Back to Journals » Journal of Asthma and Allergy » Volume 15
Role of Chemokines and Inflammatory Cells in Respiratory Allergy
Received 8 November 2022
Accepted for publication 5 December 2022
Published 21 December 2022 Volume 2022:15 Pages 1805—1822
DOI https://doi.org/10.2147/JAA.S395490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Youwei Bao, Xinhua Zhu
Department of Otolaryngology Head & Neck Surgery, the Second Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China
Correspondence: Xinhua Zhu, Email [email protected]
Abstract: The idea of “one airway, one disease” has been gaining importance in the last decade. In the upper and lower airways, allergic mechanisms interact with each other. In the initial stage of respiratory allergic inflammation, allergens contact the respiratory epithelium, which produces chemokines and inflammatory factors, which cause allergic reactions by binding to the corresponding receptors and chemotactic various inflammatory cells to reach the epithelium and tissues. It also drives inflammatory cells to activate and produce more inflammatory factors, thus producing a cascade amplification effect. Inflammatory cell aggregation and activation are very complex and interact with each other in a lattice structure. By blocking the action of various chemokines, inflammatory cell aggregation is reduced, and ultimately the symptoms of respiratory allergy are alleviated. Chemokines can serve as cues for coordinated recruitment of immune cells into and out of tissues, as well as directing the spatial organization of immune cells within tissues and cellular interactions. Chemokines are critical in directing immune cell migration and thus have an important role in the direction of respiratory allergy: however, chemokines are also involved in the production and recruitment of immune cells that contribute to respiratory allergy. In this article, linking the upper and lower respiratory tracts. We review the role of the chemokine system in the respiratory immune response and discuss how respiratory disease modulates overall chemokines to shape the type and outcome of the immune response to the treatment of respiratory allergic disease so that we can further deepen our knowledge of chemokines in the direction of respiratory allergy. In the future, we can do drug research and development based on this network structure and explore new research directions.
Keywords: respiratory allergy, allergic rhinitis, allergic asthma, chemokines
Introduction
Chemokines are 8–12 kda secreted proteins that regulate directed cell migration (chemotaxis), adhesion, cellular localization and cell-cell interactions by binding to G Protein-Coupled Receptors (GPCRs), the so-called classical chemokine receptors (Griffithetal). Chemokines bind to seven transmembrane receptors of the atypical chemokine receptors (ACKRs). We currently classify chemokines into four categories: CC, CXC, C and CX3C, determined by the approach of inserting other amino acids between the first two cysteines near the N terminus. The chemokine system consists of approximately 50 chemokine ligands, 20 signaling GPCRs and 4 ACKRs and plays an important role in development, homeostasis, inflammation, infection and pathological processes including immune responses. In recent years airway allergy has gradually been emphasized, and respiratory allergy includes diseases such as allergic rhinitis, allergic asthma, and airway hyperreactivity. All of which are interconnected by allergic pathogenic mechanisms because they belong to the same airway.
Inflammatory cells After allergen contact with the respiratory mucosa, the allergen is presented by the APC to T cells, which differentiate into TC (CD8) cells and TH (CD4) cells. TH cells can differentiate into many TH cells. The most important ones are TH1 and TH2 cells, which secrete many cytokines to produce allergic chain reactions. In addition to CD4+ T cells, other cells such as eosinophils, basophils, lymphocytes, neutrophils, mast cells and monocytes are also involved in this process, with eosinophil infiltration being the main feature and chemokines playing an important role in the activation and migration of these inflammatory cells into the airway. With the discovery of a large number of chemokines and their receptors and the study of their association with the pathogenesis of asthma, chemokines and chemokine receptors have the potential to become targets for anti-inflammatory therapy.
Study of Chemokines and Receptors on Allergic Rhinitis
Allergic rhinitis (AR) is a nasal condition caused by an inflammatory response mediated by immunoglobulin E (IgE) following exposure of the nasal mucosa to allergens. Chemokines play an important role in both the immediate and late stages of the allergic inflammatory process, inducing the activation and migration of immune system cells (including mast cells and eosinophils) to target organs and activating macrophages to trigger the synthesis of allergen-specific IgE by B cells.1 The pathogenesis of allergic rhinitis is accompanied by the aggregation and activation of eosinophils, basophils, mast cells and T lymphocytes. The aggregation of these cells is due to the action of various inflammatory transmitters, including chemokines, which are secreted by various cells of the nasal mucosal epithelium. Chemokines therefore cause the development of allergic rhinitis by binding to the corresponding chemokine receptors and chemotacticizing various inflammatory cells to reach the nasal mucosa. The origin of chemokines and the inflammatory cells on which they act are different, but the two chemokines that are more closely related in the development of allergic rhinitis are CCL and CXCL. All chemokines act by binding to their corresponding chemokine receptors. The type of chemokine receptors expressed on the surface of different inflammatory cells determines that different chemokines target different inflammatory cells. In patients with allergic rhinitis, local CD4+T lymphocytes in the nasal mucosa are mainly differentiated towards Th2, resulting in a disproportionate Th2 to Thl ratio. In contrast, Th2 Iymphocytes release Th2-like cytokines that contribute to the development of allergic rhinitis. Studies on chemokine receptors on the surface of Th2 and Thl cells have revealed that Th2 cells mainly express CCR4 and CCR8 while Thl cells mainly express CXCR3 and CCR5, while it is controversial whether Th2 cells express CCR3 on their surface.
Chemokines and Inflammatory Cells and Asthma
Clinicians currently diagnose asthma by features such as degree of airway obstruction, bronchial hyperresponsiveness and airway inflammation. Allergen-induced mechanisms mediate approximately 80% of childhood asthma and approximately 40–50% of adult asthma,2,3 and early diagnosis and treatment with medications have important effects on the prognosis of asthma in middle-aged and older adults.4
The allergic cascade reaction plays a crucial role in the mechanism of allergic asthma. Allergens bind to mast cells via IgE, which then activates mast cells and subsequently leads to the release of inflammatory mediators such as histamine, synthesis of prostaglandins and leukotrienes, and transcription of cytokines from mast cells. These mediators cause the so-called early asthma response, characterized by airway smooth muscle contraction, vascular leakage and mucus production. This early response is immediate and lasts 30–60 minutes, followed by a late response after 4 to 6 hours. The late stage is characterized by excessive airway inflammation leading to narrowing of the airway induced by various mediators originating from inflammatory cells such as T lymphocytes and eosinophils.5
This symptom of obstruction of the airways in the lungs is a typical feature of asthma. Epithelial and smooth muscle cells are crucial to the pathogenesis of asthma.6 The airway epithelium is the primary barrier against inhaled pathogens and particles,7 and the epithelium on both sides of the airway plays an important role in maintaining airway patency and host defense. The epithelium triggers responses to inhaled or inhaled substances (including allergens, viruses and bacteria), while cytokines of epithelial origin are important in the recruitment and activation of immune cells in the airways. It triggers airway inflammation and the production of mucus, which is an important factor in airway obstruction. Another major cause is the contraction of airway smooth muscle.8,9 For more than a decade, Researchers have been conducting clinical studies involving cultured cells, animal models and humans, finding different mechanisms of airway epithelial and smooth muscle cell pathology providing many insights. Zazara, Dimitra E used bone marrow chimeric (BMC) mice that have prenatal stress-exposed lungs or prenatal stress-exposed immune (hematopoietic) system and induced by ovalbumin allergic asthma. Next-generation sequencing of the lungs (RNA sequencing) and assessment of airway epithelial barrier function in ovalbumin-sensitized control and prenatally stressed offspring were also performed. Enhanced airway hyperresponsiveness, inflammation and fibrosis in offspring mice were found to be present only in female BMC mice with prenatal stress-exposed lungs.10 Song found reduced expression of SIRT3 in the bronchial tissue of asthmatic mice, the move that increased bronchial epithelial cell survival and reduced the proportion of apoptotic cells in asthmatic mice, with a consequent reduction in cytokines (TNF-α, IL-4, IL −5 and IL-13) in bronchoalveolar lavage fluid.11 The above experiments confirm the association of epithelial cells to asthma.
