Back to Journals » Therapeutics and Clinical Risk Management » Volume 12
Role of biologics in severe eosinophilic asthma – focus on reslizumab
Authors Pelaia G , Vatrella A , Busceti MT, Gallelli L , Preianò M, Lombardo N , Terracciano R, Maselli R
Received 3 May 2016
Accepted for publication 9 June 2016
Published 1 July 2016 Volume 2016:12 Pages 1075—1082
DOI https://doi.org/10.2147/TCRM.S111862
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Girolamo Pelaia,1 Alessandro Vatrella,2 Maria Teresa Busceti,1 Luca Gallelli,3 Mariaimmacolata Preianò,3 Nicola Lombardo,1 Rosa Terracciano,3 Rosario Maselli1
1Department of Medical and Surgical Sciences, Section of Respiratory Diseases, University “Magna Græcia” of Catanzaro, Catanzaro, Italy; 2Department of Medicine and Surgery, Section of Respiratory Diseases, University of Salerno, Salerno, Italy; 3Department of Health Science, University “Magna Græcia” of Catanzaro, Catanzaro, Italy
Abstract: Within the context of the heterogeneous phenotypic stratification of asthmatic population, many patients are characterized by moderate-to-severe eosinophilic asthma, not adequately controlled by relatively high dosages of inhaled and even oral corticosteroids. Therefore, these subjects can obtain significant therapeutic benefits by additional biologic treatments targeting interleukin-5 (IL-5), given the key pathogenic role played by this cytokine in maturation, activation, proliferation, and survival of eosinophils. In particular, reslizumab is a humanized anti-IL-5 monoclonal antibody that has been found to be an effective and safe add-on therapy, capable of decreasing asthma exacerbations and significantly improving disease control and lung function in patients experiencing persistent allergic or nonallergic eosinophilic asthma, despite the regular use of moderate-to-high doses of inhaled corticosteroids. These important therapeutic effects of reslizumab, demonstrated by several controlled clinical trials, have led to the recent approval by US Food and Drug Administration of its use, together with other antiasthma medications, for the maintenance treatment of patients suffering from severe uncontrolled asthma.
Keywords: IL-5, eosinophilic asthma, reslizumab, chronic obstructive pulmonary disease, MAPK, Janus kinases
Introduction
Asthma is a chronic respiratory disease, clinically manifesting as wheezing, cough, shortness of breath, and chest tightness, which is characterized by bronchial obstruction mainly due to inflammatory and structural changes leading to airway hyperresponsiveness and acute bronchoconstriction.1,2 This widespread airway disorder affects over 300 million people worldwide, which may increase to more than 400 million by 2020.3,4 Rather than a single disease entity, asthma is currently believed to be a heterogeneous complex of multiple clinical and pathobiologic phenotypes characterized by different responses to pharmacological therapies.5,6 The majority of asthmatic patients can achieve a good control of their symptoms using standard treatments including inhaled corticosteroids and bronchodilators such as β2-adrenergic agonists, eventually integrated with oral leukotriene inhibitors and/or tiotropium.7–9
However, despite an optimized inhaled therapy, a minority of subjects with severe disease are not adequately controlled and experience frequent exacerbations. Moreover, asthma severity in these difficult-to-treat patients is often further worsened by the coexistence of one or more comorbidities, including chronic rhinitis and sinusitis, gastroesophageal reflux, obesity, obstructive sleep apnea, and even chronic obstructive pulmonary disease.10 Although when considering the overall population of asthmatic subjects, patients suffering from severe disease constitute a relatively small percentage, ranging from 5% to 10%; however, they consume a huge share of economic resources, amounting to about 50% of the global asthma budget.11–13 This very high cost of severe asthma is caused by a higher utilization of health care services including unscheduled consultations and emergency visits, and also additional consumption of drugs as well as frequent hospitalizations for recurrent exacerbations. Furthermore, severe asthma is associated with significant losses of school- and work-days, and asthmatic subjects with uncontrolled disease also often experience anxiety and depression.14 Therefore, patients expressing asthma phenotypes refractory to conventional treatments are characterized by the most urgent unmet medical needs, which thus require a closer attention to the assessment, monitoring, and therapeutic management of their disease. Hence, particularly in severe asthma, an accurate phenotypic characterization should be pursued to identify the relevant cellular and molecular targets involved in disease pathobiology. Through such a personalized strategy, it would be possible to tailor individual therapies aimed to achieve an adequate and