PD-L1expression in the present study were inconsistent with those of some other studies, and there is no unified standard for the positivity of PD-L1 expression. Finally, currently, there is no standardized PD-L1 immunohistochemistry assay available; hence, caution should be exercised in the interpretation of these results.
Conclusion
The present study found that in patients of hypopharyngeal cancer RT could increase the levels of PD-L1 expression, as well as CD4+ and CD8+ T cell infiltration in tumor tissue. This suggested that RT induced favorable changes within the tumor microenvironment in hypopharyngeal cancer for additional blockade of PD-L1. Moreover, PD-L1 expression was associated with GLUT-1 expression, and in vitro experiments showed that inhibiting GLUT-1 expression could interfere with the increase of PD-L1 expression after RT. Therefore, we hypothesized that RT interferes with tumor cell glycolysis and alleviates competition for glucose with T cells. GLUT-1 is a key factor involved in the upregulation of PD-L1 expression after RT. However, this conclusion warrants further investigation. Furthermore, the present study also demonstrated for the first time that high PD-L1 expression and high CD4+ T cell infiltration were associated with a favorable OS in patients with hypopharyngeal cancer.
The English in this document has been checked by at least two professional editors in The Charlesworth Group (http://cwauthors.com/). We have attached a certificate in the supplementary.
Abbreviations
RT, radiotherapy; PD-L1, programmed cell death ligand 1; PD-1, programmed cell death 1; GLUT-1, glucose transporter 1; OS, overall survival; HNSCC, Head and neck squamous cell carcinoma; HK-2, hexokinase 2; ESCC, esophageal squamous cell carcinoma; TILs, Tumor Infiltrating Lymphocytes; MRI, magnetic resonance imaging; RT-PCR, Reverse transcription polymerase chain reaction; RIPA, Radio Immunoprecipitation Assay; NCCN, National Comprehensive Cancer Network.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The institutional review board of The First Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, Zhejiang, China) approved the present study. Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Acknowledgment
We acknowledge everyone who assisted in this research.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81372903 and 81172562).
Disclosure
The authors declare that they have no conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi:10.3322/caac.21349
3. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi:10.1038/nature10673
4. Ferris RL, Blumenschein G
5. Chang YL, Yang CY, Huang YL, Wu CT, Yang PC. High PD-L1 expression is associated with stage IV disease and poorer overall survival in 186 cases of small cell lung cancers. Oncotarget. 2017;8(11):18021–18030. doi:10.18632/oncotarget.14935
6. Filatenkov A, Baker J, Mueller AM, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21(16):3727–3739. doi:10.1158/1078-0432.CCR-14-2824
7. Takamori S, Toyokawa G, Takada K, Shoji F, Okamoto T, Maehara Y. Combination therapy of radiotherapy and anti-PD-1/PD-L1 treatment in non-small-cell lung cancer: a mini-review. Clin Lung Cancer. 2018;19(1):12–16. doi:10.1016/j.cllc.2017.06.015
8. Schoenhals JE, Seyedin SN, Tang C, et al. Preclinical rationale and clinical considerations for radiotherapy plus immunotherapy: going beyond local control. Cancer J. 2016;22(2):130–137. doi:10.1097/PPO.0000000000000181
9. Verbrugge I, Hagekyriakou J, Sharp LL, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72(13):3163–3174. doi:10.1158/0008-5472.CAN-12-0210
10. Ling DC, Bakkenist CJ, Ferris RL, Clump DA. Role of immunotherapy in head and neck cancer. Semin Radiat Oncol. 2018;28(1):12–16. doi:10.1016/j.semradonc.2017.08.009
11. Jiang Z, Liu Z, Li M, Chen C, Wang X. Increased glycolysis correlates with elevated immune activity in tumor immune microenvironment. EBioMedicine. 2019;42:431–442. doi:10.1016/j.ebiom.2019.03.068
12. Li D, Chen R, Wang YW, Fornace AJ
13. Jiang C, Zhu Y, Tang S, et al. High PD-L1 expression is associated with a favorable prognosis in patients with esophageal squamous cell carcinoma undergoing postoperative adjuvant radiotherapy. Oncol Lett. 2019;17(2):1626–1634.
14. Shen LF, Zhao X, Zhou SH, et al. In vivo evaluation of the effects of simultaneous inhibition of GLUT-1 and HIF-1alpha by antisense oligodeoxynucleotides on the radiosensitivity of laryngeal carcinoma using micro 18F-FDG PET/CT. Oncotarget. 2017;8(21):34709–34726. doi:10.18632/oncotarget.16671
15. Yang B, Liu T, Qu Y, et al. Progresses and perspectives of anti-PD-1/PD-L1 antibody therapy in head and neck cancers. Front Oncol. 2018;8:563. doi:10.3389/fonc.2018.00563
16. Ukpo OC, Thorstad WL, Lewis JS
17. Zheng A, Li F, Chen F, et al. PD-L1 promotes head and neck squamous cell carcinoma cell growth through mTOR signaling. Oncol Rep. 2019;1791–2431.