Asthma cannot develop without the cooperation of chemokines and various inflammatory cells. Among the chemokines, well known are the CC family chemokines and CXC family chemokines. In addition to this, respiratory epithelial cells express other chemokines, including the thymic stromal lymphopoietin (TSLP) and interleukin 33.12,13 Respiratory epithelial cells express and secrete chemokines and so play a key role in respiratory inflammation in asthma. Respiratory epithelial cells rapidly recognize and respond to microbes, tissue damage or cellular stress through the expression of pattern recognition receptors (PRRs). The release of chemokines, cytokines and antimicrobial peptides is achieved through the activation of the PRR in epithelial cells. The process that attracts and activates acquired and innate immune cells.14–16 These inflammatory factors cause an immediate phase response to immunoglobulin (IgE)-mediated airway inflammation and induce chronic persistent airway inflammation via eosinophils (considered as the main cells in allergic asthma) and helper T-cell (Th)2 lymphocytes.17
There are numerous treatment options for IGE antibodies. l.B. Bacharier et al in 2021 with Dupilumab18 found to achieve therapeutic effects in asthma, effectively inhibiting IGE and reducing the symptoms and duration of asthma onset.5 In the pathogenesis of asthma, the secretion of IL-33, CCL20 and TSLP is significantly increased in response to stress from allergens and other pathogenic substances. The C-C chemokine receptors IL-33R, CCR6 and TSLPR are expressed on immature DCs with IL-33, CCL20 and TSLP receptors, respectively. Because of the receptors, they activate IDCs and promote their maturation.19–21 Toki, S found that thymic interstitial lymphopoietin (TSLP) and IL-33 signaling mutually enhanced each other’s protein release and expression in the lung after Alt-Ext attack and each other’s receptor expression on pulmonary ILC2 in mouse experiments, thereby enhancing ILC2 activation and inducing congenital allergic inflammation.
One of the most important manifestations of allergic and non-allergic asthma is the development of airway hyperresponsiveness. Airway hyperresponsiveness is defined as an excessive bronchoconstrictive response not only to allergens but also to nonspecific stimuli, including cold air, moisture, or chemicals such as acetylcholine.22 The degree of airway hyperresponsiveness is associated with the severity of asthma. Stimuli that induce airway hyperresponsiveness simultaneously cause airway inflammation, and inhibition of this airway inflammation attenuates airway hyperresponsiveness. Furthermore, in asthmatic patients, the level of airway hyperresponsiveness is usually associated with the clinical severity of asthma and the need for medication. To date, several interrelated mechanisms for generating airway hyperresponsiveness have been proposed. First, airway inflammation mediated by eosinophils induces mediators that damage epithelial airway tissue. This exposes sensory nerve endings, which will be more susceptible to exogenously triggered stimuli and therefore contribute to the development of airway hyperresponsiveness. However, the role of eosinophils in the induction of airway hyperresponsiveness remains controversial, as it has been shown that airway hyperresponsiveness can occur in the absence of eosinophilic inflammation. In contrast, recent studies of mice with genetic deletions that eliminate eosinophils have reinforced their role in the asthma disease process.In addition, T cell-mediated immune responses may be the causative mechanism of airway hyperresponsiveness, as T cells constitute a large proportion of inflammatory cells in the airways. T cells may directly influence the development of airway hyperresponsiveness through the secretion of IL-13. Thus, two pathways have been found to be considered for airway hyperresponsiveness: 1. the eosinophil pathway; and 2. the T-cell and mast cell pathway. The specific mediating mechanisms are not yet fully understood and this requires further research.23
The Specific Effects of the Main Chemokines in the Process of Respiratory Allergic Reactions
Table 1 analyzes the CCR and CXCR class chemokines and their corresponding receptors.
 |
Table 1 Detailed Table of Chemokines and Receptors |
CCL1(I-309)
Is a potent Th2 lymphocyte primer CCL1 represents the major CC secreted by ige-activated mast cells, and high concentrations of CCL1 are found in asthmatic airways.
Rantes (CCL5)
Is a chemokine of the CCL class that has a chemotactic effect on both T lymphocytes and eosinophils.24,25 It is now known that direct stimulation of the nasal mucosa by CCL5 can lead to a large number of eosinophils, basophils and lymphocytes wandering from the blood vessels to the localized nasal mucosal tissue.26 Application of this CCL5 antagonist can significantly inhibit the recruitment of inflammatory cells such as eosinophils and T lymphocytes, thus suppressing the development of allergic rhinitis.27,28 Previous studies have shown that polymorphisms of RANTES are associated with asthma susceptibility. Zhang, YG29 found that the RANTES gene-403G/A polymorphism may be a risk factor for patients with atopic asthma. To further evaluate the impact of gene-gene and gene-environment interactions on RANTES polymorphisms and asthma risk, more studies with more patients are needed.
Eotaxin (CCL-11,24,26)
Are also chemokine belonging to the CCL class that chemotacticizes eosinophils. Its members are produced by lung and bronchial epidermal cells, induced by interleukin-4 and blocked by gamma interferon. From the discovery of Eotaxin in 1997 to the present, eotaxin-1 (CCL11), eotaxin-2 (CCL24) and eotaxin-3 (CCL26) have been found. The specific effects of this chemokine have been identified as follows: Eotaxin promotes adhesion, migration and aggregation of eosinophils; activation of eosinophils and release of inflammatory transmitters leading to the development of allergic rhinitis and tissue destruction. Eotaxin mobilizes eosinophils from the bone marrow into the blood.30–32 Under the action of numerous eosinophil chemokines, a cascade amplification effect occurs through β-2 integral protein adhesion, reactive oxygen species production, intracellular EG2 content and eosinophil production of RANTES. In allergic asthma, part of the asthma is eosinophilic asthma. Chemokines such as eotaxins and MCP-4 play a key role in the selective recruitment of eosinophils to sites of inflammation in allergy and asthma. Chemokines have been widely recognized as causative mediators of acute and chronic inflammation and tissue damage in allergy and asthma.33
CCL17[Thymic and Activation-Regulated Chemokine (TARC)]
Recruitment, activation phase of Th2 cells expressing CCR4 requires the action of CCL17. We now know that elevated levels of CCL22 are associated with asthma. We now know that CCL17 has a more powerful role and that its elevated levels imply the development of allergic symptoms. The current study found that CCL17-airway epithelial cell (CCR4)-CGRP represents a new inflammatory pathway, which implies a new therapeutic direction for patients with asthma and allergic diseases.34 Abelius, MS35 found that CB CCL17 and CCL22 levels, and TH2/TH1 chemokine ratios were significantly different between non-sensitized and sensitized children: higher in sensitized children. This finding also validates the hypothesis that the exact mechanism needs to be investigated.
CCL22 [Monocyte-Derived Chemokine (MDC)]
Induces selective migration of Th2 cells (role in Th2 cell homing and recruitment of CC chemokine receptor 4 in allergen-induced inflammation).35,36 In addition CCL5-induced migration of macrophages via CCR4 can be regulated by the natural agonists CCL17 and CCL22, which are upregulated at sites of allergic inflammation.37 High serum levels of CCL22 in patients with allergic rhinitis who are generally sensitized to pollen suggest a possible role of CCL22 in the pathogenesis of allergic rhinitis. Esaki38 found significant high levels of CCL22/MDC in children correlated with IgE related to egg allergens and Th2 chemokines. Yeh found39 that cord blood CCL22 levels were positively correlated with IgE sensitization at 2 years of age. Chiu40 found that low maternal and infant CCL22 chemokine levels were negatively associated with early childhood mite allergy and asthma. These studies also suggest that CCL22 plays an important role in TH2-associated allergic reactions.
CX3CL1 (Fraktaline)
Promotes TH2 cell survival in airway allergic reactions such as allergic rhinitis and asthma.41 El-Shazly42 found that CX3CL1/CCL26-tyrosine kinase-NK cells this mechanism promotes NK cell migration to the upper airways in allergic reactions. The CX3CL1/CX3CR1 axis has also been shown to be associated with the development of allergic asthma in studies in mice. CX3CL1 levels are elevated in patients with allergic asthma after segmental allergen excitation. Fraktaline is now increasingly studied, for example in celiac disease, idiopathic dermatitis, allergic purpura, and atherosclerosis, with increasing attention to this chemokine as a potential diagnostic biomarker.41,43–45
The Function of CXCL8 (IL-8)
Is mainly to recruit neutrophils to the site of injury or infection. It has now been found that neutrophils in the presence of IL-8 may contribute to the aggregation of eosinophils in the airways of asthma. So far, however, the mechanism has not been fully investigated.46,47 Larson47 found that IgE-bound monocytes from allergic individuals have an enhanced capacity to produce IL-8, which may contribute to the recruitment of innate immune cells during IgE-mediated allergy and promote inflammation during repeated allergen exposure. Lapointe46 found enhanced ERK1/2 phosphorylation and CXCL8 secretion in LTD4-stimulated human monocytes transfected with PTPε-specific siRNA, providing support for a regulatory/inhibitory role of PTPε in CysLT1R signaling. Tanabe48 found that IL-33 increased CXCL8/IL-8 secretion by cupulocytes via the ST2R-ERK pathway as a mechanism for enhanced airway inflammation in asthma. These aspects of the study are not yet fully understood.