persistent control of symptoms, as well as to decrease the risk of future exacerbations and to slow down the progression of lung function decline.15 Within this context, the present and future cornerstone of patient-centered treatment of severe asthma is based on the use of biological drugs.16–19 Biologic therapies usually include monoclonal antibodies, soluble receptors, and genetically altered cytokines. Because biologic treatments for asthma point to specific molecular and cellular targets, eligible patients should be identified through a search for reliable and easily assessable biomarkers. Among these, the most commonly measured in asthmatic patients are serum IgE, sputum or blood eosinophils (the latter being used more often), fractional exhaled nitric oxide, and periostin, a matricellular protein produced by both inflammatory and airway structural cells.20,21
Several inflammatory phenotypes of asthma have been characterized, which include eosinophilic, neutrophilic, mixed, and paucigranulocytic patterns.2,22 Eosinophils are the inflammatory cells most frequently infiltrating the airways of asthmatic patients, and they are crucially implicated in the development of both allergic and nonallergic asthma.23,24 Eosinophilic asthma originates from the activation of immunopathologic and proinflammatory pathways, mainly coordinated by T-helper (Th)2 lymphocytes, which release interleukins (IL)-5, 4, and 13. In addition to being driven by adaptive immune responses, airway eosinophilia can also arise from innate immune mechanisms, which are mediated by intercellular communications involving bronchial epithelial cells and innate lymphoid cells.25,26
Because of the central role played by IL-5 in maturation, activation, proliferation, and survival of eosinophils, this cytokine is a key target for the treatment of eosinophilic asthma.27–30 In this regard, it is noteworthy that, among the pleiotropic effects of corticosteroids, inhibition of IL-5 synthesis is one of the most important mechanisms underlying the very effective antiasthma action of these drugs.31 Corticosteroids are indeed powerful inducers of eosinophil apoptosis;32,33 nevertheless, despite a regular or almost continuous use of inhaled and even systemic corticosteroids, some subgroups of asthmatic subjects display persistent bronchial and/or blood eosinophilia, associated with an inadequate control of asthma.34 Therefore, these patients can potentially benefit from additional therapies based on the use of biological drugs targeting IL-5. Moreover, previous studies showed that neutrophilic asthma may be related to corticosteroid resistance. Indeed, it is known that corticosteroids inhibit neutrophil apoptosis and contribute to activation of these cells, suggesting that corticosteroid treatment itself could have some role in the development of neutrophilia, thus contributing to worsening of severe asthma.35
Role of IL-5 in eosinophilic asthma
IL-5 plays a pivotal pathogenic role in eosinophilic asthma. In asthmatic airways, the main cellular sources of IL-5 include Th2 lymphocytes, CD4+ invariant natural killer T-cells, Type 2 innate lymphoid cells, mast cells, and eosinophils themselves.26,36–39 Production of Th2 cytokines, also including IL-5, is markedly enhanced by IL-25.40 In patients with allergic asthma, the bone marrow is able to respond to allergen challenge with an enhanced capacity of producing eosinophils, and this effect is associated with higher concentrations of IL-5 mRNA in subjects experiencing dual early and late asthmatic responses, when compared to patients showing only early bronchoconstrictive reactions.41 The stimulatory action of IL-5 on eosinophil differentiation extends from the bone marrow to the airways; indeed, increased levels of IL-5, eosinophil progenitors, and mature eosinophils have been detected in the induced sputum of dual-responder asthmatics.42 IL-5 synergizes with powerful eosinophil-chemotactic chemokines, namely, eotaxins 1, 2, and 3, in eliciting airway eosinophilia and bronchial hyperresponsiveness.30 Furthermore, significantly increased sputum levels of IL-5 and eotaxins have been found in patients experiencing acute asthma exacerbations when compared with both healthy controls and subjects with mild persistent disease.43 IL-5 and eotaxins cooperate in favoring eosinophil accumulation into the airways, especially during asthma exacerbations, and this effect is at least in part dependent on IL-5-induced inhibition of eosinophil apoptosis.44,45 Sputum levels of IL-5 were indeed found to be inversely correlated with the numbers of apoptotic eosinophils in both stable patients and asthmatic subjects experiencing exacerbations. Also in nonallergic, late-onset eosinophilic asthma, IL-5 exerts a key pathogenic role.24 In this particular asthmatic phenotype, large amounts of IL-5 are produced by Type 2 innate lymphoid cells,46 in the absence of active allergic pathways triggered by Th2 lymphocytes.