18. Zandberg DP, Strome SE. The role of the PD-L1: PD-1pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50(7):627–632. doi:10.1016/j.oraloncology.2014.04.003
19. Fukushima Y, Someya M, Nakata K, et al. Influence of PD-L1 expression in immune cells on the response to radiation therapy in patients with oropharyngeal squamous cell carcinoma. Radiother Oncol. 2018;129(2):409–414. doi:10.1016/j.radonc.2018.08.023
20. Vassilakopoulou M, Avgeris M, Velcheti V, et al. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. 2016;22(3):704–713. doi:10.1158/1078-0432.CCR-15-1543
21. Liu YJ, Tsang NM, Hsueh C, et al. Low PD-L1 expression strongly correlates with local recurrence in epstein-barr virus-positive nasopharyngeal carcinoma after radiation-based therapy. Cancers (Basel). 2018;10:10. doi:10.3390/cancers10100374
22. Hecht M, Buttner-Herold M, Erlenbach-Wunsch K, et al. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur J Cancer. 2016;65:52–60. doi:10.1016/j.ejca.2016.06.015
23. Lin YM, Sung WW, Hsieh MJ, et al. High PD-L1 expression correlates with metastasis and poor prognosis in oral squamous cell carcinoma. PLoS One. 2015;10(11):e0142656. doi:10.1371/journal.pone.0142656
24. Tokito T, Azuma K, Kawahara A, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14. doi:10.1016/j.ejca.2015.11.020
25. Kos Z, Roblin E, Kim RS, et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer. 2020;6:17. doi:10.1038/s41523-020-0156-0
26. Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi:10.1038/nature14292
27. Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–265. doi:10.1093/jnci/djs629
28. Kikuchi M, Clump DA, Srivastava RM, et al. Preclinical immunoPET/CT imaging using Zr-89-labeled anti-PD-L1 monoclonal antibody for assessing radiation-induced PD-L1 upregulation in head and neck cancer and melanoma. Oncoimmunology. 2017;6(7):e1329071. doi:10.1080/2162402X.2017.1329071
29. Wu CY, Yang LH, Yang HY, et al. Enhanced cancer radiotherapy through immunosuppressive stromal cell destruction in tumors. Clin Cancer Res. 2014;20(3):644–657. doi:10.1158/1078-0432.CCR-13-1334
30. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. doi:10.1172/JCI67313
31. Dovedi SJ, Cheadle EJ, Popple AL, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res. 2017;23(18):5514–5526. doi:10.1158/1078-0432.CCR-16-1673
32. Herter-Sprie GS, Koyama S, Korideck H, et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight. 2016;1(9):e87415. doi:10.1172/jci.insight.87415
33. Kelly RJ, Zaidi AH, Smith MA, et al. The dynamic and transient immune microenvironment in locally advanced esophageal adenocarcinoma post chemoradiation. Ann Surg. 2018;268(6):992–999. doi:10.1097/SLA.0000000000002410
34. Keung EZ, Tsai JW, Ali AM, et al. Analysis of the immune infiltrate in undifferentiated pleomorphic sarcoma of the extremity and trunk in response to radiotherapy: rationale for combination neoadjuvant immune checkpoint inhibition and radiotherapy. Oncoimmunology. 2018;7(2):e1385689. doi:10.1080/2162402X.2017.1385689
35. Oweida A, Lennon S, Calame D, et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology. 2017;6(10):e1356153. doi:10.1080/2162402X.2017.1356153
36. Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831–838. doi:10.1158/2326-6066.CIR-14-0069
37. Gottfried E, Kreutz M, Mackensen A. Tumor metabolism as modulator of immune response and tumor progression. Semin Cancer Biol. 2012;22(4):335–341. doi:10.1016/j.semcancer.2012.02.009
38. Sabharwal SS, Rosen DB, Grein J, et al. GITR agonism enhances cellular metabolism to support CD8(+) T-cell proliferation and effector cytokine production in a mouse tumor model. Cancer Immunol Res. 2018;6(10):1199–1211. doi:10.1158/2326-6066.CIR-17-0632
39. Li HH, Wang YW, Chen R, Zhou B, Ashwell JD, Fornace AJ
40. Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi:10.1016/j.cell.2015.08.016
41. Cascone T, McKenzie JA, Mbofung RM, et al. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 2018;27(5):977–987. doi:10.1016/j.cmet.2018.02.024
42. Ruf M, Moch H, Schraml P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int J Cancer. 2016;139(2):396–403. doi:10.1002/ijc.30077
43. Chang YL, Yang CY, Lin MW, Wu CT, Yang PC. High co-expression of PD-L1 and HIF-1alpha correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur J Cancer. 2016;60:125–135. doi:10.1016/j.ejca.2016.03.012
44. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13(5):273–290.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.