IP-10/CXCL10
Interferon (IFN)-gamma-inducible protein 10 (IP-10/CXCL10) is a chemokine. It has an important role in infectious, autoimmune and neoplastic diseases. It induces integrin activation, generates migration of activated T cells, monocytes and natural killer (NK) cells, activates these cells upon binding to CXCR3 receptors, recruits effector cells to sites of inflammation and is involved in the regulation of migration, activation and differentiation of multiple immune cells. For these reasons, it may regulate inflammation at multiple levels.49 Tworek50 showed elevated concentrations of IP-10 and MIG in nasal lavage fluid from allergic patients, suggesting that these chemokines may play a role in chronic allergic inflammation. Several studies have also evaluated the effects of different immunomodulatory drugs on allergic rhinitis, showing that increases in local and peripheral IFN-γ and IP-10 are associated with symptom reduction. In recent years, attention has been focused on the role of IP-10 and the measurement of IP-10 serum levels to determine its relationship with allergic rhinitis and allergic asthma.49,51 Thus, studies antagonizing IP-10-mediated immune responses have great potential to play a role in the future.52
CXCL12 (SDF-1α)
Is a CXCL-like chemokine that has been more frequently studied in allergic rhinitis. Consistently expressed in a wide variety of normal tissues and cells, CXCL12 was not usually considered as an inflammatory chemokine in the past. Histamine released from allergen-activated mature mast may promote allergic inflammation in mast cell-rich tissues by enhancing the recruitment of its precursor (H4), in CXCL12-expressing tissues.53 Recent studies have found that CXCL12 is indeed closely associated with allergic airway disease, as the CXCL12/CXCR4 axis has been found to be associated with allergic airway disease in recent years: CXCR4 over expression is present in leukocytes localized to allergic airway disease. There is a correlation between the severity of allergic inflammation and the number of CXCR4+ cells; application of either anti-CXCR4 or anti-CXCL12 antibodies inhibits the allergic inflammatory response.54,55 Tan56 found that deletion of Twist1 in the mesenchymal compartment promotes increased fibrosis in experimental lung injury by enhancing CXCL12 expression. This direction has not been fully investigated and further studies are still needed.
Monocyte Chemotactic Proteins (MCPs)
Are also a class of CCL-like chemokines, and five MCPs (MCP-1 to MCP-5) have been identified in respiratory allergy, with MCP-1, MCP-3, and MCP-4 being the most closely associated. MCP+ cells in the nasal mucosa epithelium are mainly mononuclear macrophages followed by eosinophils and T lymphocytes. Histamine induces the expression of CCL-like chemokines (including MCP-1, MCP-3, Eotaxins, and Rantes) in the nasal mucosa of patients with allergic rhinitis such that there may be a histamine-MCPs axis playing an important role in the pathogenesis of allergic rhinitis.57–59 Allergens - nasal mucosa - chemokines (MCPs) - inflammatory cells - chemokines (MCPs, etc.) -. -allergic symptoms, which thus forms a vicious circle making allergic rhinitis worse and recurrent. This mechanism has been investigated. For example, Meiling Chen60 et al used histamine H1 receptor antagonists to inhibit the production of chemokines in the guinea pig model of ovalbumin-induced allergic rhinitis and histamine-induced human nasal epithelial cells by inhibiting ERK1/2 and NF-kappaB signaling cascades, and found that MCP levels could be suppressed, thereby reducing histamine levels.
Several CCL-like chemokines (Rantes, Eotaxin, MCP-1, MCP-3, etc.) that are closely related to allergic rhinitis share a common receptor, CCR324 and travel to the inflammatory localization mainly through CCR3 chemotaxis of eosinophils, etc. Therefore, anti-CCR3 monoclonal antibodies may be one of the most useful drugs.1,57,61,62 The current use of CCR3 antibodies to alleviate the symptoms of allergic rhinitis is a promising direction.
Effect of Chemokines on Specific Inflammatory Cells
After allergen contact with the respiratory mucosa, the allergen is presented by the APC to T cells, which differentiate into TC (CD8) cells and TH (CD4) cells. TH cells can differentiate into many TH cells. The most important ones are TH1 and TH2 cells, which secrete many cytokines to produce allergic chain reactions. In addition to CD4+ T cells, other cells such as eosinophils, basophils, lymphocytes, neutrophils, mast cells and monocytes are also involved in this process, with eosinophil infiltration being the main feature and chemokines playing an important role in the activation and migration of these inflammatory cells into the airway (Figure 1).
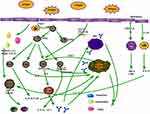 |
Figure 1 Inflammatory cells and some chemokines, the role of cytokines in respiratory epithelial allergy. |
Respiratory Epithelial Cells
Epithelial cells not only act as a physical barrier, but also act as important modulators of the immune response by producing chemokines. The role of epithelial cells in asthma has been much studied, but the specific role of epithelial cells in allergic rhinitis is not fully understood. CCL5, CCL11 and CCL13, important inducers of eosinophils, are significantly upregulated in the epithelial cells of asthma patients. Tumor necrosis factor (TNF)-α and IL-1, which are present in the epithelial environment within hours after allergen stimulation, are able to induce chemokine production in epithelial cells in vitro. In addition, respiratory viruses commonly associated with asthma attacks initially infect epithelial cells, which in response to infection produce a variety of inflammatory cytokines and chemokines, including CCL2, CCL3, CCL5, CCL11, CXCL1, CXCL5, and CXCL8.5,55,63 This leads to the recruitment of T cells, NK cells, macrophages, eosinophils and neutrophils, which would lead to viral clearance. However, this may also lead to existing airway inflammation and dysfunction in asthma by amplifying pre-existing allergic inflammation.5 Experiments by Zazara10 and Song11 confirmed the association of epithelial cells to asthma. Reducing the incidence and extent of asthma through upregulation of epithelial cell apoptosis would be a therapeutic direction for asthma.
The basal stem cells of the nasal mucosal epithelium can differentiate into different cell subpopulations: secretory cells and ciliated cells, which with their help can protect the respiratory tract. Similar to asthma, impaired integrity of nasal mucosal epithelial cells in patients with allergic rhinitis will exacerbate the allergic response, as it is more conducive to the action of inflammatory cells.64,65 In recent years, there has been an increasing number of studies in this area. For example, Steelant, B found in in vitro experiments that the integrity of the nasal mucosal epithelial barrier could be reduced by histamine and nasal secretions of AR. Treatment with TNF-α or anti-IL-4Rα monoclonal antibodies restored TH1 and TH2-induced epithelial barrier dysfunction, respectively. In a mouse model of house dust mite allergic airway inflammation, antagonism of IL-4 prevented disruption of the mucosal barrier and downregulation of tight junctions.66 Fukuoka, A found that inhibition of AR symptoms using human cystatin SN was found to protect the nasal TJ barrier by inhibiting the activity of allergenic proteases.67 This also confirms that the nasal mucosa plays a protective role in allergic rhinitis. As for cytokines and chemokines secreted by the nasal mucosa, they have not been thoroughly studied. They may be used as therapeutic targets for the treatment of allergic rhinitis in the future.
Leukocyte Extravasation Involves a Series of Events
Binding of adhesion molecules to receptors expressed on leukocytes. Leukocytes are dependent on the activation of leukocyte chemokines through this process of integrins. Thus, the recruitment and activation of basophils, eosinophils and lymphocytes during the inflammatory response is likewise achieved through the secretory function of airway endothelial cells. For example, induction of TNF-α and IFN-γ produces CCL5 from nasal mucosal endothelial cells in patients with allergic rhinitis. And may provide a mechanism by which respiratory viral infections can cause leukocytes to migrate out of circulation, leading to airway inflammation and immune modulation.5
Pulmonary Fibroblasts Act
Firstly, they are involved in building the integrity of lung tissue, and secondly, in repair during inflammation. Fibroblasts can not only influence several chemokines. They can also participate in the local immune response by secreting a series of inflammatory cytokines and chemokines.68 For example, after stimulation with TNF-α in vitro, IFN-γ releases CCL5 together with TNF-α, while IL-4 releases CCL11 together with TNF-α. Fibroblasts stimulate eosinophil migration by releasing CCL5 and CCL11. Both IL-4 and IL-13 show upregulation of CCL2, which recruits macrophages and basophils, leading to asthma. Furthermore, in a model of pulmonary fibrosis, CCR2 regulates fibroblast recruitment and activation, suggesting a role for CCR2 in asthma airway remodeling. Wang found that the TGF-β1/SMOC2/AKT and ERK axes regulate lung fibroblast proliferation, migration and fibroblast-to-fibroblast conversion for asthma development, which may provide new therapeutic targets for asthma management.69
Mast Cells
Mast cells are produced in the bone marrow and mature under the influence of stem cell factors as well as other cytokines such as IL-3, IL-4, IL-9 and IL-10. Mast cells in the body play a central role in the allergic response because of the ability to secrete histamine mediators. Mast cells are involved in systemic immune responses, such as allergic rhinitis and asthma processes, through their ability to secrete various cytokines and mediators. Such released chemokines include CCL2, CCL3 and CCL11, all of which are involved in leukocyte attraction during inflammation, linking mast cell secretion to the initial phase of allergic rhinitis and the late response of asthma. In the lungs, mast cells are present in airway smooth muscle, submucosa and airway epithelium. In patients with asthma, the number of mast cells in these tissues is significantly increased, and certainly chemokines may be associated with this increase. It has been shown that human mast cells express CCR1, CCR3, CCR4, CCR5, CCR7, CXCR1, CXCR2, CXCR3, CXCR4 and CXCR16.70
Mast cells activate themselves after allergens and other non-immune stimuli by releasing endocrine secretions, proteases and cytokines. After an endogenous or exogenous stimulus, IgE binds to IgE receptors (FcεRI) on the cell membrane, after which the same allergen cross-links the cell-bound IgE and triggers the release of pre-formed prostaglandins, histamine and cytokines. Two main activation pathways have been identified in mast cells: 1. IgE-dependent immunological mechanisms: activation of Lyn, Syk and Fyn signaling pathways, 2. non-immunological mechanisms: eg c-Kit receptors, complement receptors (C3aR, C5aR), Toll-like receptors (TLRs), chemokine receptors (CCR3)), etc. Upon activation mast cells release a variety of inflammatory mediators. They affect vascular endothelial cells by :1) altering the permeability and adhesion of blood vessels. They cause other circulating inflammatory cells to attach to endothelial cells and migrate to surrounding tissues .(ii) Cytokines and lipid mediators can also directly affect numerous lymphocytes and macrophages in the body. In addition, mast cells produce several neutral proteases, including trypsin-like and chymotrypsin, which may damage and activate the bronchial epithelium and may promote airway wall remodeling.71 Therefore, targeting intervention on mast cells to reduce the symptoms of allergic rhinitis. Zhou72 used baicalin also inhibited the production of inflammatory cytokines such as IL-1β, IL-6, IL-8 and TNF-α and inhibited the phosphorylation of JAK2, STAT5, IKKβ, IκBα and NF-κB (p65) LPS-stimulated subunits in human mast cells. Shao found73 that Shenqi balanced the ratio of Th1/Th2(IFN-γ/IL-4) in OVA and stimulated splenic lymphocytes.