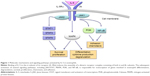
On eosinophils, the cellular effects of IL-5 are mediated by its binding to a membrane receptor including a ligand-specific α subunit (IL-5Rα) and a nonspecific signaling βc subunit (Figure 1), which also interacts via a shared extracellular domain with two other hematopoietic cytokines IL-3 and granulocyte-macrophage colony stimulating factor.47,48 High-affinity binding of IL-5 to IL-5Rα is followed by ligation of this activated IL-5/IL-5Rα complex to the βc subunit, which probably triggers signal transduction via dimerization of its cytoplasmic domain.49,50 The signaling pathways activated by the interaction of IL-5 with its receptor involve several transducing enzymes, mainly including intracellular kinases such as Janus kinases (JAK), mitogen-activated protein kinases, Lyn tyrosine kinase, Raf-1 kinase, and phosphoinositide 3-kinase (PI3K) (Figure 1).29
In the absence of IL-5, JAK2 and JAK1 are constitutively associated with IL-5Rα and the βc chain, respectively.51 Upon IL-5 binding, the receptor construct undergoes a dynamic conformational change, leading to the association of JAK1 with IL-5Rα.51 Therefore, IL-5 activates both JAK1 and JAK2, thus triggering the assembly of a functional IL-5Rα-βc complex. As a result of this IL-5-dependent stimulatory process, JAK2 induces the activation of signal transducers and activators of transcription (STAT)1, 3, and 5, which enhance the expression of pim-1, cyclin D3, and other IL-5-inducible genes involved in cell cycle progression and eosinophil proliferation.52,53 JAK2 is also implicated, via a cooperative action with Lyn and Raf-1 kinases, in IL-5-mediated inhibition of eosinophil apoptosis, thereby contributing to cell survival.54 Moreover, Raf-1 plays a key role in eosinophil activation and degranulation.54
Within the context of the intricate IL-5-stimulated signaling network activated by the βc receptor subunit, a central role is played by both extracellular-signal-regulated kinase (ERK) and p38 subgroups of mitogen-activated protein kinases (MAPK). In particular, Ras/Raf-1/MEK-mediated activation of ERK is crucially responsible for induction of c-fos gene expression and eosinophil differentiation, proliferation and survival, and also for the release of leukotriene C4.55–58 Furthermore, p38 MAPK mainly induces, also acting through activation of the transcription factor NF-κB, cytokine production by eosinophils, as well as eosinophil adhesion and chemotaxis occurring during allergic inflammation.58–60 IL-5-induced interaction of eosinophils with intercellular adhesion molecule-1 is also promoted by phosphoinositide 3-kinase, and this effect is mediated by downstream stimulation of protein kinase C and phosphorylation-dependent activation of ERK1/2.61
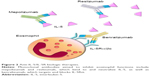
Given the pivotal role played by IL-5 in eosinophil functions and asthma pathobiology, this cytokine and its receptor are suitable targets of biological therapies and are being evaluated for treatment of eosinophilic asthma.62 In this regard, several preclinical studies have been carried out in experimental animal models of asthma. Indeed, the anti-IL-5 antibody TRFK-5 suppressed airway eosinophilia in allergen-sensitized mice.63 Moreover, in nonhuman primate models of asthma, TRFK-5 inhibited the influx of eosinophils into bronchi and the associated airway hyperresponsiveness.64 Later, other monoclonal antibodies directed against IL-5 (mepolizumab and reslizumab) or IL-5Rα (benralizumab) have been developed and evaluated in clinical trials (Figure 2).16,65–67
Reslizumab: mechanism of action, efficacy, and safety
Reslizumab is an IgG4/κ monoclonal antibody, also known as SCH-55700, which was humanized from the rat monoclonal IgG2a antibody JES1-39D10 via a synthetic process based on recombinant technology using complementarity-determining region grafting, aimed to incorporate rat antigen recognition sites for human IL-5 onto a human IgG4 structure.68–70 Reslizumab has a molecular weight of 146 kDa and binds with high affinity to an epitope region corresponding to amino acids 89–92 of human IL-5, thus preventing this cytokine from binding to IL-5Rα.71–73