In asthma, mast cells are in an activated state in the asthmatic bronchial mucosa and have been releasing mediators and cytokines and spontaneously releasing IgE-related mediators.71 Airway smooth muscle, respiratory mucosal glands and airway epithelial cells, three structures that are affected by mast cells. For example, the number and activation status of mast cells in mucosal glands are importantly related to the degree of respiratory mucus obstruction, whereas the level of mast cells in airway smooth muscle is related to AHR.71 HLMC highly express CCR3, CXCR1, CXCR3 and CXCR4, and the corresponding ligands CCL11, CXCL8, CXCL10, CXCL12 together regulate the chemotaxis of mast cells.70 Recently we found that mast cells induce IgE synthesis in B cells and differentiation of Th2 lymphocytes. The current study found that mast cells may have a substantial effect on tissue remodeling, especially in the respiratory tract, through the release of enzymes such as trypsin-like and growth factors, on smooth muscle hypertrophy and mucus hypersecretion.71
The role of airway mast cells in allergy is crucial, and research on them has focused on the relationship between the nose and lungs, such as allergic rhinitis and asthma. The pathogenic modalities are immunomodulatory, pro-inflammatory and pro-fibrotic activities. Another point of interest for mast cells is heterogeneity. Mast cells from different tissues are specific. MCTC or MCT, the lack of FcεRI expression in alveolar mast cells compared to mast cells from other parts of the body has been found through studies, which may be related to homeostatic regulation of the pulmonary vascular system, alveolar defense or distal coordination of the immune response in the lung.
T Cells
T cells are divided into cytotoxic T cells (Tc cells) and Th cells.Tc are called CD8+ cells because of the CD8 antigenic sites on the surface, which recognize specific antigens and form complexes with MHC1 proteins to initiate transmembrane signals. Tc cells can kill cells presenting exogenous antigens on their surface. There are two main types of Th cells, Th1 and Th2,16 which enhance immune response, stimulate the proliferation of other T cells and activate other lymphocytes (B cells). Th is called CD4+ cells because the cell surface expresses CD4 protein. It is now found that TH1 cells, TH2 cells, TH9 cells, TH17 cells, they all produce related cytokines to have a stimulating effect on mast cells and basophils (Figure 2).
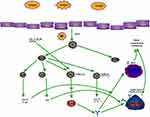 |
Figure 2 Plot of the relationships between TH cells and their secreted cytokines and associated inflammatory cells. |
Th1 cells have CCR1, CCR5, CXCR3 and CXCR6 receptors, so it is mainly associated with CXC chemokines, especially CXCL9, 10 and 11. The main function of Th1 is to activate macrophages, mediate immune response, and assist in antibody production, and is a key effector cell in type IV metaplasia, because it secretes IL-2, IL-12, α interferon, β interferon. The main function of Th1 is to activate macrophages to mediate immune response and assist in antibody production.
TH2 cells play a dominant role in allergies and are involved in all the different types and processes of the body’s immune response, and are a very important cell population in the collective immune system. Th2 cells are closely related to CC chemokines because they secrete IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, and granulocyte colony-stimulating factor, especially IL-4, and therefore have the function of: inducing Ig antibody production by B cells and participating in the immune response Therefore, it has the function of: inducing Ig antibody production by B cells and participating in immune response. TH2 cells are important players in allergic rhinitis and asthma. In addition, other TH cells are involved, such as Treg, TH3, TH9, TH10, TH17, Breg. T0, T1 and T2 cells can express different chemokine receptors, so they receive different chemokine influences and have different effects. For example, TH1 cells express CCR3 and CCR5, and TH1 produces CCL5; TH2 expresses CCR3, CCR4, CCR8, CXCR4, and produces CCL1 and CCL22. Th2 cells mediate cellular immune and inflammatory responses, anti-viral and anti-cellular parasitic bacterial infections, and participate in graft rejection by secreting IL-2, INF-γ, and TNF-β; Th2 cells are involved in B-cell proliferation, antibody production and hypersensitivity through secretion of IL-4, IL-5, IL-6 and IL-3. recruitment of Th2 cells and production of Th2-type cytokines form the main features of respiratory inflammation in asthma. Surface CCR3 and CCR4 receptors on Th2 cells bind directly to chemokines secreted by epithelial cells. CCR4 interacts with CCL2274 and CCR3 interacts with CCL13.75 Th2 cells infiltrate into the site of inflammation where they secrete cytokines including IL-4, IL-5, IL-13 and TNF-α. In addition to these, vascular endothelial cell surface adhesion molecules, TSLP, CCL17, and CCL22 induce Th2 cell aggregation and infiltration.
The Th9 cell subpopulation secretes mainly IL-9, which has been well studied since its discovery in 1988. It is known that in AR and asthma, IL-9 acts on tissues and inflammatory cells to promote the production of mast cells, eosinophils and lymphocytes, which in turn stimulates the secretion of IgE, as well as promoting mast cell expression and enhancing the inflammatory response. In patients with asthma combined with AR, the level of IL-9 is influenced by allergen-specific IgE, and the level of IL-9 in peripheral blood mononuclear cells is significantly higher than that in normal subjects.
Th17 cells mainly secrete the pro-inflammatory factor IL-17, which in turn regulates IL-5 levels, influences immune function and promotes the development of autoimmune diseases and chronic inflammation. Th17 lymphocytes are associated with CXC family chemokines: CXCL1(GROα), CXCL2(GROβ), CXCL3(GROγ), CXCL5[epithelial-derived neutrophil activating peptide 78(ENA-78)], CXCL6[granulocyte chemotactic protein 2(GCP2)], and CXCL8(IL-8). These chemokines and CXCL7 belong to the ELR+CXC chemokine family (characterized by a highly conserved N-terminal ELR (glutamate-leucine-arginine) triad and CXCR2 receptor agonists). Their main role is to attract and activate neutrophils. Cytokines such as IL-6, IL-17, IL-23, and IL-10 secreted by Th17 cells are involved in various activities such as collective mobilization, recruitment, and activation of neutrophils, and IL-17 induces the expression of pro-inflammatory cytokines, chemokines, and matrix metalloproteinases, which in turn cause tissue cell infiltration and tissue destruction. Pro-inflammatory cytokines (eg IL-6) affect IL-21 and together upregulate IL-23 receptors, and the upregulation of IL-23 ultimately affects the effector function of Th17, leading to disease.33 IL-23 and IL-27 are both members of the IL-12 family, and IL-23 does not affect Th17 production, but does affect Th17 survival and plays an important role in Th17 cell activation IL-27 inhibits the differentiation of naive CD4+ T cells into Th2 and Th17 on the one hand, and promotes the secretion of IL-10 from activated Treg cells to suppress the inflammatory response on the other. Elevated levels of Th17/Treg cells are important in AR.
T follicular helper cells (TFH) Recent discoveries have shifted our understanding of asthma from a simple TH2 cell-dependent disease to a heterogeneous disease regulated by multiple T cell subsets, including T follicular helper cells (TFH). Follicular T helper (Tfh) cells may play a role in the development of excessive IgE accumulation. Blood chemokine (CXC motif) receptor 5(CXCR5) CD4 T cells, called circulating Tfh, share functional characteristics with Tfh cells in the germinal center. Circulating Tfh cells are divided into three subsets: Tfh1(CXCR3 CC chemokine receptor [CCR]6), Tfh2(CXCR3CCR6), and Tfh17(CXCR3CCR6). Gong studies found alterations in the distribution of circulating Tfh subsets, suggesting that this cell subset may play an important role in the asthma pathogenesis.
Eosinophils
EOS is an intrinsic class of immune cells in the human body, and mature human eosinophils contain eosinophil basic protein MBP, eosinophil cationic protein ECP, eosinophil-derived neurotoxin, and eosinophil peroxidase. These contents have been found to act on airway epithelial cells and cardiomyocytes and play a role in tissue remodeling, which may lead to asthma or tissue damage and organ dysfunction. EOS and IL-33/ST276 are implicated in the pathogenesis of AR.IL-33 directly activates and drives the migration of ILC2s in the bone marrow and secretes IL-5, which activates eosinophil migration. Eosinophils express CCR3, CCR1, CXCR1, and CXCR2 on the surface of eosinophils, and the ligand of CXCR4, stromal cell-derived factor-1 (SDF-1), is able to induce migration with eotaxin.77 Regulated by activation, normal T cells express and secrete RANTES, CCL7, CCL11 and CCL13, all of which act through CCR3. The chemokine family, including CCL11, CCL24 and CCL26, binds only to CCR3, and they induce eosinophil motility (Figure 3).
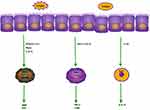 |
Figure 3 Mechanism of action of eosinophils, neutrophils and ILC2 in allergy. |
In recent years, our understanding of eosinophils as complex immunomodulatory cells has been increasing. Eosinophils can also function as antigen presenting cells (APCs).80 Namely, eosinophils express major histocompatibility complex class II and co-stimulatory molecules on their surface. It was also found that eosinophils can be transported to regional lymph nodes and act as professional APCs in various experimental settings.
In the asthma direction, airway eosinophils showed reduced cell surface CCR3 and increased expression of CCR4, CCR9 and CXCR3 compared to their circulating counterparts. Furthermore, the expression of CCR3, CCR4 and CXCR3 was significantly correlated with the percentage of eosinophils in the BAL fluid within 48 h after allergen exposure.78 Based on the properties of eosinophils in asthma, many asthmatic patients have an eosinophil endothelial phenotype and therefore tailoring asthma medications to airway eosinophil levels (sputum eosinophils or exhaled nitric oxide, FeNO) may improve the prognosis of asthma.79 The recruitment of circulating eosinophils to the lungs is characteristic of allergic airway inflammation. Chemokine receptors may play a role in this complex process. Airway eosinophils have reduced cell surface CCR3 and increased expression of CCR4, CCR9 and CXCR3 compared to their circulating counterparts. Furthermore, at 48 hours, the expression of CCR3, CCR4 and CXCR3 significantly correlated with the percentage of eosinophils in BAL fluid.78 Based on the properties of eosinophils in asthma, many asthmatics have an eosinophil endothelial phenotype and therefore tailoring asthma medications to airway eosinophil levels (sputum eosinophils or exhaled nitric oxide, FeNO) may improve the prognosis of asthma.79
For eosinophils, we treat allergic rhinitis and asthma by promoting their apoptosis or by inhibiting their activity. The most used and recognized safest modality remains the use of glucocorticoids81 to relieve acute symptoms. In addition, there are other therapeutic modalities such as targeting CCR3, Zhu, XH82 RNA interference therapy against CCR3 administered topically through the nose can inhibit the eosinogenesis, migration and invasion process of eosinophils in allergic rhinitis, thus reducing the role of eosinophils and consequently the inflammatory effect of allergic rhinitis. It may be a new therapy for allergic inflammation of the respiratory tract.83 In addition, the use of knockdown CCR3 gene inhibited the proliferation and maturation of EOS and promoted its apoptosis, providing a theoretical basis for CCR3 as a target gene for the treatment of allergic rhinitis.82 Shao84 found that Twist1 maintained apoptotic resistance of eosinophils in the nasal mucosa of allergic rhinitis. High Ras activation was detected in EOS isolated from AR nasal mucosal tissue.IgG sensitization induced Ras activation and Twist expression in EOS, which conferred apoptotic resistance to EOS cells. In asthma, Yan27 used Wentong Tang to accelerate EOS apoptosis by regulating anti-apoptotic and pro-apoptotic related factors to reduce asthma inflammation and alleviate the disease. Tang 85 used acupuncture points to reduce the airway inflammatory response in asthmatic rats, which may be related to the downregulation of p38MAPK signaling, ICAM-1 and IL-4 mRNA expression to reduce EOS aggregation, It may be related to the reduction of EOS aggregation and promotion of EOS apoptosis by downregulating p38MAPK signaling, ICAM-1 and IL-4 mRNA expression.
Basophils
Basophils, which are bone marrow stem cells, are important cells that produce IL-4 and IL-13 and are involved in allergic reactions. IL-13 plays an important role in basophil development as IL-13-basophil (FcεRI)-inflammatory mediators (histamine, leukotrienes). Subsequently, IL-4, IL-13 and other Th2-like cytokines and chemokines are released to initiate allergen-specific Th2 immune responses and participate in immune regulation. Basophils are involved in acute and chronic inflammatory responses, antiparasitic immunity, as well as regulation of innate and acquired immunity. These processes are inseparable from the presence of a number of immune molecules on the surface of basophils that are associated with intrinsic immunity, including pattern recognition receptors such as Toll-like receptor TLR2, TLR3, TLR4, TLR6, TLR7, TLR9, and surface chemokine receptors such as CCR1, CCR2, CCR3, CCR5; CXCR1, CXCR2, CXCR4; CRTH2, etc (Figure 3).
Due to the small proportion of basophils in circulating peripheral blood cells, the lack of specific cell surface markers, and the absence of appropriate animal models (eg basophil-deficient mice), their biological properties are still not well studied. There have been many advances in the detection of asthma by basophils. The high expression of IL-25 and IL-33 receptors on basophil membranes in patients with severe asthma suggests that these cytokines over-stimulate basophils in severe asthma. This finding could be used as a biomarker of asthma severity.86 Basophils in a subset of patients downregulate CD123 through activation gating CD123/HLA-DR cells prior to or alone with CD203c ensures identification of the entire basophil population and accurate assessment of basophil activation, with diagnostic importance.87 Quantitative PCR-based measurements reflecting different sputum MC/basophil abundances demonstrated an association between MC/basophils and eosinophilic inflammation, spirometry and history of exacerbation in severe asthma.88
In patients with allergic rhinitis, allergen-specific immunotherapy (ASIT) is the main allergic treatment, but reliable methods to monitor the immune response to ASIT and to predict clinical efficacy are lacking. A method to determine the threshold of allergen sensitivity (CD-Sens) by basophil activation test was recently proposed. Caruso 89 confirmed the hypothesis that SLIT can alter the immune process of allergic sensitization in the first year and that CD-Sens immunological parameters measured by CD63 and CD203c expression on stimulated basophils can be used to monitor changes in the immune system. Grass pollen immunotherapy induces suppression of allergen-specific basophil responses that persist after treatment is completed and may explain long-term clinical tolerance. It also seems to be related to the persistent blocking activity of IgG antibodies.90 Ando91 found that allergen-induced basophil surface antigen expression in seasonal allergic rhinitis induced by Japanese cedar pollen exhibited with circadian rhythm. This could partially explain the temporal symptom variation in allergic rhinitis. Further studies of basophils are needed in the future and are not fully understood at present.
Dendritic Cells
Dendritic cells DCs are the most powerful specialized antigen-presenting cells known in vivo that activate resting T cells and are central to the initiation, regulation and maintenance of the immune response. Dendritic cells are broadly classified into three major groups: a small number of plasmacytoid dendritic cells (pDC), a large number of conventional dendritic cells (cDC), and a third group of dendritic cells differentiated from monocytes in inflammation (moDC).92 The involvement of DCs in the inflammatory response is divided into: 1. DCs recognize and take up antigens; 2. activate initial T cells, which differentiate into effector T cells. Dendritic cell precursors enter tissues (including the nasal cavity and lungs) and differentiate into immature dendritic cells. DCs are the main drivers of immunity and CD4+ T cell polarization in the nasal mucosa. Current treatments for AR such as glucocorticoids, immunotherapy and anti-IgE may function by modulating the function of DCs.
In asthma, they were found to express certain immunoreceptors on the bronchial epithelial surface, which makes them sensitive to pathogen-induced nonspecific stimuli. Some allergens have a structure similar to bacterial LPS; it is able to activate epithelial cells and DCs directly via Toll-like receptor 4 (TLR4). Upon stimulation, the expression of chemokine receptors changes rapidly from two receptors, CCR2 and CXCR4, to a series of receptors CCR1, CCR2, CCR5, CCR6, CXCR1, etc. When the mature phenotype is activated and altered, dendritic cells induce a single receptor, CCR7, while losing the expression of all other receptors. Dendritic cells migrate to the T-cell zone of the mediastinal lymph nodes where they activate T cells.17 TSLP also upregulates the expression of the DC surface costimulatory molecules CD40, CD80 and CD86. In the presence of secreted cytokines and membrane-expressed molecules Jagged1, OX40L, leukotriene C4, and IL-6, activated inflammatory DCs interact with initial CD4+ T cells and induce Th2, Th17, and follicular helper T cell differentiation.13,21,93,94 Depending on the stimulation, human dendritic cells are capable of producing CCL2, CCL3, CCL4, CCL17, CCL22 and CXCL8.95–97 Therefore, interfering with DC transport using inhibitors targeting DC cells is also a therapeutic target for will respiratory allergy.
ILC2
Type 2 innate lymphocytes (ILC2) are thought to be closely related to Th2 cells and they cooperate in the pathogenesis of host protection against helminths and allergic diseases. ILC2 belongs to the innate immune system and lacks antigen-specific receptors, and its activation is mainly controlled by epithelial cytokines such as TSLP, IL-25, IL-33, etc. ILC2 is located in a critical airway mucosal ILC2 is characterized by the production of type 2 cytokines (IL-13, IL-4 and IL-5) and the expression of GATA-3 transcription factors. In the last few years, studies on ILC2 have focused on the development, activation or inhibition of its activity and the classification of precursors.
ILC2s can secrete a variety of Th2 factors, which are the hub of synergy between the natural and adaptive immune systems.12,98 Th2 cytokines such as IL-4, IL-5, IL-13,12,98,99 etc. Can also interact with various immune cells (eg, macrophages, eosinophils, basophils, mast cells) and immune factors (IgE) to integrate and coordinate their functions. In addition, the activity of ILC2s is regulated by the functional integrity of the mucosal barrier, neurotransmitters, mucosal microflora, metabolites, and other substances. In AR, IL-2 is mainly derived from the nasal mucosa and peripheral blood, but low levels of IL-2 and too little nasal mucosa make the study of its function difficult. In addition to the classical pathway involving dendritic cells, this study found that the airway allergic inflammatory response can be produced independently of T cells. There are few studies on IL-C2 in allergic rhinitis, but this also implies that IL-C2 has the potential to be studied in allergic rhinitis.
In allergic asthma, IL-5 is mainly produced by ILC2 and IL-5 blockade may be a useful treatment for steroid-resistant asthma.100 Data from a study by Wu 101 showed that circulating ILC2 or MDSC and its characteristic cytokines or transcription factors are significantly enhanced. These findings suggest that Th2 polarization is closely associated with a synergistic increase in ILC2 and MDSC, which may contribute to further understanding of the contribution of these cells to the inflammatory response in asthma or other respiratory inflammatory diseases (eg COPD and RVI), such as well as to the development of new therapeutic directions. It is worthwhile to affirm that the pathway through targeted regulation of ILC2 may provide new ideas for the clinical treatment of allergic rhinitis and asthma.
Other Cells
Monocytes have CCL2, CXCL10, CCR2 and CXCR3 on their surface. Macrophage recruitment is altered by monocyte-selective chemokines.102 After activation and recruitment, monocytes release superoxide anion and lysozyme.103–105 Meanwhile, monocytes express surface-specific adhesion molecules CD11c and CD11b on their surface, which are involved in the regulation of respiratory inflammation. In addition, increased expression of integrins β2 and α4 was accompanied by upregulation of IL-1 and IL-6.106 The interaction between mucosal epithelial cells and macrophages plays a key role in metaplastic lung inflammation.107 NKT cells are a specialized group of T cells with both T cell receptors TCR and NK cell receptors on the cell surface, thus NKT respond very rapidly after antigen stimulation and produce IL-4 and IFN-γ.
Conclusions
The idea of “one airway, one disease” has been gaining importance in the last decade. In the upper and lower airways, allergic mechanisms interact with each other. In the initial stage of respiratory allergic inflammation, allergens contact the respiratory epithelium, which produces chemokines and inflammatory factors, which cause allergic reactions by binding to the corresponding receptors and chemotactic various inflammatory cells to reach the epithelium and tissues. It also drives inflammatory cells to activate and produce more inflammatory factors, thus producing a cascade amplification effect. Inflammatory cell aggregation and activation are very complex and interact with each other in a lattice structure. By blocking the action of various chemokines, inflammatory cell aggregation is reduced, and ultimately the symptoms of respiratory allergy are alleviated. This is an important target for the future treatment of respiratory allergy. At present, with more and more understanding of how chemokines affect respiratory allergies, more and more studies have been carried out on the basic mechanism of using drugs to treat AR by controlling chemokines, mainly focusing on how chemokines regulate Th1/ Th2 and Treg/Th17 cells and affect the balance and influence of related immune cells and immune factors. At present, the use of CCR1, CCR2, CCR3 to interrupt AR and ASTHMA may be a new way of treatment effect. In the future, we can make drug research and development based on this network structure and explore new research directions.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding
This work was supported by grants National Natural Science Foundation of China (82060186); Natural Science Foundation of Jiangxi Province (20202BABL206064).
Disclosure
Mr Youwei Bao reports grants, personal fees, and non-financial support from National Natural Science Foundation of China (82060186) and Natural Science Foundation of Jiangxi Province (20202BABL206064), during the conduct of the study. Prof. Dr. Xinhua Zhu reports grants from National Natural Science Foundation of China (82060186), during the conduct of the study; grants from National Natural Science Foundation of China (82060186) and Natural Science Foundation of Jiangxi Province, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Mazzi V, Fallahi P. Allergic rhinitis and CXCR3 chemokines. Clin Ter. 2017;168(1):e54–e58.
2. Liu CT. 过敏性哮喘防治的重要性与特殊性[J] [The significance and particularity of prevention and treatment of allergic asthma]. Zhonghua Nei Ke Za Zhi. 2019;58(9):628–629. Chinese.
3. Editorial Board, C.J.o.P., t.S.o.P.C.M.A. Subspecialty Group of Respiratory Diseases, and t.S.o.P.O.C.M.D.A. 儿童支气管哮喘规范化诊疗建议 (2020年版) Children‘s respiratory professional committee, [Recommendations for diagnosis and management of bronchial asthma in children (2020)]. Zhonghua Er Ke Za Zhi. 2020;58(9):708–717. Chinese.
4. Nanda A, Baptist AP, Divekar R, et al. Asthma in the older adult. J Asthma. 2020;57(3):241–252.
5. Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol. 2014;205(5):621–631.
6. Brightling CE, Bradding P, Symon FA, et al. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):1699–1705.
7. Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120(6):1233–44; quiz 1245–6.
8. Bonser LR, Erle DJ. The airway epithelium in asthma. Adv Immunol. 2019;142:1–34.
9. Calv¨¦n J, Ax E, R?dinger M. The airway epithelium-A Central player in asthma pathogenesis. Int J Mol Sci. 2020;21:23.
10. Zazara DE, Wegmann M, Giannou AD, et al. A prenatally disrupted airway epithelium orchestrates the fetal origin of asthma in mice. J Allergy Clin Immunol. 2020;145(6):1641–1654.
11. Song J, Wang J. SIRT3 regulates bronchial epithelium apoptosis and aggravates airway inflammation in asthma. Mol Med Rep. 2022;25:4.
12. Toki S, Goleniewska K, Zhang J, et al. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy. 2020;75(7):1606–1617.
13. Bunyavanich S, Melen E, Wilk JB, et al. Thymic stromal lymphopoietin (TSLP) is associated with allergic rhinitis in children with asthma. Clin Mol Allergy. 2011;9:1.
14. Mitchell PD, O’Byrne PM. Biologics and the lung: TSLP and other epithelial cell-derived cytokines in asthma. Pharmacol Ther. 2017;169:104–112.
15. Gao W, Li L, Wang Y, et al. Bronchial epithelial cells: the key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology. 2015;20(5):722–729.
16. Mitchell PD, O’Byrne PM. Epithelial-Derived Cytokines in Asthma. Chest. 2017;151(6):1338–1344.
17. Berghi NO, Dumitru M, Vrinceanu D, et al. Relationship between chemokines and T lymphocytes in the context of respiratory allergies (Review). Exp Ther Med. 2020;20(3):2352–2360.
18. Bacharier LB, Maspero JF, Katelaris CH, et al. Dupilumab in children with uncontrolled moderate-to-severe asthma. N Engl J Med. 2021;385(24):2230–2240.
19. Hong GH, Kwon HS, Moon KA, et al. Clusterin modulates allergic airway inflammation by attenuating CCL20-mediated dendritic cell recruit ment. J Immunol. 2016;196(5):2021–2030.
20. Nygaard U, Hvid M, Johansen C, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood ato pic dermatitis. J Eur Acad Dermatol Venereol. 2016;30(11):1930–1938.
21. Chauhan A, Singh M, Agarwal A, et al. Correlation of TSLP, IL-33, and CD4?+?CD25?+?FOXP3?+?T regulatory (Treg) in pediatric asthma. J Asthma. 2015;52(9):868–872.
22. O’Byrne PM. Introduction: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138(2 Suppl):1s–3s.
23. Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulm Med. 2005;11(1):35–42.
24. Kim JJ, Lee JH, Jang CH, et al. Chemokine RANTES promoter polymorphisms in allergic rhinitis. Laryngoscope. 2004;114(4):666–669.
25. Tian C, Lei X, Shui M, et al. The expression and significance of chemokines eotaxin and RANTES in the rat model of allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;28(14):1069–1071.
26. Peric A, Sotirović J, Špadijer‐Mirković C, et al. Nonselective chemokine levels in nasal secretions of patients with perennial nonallergic and allergic rhinitis. Int Forum Allergy Rhinol. 2016;6(4):392–397.
27. Yan Y, Bao HP, Li CL, et al. Wentong decoction cures allergic bronchial asthma by regulating the apoptosis imbalance of EOS. Chin Med. 2018;13:21.
28. Errahali YJ, Thomas LD, Keller TC, et al. Inhibition by new glucocorticoid antedrugs [16¦Á, 17¦Á-d] isoxazoline and [16¦Á, 17¦Á-d]-3’-hydroxy-imino formyl isoxazoline derivatives of chemotaxis and CCL26, CCL11, IL-8, and RANTES secretion. J Interferon Cytokine Res. 2013;33(9):493–507.
29. Zhang YG, Huang J, Zhang J, et al. RANTES gene polymorphisms and asthma risk: a meta-analysis. Arch Med Res. 2010;41(1):50–58.
30. Asosingh K, Vasanji A, Tipton A, et al. Eotaxin-rich proangiogenic hematopoietic progenitor cells and CCR3+ endothelium in the atopic asthmat ic response. J Immunol. 2016;196(5):2377–2387.
31. Elsner J, Kapp A. The chemokine network in eosinophil activation. Allergy Asthma Proc. 2001;22(3):139–148.
32. Rose CE, Lannigan JA, Kim P, et al. Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell Mol Immunol. 2010;7(5):361–374.
33. Gangur V, Oppenheim JJ. Are chemokines essential or secondary participants in allergic responses? Ann Allergy Asthma Immunol. 2000;84(6):569–79; quiz 579–581.
34. Bonner K, Pease JE, Corrigan CJ, et al. CCL17/thymus and activation-regulated chemokine induces calcitonin gene-related peptide in human airw ay epithelial cells through CCR4. J Allergy Clin Immunol. 2013;132(4):942–950.
35. Abelius MS, Ernerudh J, Berg G, et al. High cord blood levels of the T-helper 2-associated chemokines CCL17 and CCL22 precede allergy develo pment during the first 6 years of life. Pediatr Res. 2011;70(5):495–500.
36. Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–680.
37. Juremalm M, Olsson N, Nilsson G. CCL17 and CCL22 attenuate CCL5-induced mast cell migration. Clin Exp Allergy. 2005;35(6):708–712.
38. Esaki H, Takeuchi S, Furusyo N, et al. Levels of immunoglobulin E specific to the major food allergen and chemokine (C-C motif) ligand (CCL) 17/thymus and activation regulated chemokine and CCL22/macrophage-derived chemokine in infantile ato pic dermatitis on Ishigaki Island. J Dermatol. 2016;43(11):1278–1282.
39. Yeh KW, Chiu CY, Su KW, et al. High cord blood CCL22/CXCL10 chemokine ratios precede allergic sensitization in early childhood. Oncotarget. 2017;8(5):7384–7390.
40. Chiu CY, Su KW, Tsai MH, et al. Low mother-to-child CCL22 chemokine levels are inversely related to mite sensitization and asthma in early childhood. Sci Rep. 2018;8(1):6043.
41. Julia V. CX3CL1 in allergic diseases: not just a chemotactic molecule. Allergy. 2012;67(9):1106–1110.
42. El-Shazly AE, Doloriert HC, Bisig B, et al. Novel cooperation between CX3CL1 and CCL26 inducing NK cell chemotaxis via CX3CR1: a possible mechani sm for NK cell infiltration of the allergic nasal tissue. Clin Exp Allergy. 2013;43(3):322–331.
43. Chen T, Guo ZP, Jiao XY, et al. CCL5, CXCL16, and CX3CL1 are associated with Henoch-Schonlein purpura. Arch Dermatol Res. 2011;303(10):715–725.
44. Fern¨¢ndez-Prieto M, Bodas A, Farrais S, et al. CX3CL1-CX3CR1 axis: a new player in coeliac disease pathogenesis. Nutrients. 2019;11:10.
45. Riopel M, Vassallo M, Ehinger E, et al. CX3CL1-Fc treatment prevents atherosclerosis in Ldlr KO mice. Mol Metab. 2019;20:89–101.
46. Lapointe F, Turcotte S, Véronneau S, et al. Role of protein tyrosine phosphatase epsilon (PTP¦Å) in Leukotriene D4-induced CXCL8 expression. J Pharmacol Exp Ther. 2019;369(2):270–281.
47. Larson EM, Babasyan S, Wagner B. IgE-binding monocytes have an enhanced ability to produce IL-8 (CXCL8) in animals with naturally occu rring allergy. J Immunol. 2021;206(10):2312–2321.
48. Tanabe T. IL-33 stimulates CXCL8/IL-8 secretion in goblet cells but not normally differentiated airway cells. Clin Exp Allergy. 2014;44(4):540–552.
49. Ragusa F, Fallahi P. IP-10 in occupational asthma: review of the literature and case-control study. Clin Ter. 2017;168(2):e151–e157.
50. Tworek D, Kuna P, Młynarski W, et al. MIG (CXCL9), IP-10 (CXCL10) and I-TAC (CXCL11) concentrations after nasal allergen challenge in patie nts with allergic rhinitis. Arch Med Sci. 2013;9(5):849–853.
51. Arikoglu T, Akyilmaz E, Yildirim DD, et al. The relation of innate and adaptive immunity with viral-induced acute asthma attacks: focusing on IP- 10 and cathelicidin. Allergol Immunopathol. 2017;45(2):160–168.
52. Glatzer F, Mommert S, Köther B, et al. Histamine downregulates the Th1-associated chemokine IP-10 in monocytes and myeloid dendritic cells. Int Arch Allergy Immunol. 2014;163(1):11–19.
53. Godot V, Arock M, Garcia G, et al. H4 histamine receptor mediates optimal migration of mast cell precursors to CXCL12. J Allergy Clin Immunol. 2007;120(4):827–834.
54. Eddleston J, Christiansen SC, Zuraw BL. Functional expression of the C-X-C chemokine receptor CXCR4 by human bronchial epithelial cells: regu lation by proinflammatory mediators. J Immunol. 2002;169(11):6445–6451.
55. Liu C, Zhang X, Xiang Y, et al. Role of epithelial chemokines in the pathogenesis of airway inflammation in asthma (Review). Mol Med Rep. 2018;17(5):6935–6941.
56. Tan J, Tedrow JR, Nouraie M, et al. Loss of twist1 in the mesenchymal compartment promotes increased fibrosis in experimental lung injury by enhanced expression of CXCL12. J Immunol. 2017;198(6):2269–2285.
57. Baumann R, Rabaszowski M, Stenin I, et al. Comparison of the nasal release of IL-4, IL-10, IL-17, CCL13/MCP-4, and CCL26/eotaxin-3 in allergic r hinitis during season and after allergen challenge. Am J Rhinol Allergy. 2013;27(4):266–272.
58. Bruno G. Cetirizine, a second-generation H1 antagonist, modulates Rantes and MCP-1 levels in allergic rhinitis. Int J Immunopathol Pharmacol. 2002;15(2):113–118.
59. Grger M, Klemens C, Wendt S, et al. Mediators and cytokines in persistent allergic rhinitis and nonallergic rhinitis with eosinophilia sy ndrome. Int Arch Allergy Immunol. 2012;159(2):171–178.
60. Chen M, Zhou P, He G, et al. Desloratadine citrate disodium injection, a potent histamine H(1) receptor antagonist, inhibits chemokine production in ovalbumin-induced allergic rhinitis Guinea pig model and histamine-induced human nasal epithelial cells via inhibiting the ERK1/2 and NF-kappa B signal cascades. Eur J Pharmacol. 2015;767:98–107.
61. Berghi O, Dumitru M, Caragheorgheopol R, et al. The relationship between chemokine ligand 3 and allergic rhinitis. Cureus. 2020;12(4):e7783.
62. Castan L, Magnan A, Bouchaud G. Chemokine receptors in allergic diseases. Allergy. 2017;72(5):682–690.
63. Smit JJ, Lukacs NW. A closer look at chemokines and their role in asthmatic responses. Eur J Pharmacol. 2006;533(1–3):277–288.
64. Kulkarni HS, Liszewski M K, Brody S L, et al. The complement system in the airway epithelium: an overlooked host defense mechanism and therapeutic target? J Allergy Clin Immunol. 2018;141(5):1582–1586.e1.
65. Ordovas-Montanes J, Dwyer D F, Nyquist S K, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560(7720):649–654.
66. Steelant B, Seys S F, Van Gerven L, et al. Histamine and T helper cytokine-driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol. 2018;141(3):951–963.e8.
67. Fukuoka A, Matsushita K, Morikawa T, et al. Human cystatin SN is an endogenous protease inhibitor that prevents allergic rhinitis. J Allergy Clin Immunol. 2019;143(3):1153–1162.e12.
68. Bissonnette Y, Madore A-M, Chakir, J et al. Fibroblast growth factor-2 is a sputum remodeling biomarker of severe asthma. J Asthma. 2014;51(2):119–126.
69. Wang Y, Yang, H, Su, X, et al. TGF-¦Â1/SMOC2/AKT and ERK axis regulates proliferation, migration, and fibroblast to myofibroblast tra nsformation in lung fibroblast, contributing with the asthma progression. Hereditas. 2021;158(1):47.
70. Pawankar R. Mast cells in allergic airway disease and chronic rhinosinusitis. Mast Cells Allergic Dis. 2005;87:111–129.
71. Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012;106(1):9–14.
72. Zhou YJ, Wang Hu, Sui H-H, et al. Inhibitory effect of baicalin on allergic response in ovalbumin-induced allergic rhinitis Guinea pigs and lipopolysaccharide-stimulated human mast cells. Inflamm Res. 2016;65(8):603–612.
73. Shao YY, Zhou Y-M, Hu M, et al. The anti-allergic rhinitis effect of traditional Chinese medicine of shenqi by regulating mast cell D egranulation and Th1/Th2 cytokine balance. Molecules. 2017;22:3.
74. Zhang K, Liu Y, Yang X, et al. HBV promotes the recruitment of IL-17 secreting T cells via chemokines CCL22 and CCL17. Liver Int. 2020;40(6):1327–1338.
75. He M, Song G, Yu Y, et al. LPS-miR-34a-CCL22 axis contributes to regulatory T cell recruitment in periapical lesions. Biochem Biophys Res Commun. 2015;460(3):733–740.
76. Fan H, Li M, Qin TJ, et al. 变应性鼻炎患者外周血嗜酸性粒细胞及IL-33水平的表达 [Expression of eosinophils and IL-33 levels in peripheral blood of patients with allergic rhinitis]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;31(18):1427–1430. Chinese.
77. Nagase H. Regulation of chemokine receptor expression in eosinophils. Int Arch Allergy Immunol. 2001;125(Suppl 1):29–32.
78. Liu LY. Chemokine receptor expression on human eosinophils from peripheral blood and bronchoalveolar lavage f luid after segmental antigen challenge. J Allergy Clin Immunol. 2003;112(3):556–562.
79. Petsky HL, Cates C J, Kew K M, et al. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): a sy stematic review and meta-analysis. Thorax. 2018;73(12):1110–1119.
80. Akuthota P, Wang H, Weller PF. Eosinophils as antigen-presenting cells in allergic upper airway disease. Curr Opin Allergy Clin Immunol. 2010;10(1):14–19.
81. Luo H. 根据变应性鼻炎患者鼻分泌物嗜酸性粒细胞计数调整类固醇治疗的临床意义 [Relationship between eosinophils in nasal discharge and responses to treatment of inhaled glucocorti costeroid in patients with persistent allergic rhinitis]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26(11):494–498. Chinese.
82. Zhu XH, Wang J L, Huang Q L, et al. 敲除CCR3基因对小鼠嗜酸性粒细胞的作用实验观察 [Effect of CCR3 gene knockout on eosinophils in mice]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;31(24):1913–1918. Chinese.
83. Zhu XH, Liao B, Liu Ke, et al. Effect of RNA interference therapy on the mice eosinophils CCR3 gene and granule protein in the murin e model of allergic rhinitis. Asian Pac J Trop Med. 2014;7(3):226–230.
84. Shao JB, Luo X-Q, Mo L-H, et al. Twist1 sustains the apoptosis resistance in eosinophils in nasal mucosa of allergic rhinitis. Arch Biochem Biophys. 2021;702:108828.
85. Tang XY, Chen PB, Du DJ, et al. 穴位埋线对哮喘大鼠肺组织中的pMASK信号通路及细胞间黏附因子-1、白细胞介素-4、嗜酸性粒细胞的影响 [Acupoint catgut embedment may reduce airway inflammation reaction by down-regulating ICAM-1 and EOS by suppressing p38MAPK signaling in lung tissue of asthmatic rats]. Zhen Ci Yan Jiu. 2022;47(2):129–134. Chinese.
86. Boita M, Heffler E, Omedè P, et al. Basophil membrane expression of epithelial cytokine receptors in patients with severe asthma. Int Arch Allergy Immunol. 2018;175(3):171–176.
87. Santos AF, Bécares N, Stephens A, et al. The expression of CD123 can decrease with basophil activation: implications for the gating strategy o f the basophil activation test. Clin Transl Allergy. 2016;6:11.
88. Winter NA, Qin L, Gibson P G, et al. Sputum mast cell/basophil gene expression relates to inflammatory and clinical features of severe ast hma. J Allergy Clin Immunol. 2021;148(2):428–438.
89. Caruso M, Cibella F, Emma R, et al. Basophil biomarkers as useful predictors for sublingual immunotherapy in allergic rhinitis. Int Immunopharmacol. 2018;60:50–58.
90. Zidarn M, Košnik M, Šilar M, et al. Sustained effect of grass pollen subcutaneous immunotherapy on suppression of allergen-specific basop hil response; a real-life, nonrandomized controlled study. Allergy. 2015;70(5):547–555.
91. Ando N, Nakamura Y, Ishimaru K, et al. Allergen-specific basophil reactivity exhibits daily variations in seasonal allergic rhinitis. Allergy. 2015;70(3):319–322.
92. Froidure A, Pilette C. Les cellules dedritiques humaines dans I’ ashma et la rhinite allergique [Human dendritic cells in allergic asthma and rhinitis]. Med Sci. 2015;31(2):151–158. French.
93. Murrison LB, Ren X, Preusse K, et al. TSLP disease-associated genetic variants combined with airway TSLP expression influence asthma risk. J Allergy Clin Immunol. 2022;149(1):79–88.
94. Papli¨½ska-Goryca M, Nejman-Gryz P, Proboszcz M, et al. Expression of TSLP and IL-33 receptors on sputum macrophages of asthma patients and healthy subjects. J Asthma. 2020;57(1):1–10.
95. Kahl J. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006;116(3):783–796.
96. Lu M. Inhibition of p21-activated kinase 1 attenuates the cardinal features of asthma through suppressing t he lymph node homing of dendritic cells. Biochem Pharmacol. 2018;154:464–473.
97. Perros F. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL2 2 in attracting Th2 cells and inducing airway inflammation. Allergy. 2009;64(7):995–1002.
98. Huang W, Song Y, Wang L. Wenshen decoction suppresses inflammation in IL-33-induced asthma murine model via inhibiting ILC2 ac tivation. Ann Transl Med. 2019;7(20):570.
99. Zhu J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine. 2015;75(1):14–24.
100. Matsuda M, Tanaka Y, Shimora H, et al. Pathogenic changes in group 2 innate lymphoid cells (ILC2s) in a steroid-insensitive asthma model of mice. Eur J Pharmacol. 2022;916:174732.
101. Wu Y, Yan Y, Su Z, et al. Enhanced circulating ILC2s accompany by upregulated MDSCs in patients with asthma. Int J Clin Exp Pathol. 2015;8(4):3568–3579.
102. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14(6):392–404.
103. Coma G, Peña R, Blanco J, et al. Treatment of monocytes with interleukin (IL)-12 plus IL-18 stimulates survival, differentiation and t he production of CXC chemokine ligands (CXCL)8, CXCL9 and CXCL10. Clin Exp Immunol. 2006;145(3):535–544.
104. Hansen AS, Biltoft M, Bundgaard B, et al. CD46 activation induces distinct CXCL-10 response in monocytes and monocyte-derived dendritic cells. Cytokine. 2019;113:466–469.
105. Wang J, Vodovotz Y, Fan L, et al. Injury-induced MRP8/MRP14 stimulates IP-10/CXCL10 in monocytes/macrophages. FASEB j. 2015;29(1):250–262.
106. Sushak L, Gabure S, Maise J, et al. Dibutyltin alters immune cell production of the pro-inflammatory cytokines interleukin (IL) 1¦Â and IL −6: role of mitogen-activated protein kinases and changes in mRNA. J Appl Toxicol. 2020;40(8):1047–1059.
107. Niessen NM, Baines K J, Simpson J L, et al. Neutrophilic asthma features increased airway classical monocytes. Clin Exp Allergy. 2021;51(2):305–317.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