The first clinical study aimed to assess the efficacy of reslizumab in asthma treatment was carried out by Kips et al74 in a small group of asthmatic subjects. This Phase II, double-blind, randomized, and dose-ranging pilot trial evaluated the biological, clinical, and functional effects, as well as the safety and pharmacokinetic profiles of reslizumab. Enrolled patients were recruited on the basis of their severe persistent asthma, treated with oral glucocorticoids or high doses of inhaled corticosteroids, regardless of the underlying inflammatory phenotypes. Reslizumab was compared with placebo (n=8) and administered as a single intravenous infusion at four rising doses of 0.03 mg/kg (n=2), 0.1 mg/kg (n=4), 0.3 mg/kg (n=6), or 1.0 mg/kg (n=12), respectively. When compared with placebo, reslizumab doses ≥0.3 mg/kg significantly reduced eosinophil counts in peripheral blood with respect to baseline values, thus inducing mean decreases in circulating eosinophils ranging from 52.5% at 48 hours to 18.9% at day 30. Moreover, reslizumab lowered sputum eosinophil numbers in three of four patients with documented bronchial eosinophilia. However, no significant changes were detected in both symptom control and physician evaluation of overall clinical status. With regard to lung function, in comparison with placebo reslizumab elicited a transiently significant increase in forced expiratory volume in one second (FEV1), recorded 24 hours after administration of the 0.3 mg/kg dosage. Although a trend toward FEV1 improvement also persisted at subsequent time points, no dose of reslizumab was able to induce further significant FEV1 changes. On day 30, FEV1 increases with respect to baseline values were 11.2% in the 0.3 mg/kg group, 8.6% in the 1.0 mg/kg arm, and 4.0% in patients assigned to placebo treatment. Furthermore, no meaningful variations were observed in terms of either FEV1/FVC (forced vital capacity) ratio or peak expiratory flow. With regard to safety, all single doses of reslizumab were well tolerated, and no significant alterations of vital signs or laboratory parameters were found. The most common adverse events included headache and fatigue, which were reported with the same frequency also in the placebo group. Similarly, no significant differences were detected with regard to asthma worsening, which occurred in three of 12 patients treated with 1.0 mg/kg of reslizumab, and in one of eight subjects of the placebo arm. In one patient treated with the dosage of 1.0 mg/kg, nonneutralizing serum antibodies to reslizumab were found. The plasma levels of reslizumab were dose proportional. At 6.9 hours after administration of the 1.0 mg/kg dose, the pharmacokinetic profile of reslizumab was characterized by a mean maximal concentration of 30.3 μg/mL. After the same drug dosage, mean concentrations of 0.87 and 0.43 μg/mL were detected on days 90 and 120, respectively. The elimination half-life ranged between 24.5 and 30.1 days.
Later, in a Phase II multicenter, double-blind study performed in Northern America (United States and Canada) and specifically targeted to eosinophilic asthma, Castro et al75 evaluated the effects of reslizumab in 106 patients with inadequately controlled disease despite the use of high doses of inhaled corticosteroids. Patient enrollment was made on the basis of an eosinophil percentage of at least 3% in induced sputum. In particular, 53 subjects were randomly assigned to treatment with placebo, and the other 53 patients were treated with four intravenous infusions of 3.0 mg/kg of reslizumab, administered every 4 weeks for 12 weeks, respectively. With respect to baseline counts, at the end of treatment, reslizumab induced a significant, median 95.4% reduction of sputum eosinophils. In clinical terms, this effect was associated with a positive trend toward better asthma control, which however did not reach the threshold of statistical significance; greater improvements in asthma control were achieved by a subgroup of patients characterized by the highest levels of blood and sputum eosinophils, associated with nasal polyposis. In comparison with placebo, a positive but not significant difference in favor of the reslizumab arm was also observed in the rate of asthma exacerbations. With regard to lung function, when compared to placebo reslizumab elicited significant improvements in both FEV1 (mean FEV1 increase: 180 mL) and FVC. This trial confirmed the good tolerability profile of reslizumab. Indeed, in reslizumab and placebo arms, the proportions of patients who experienced adverse events were very similar. The most frequently reported adverse event was nasopharyngitis.
More recently, two large, multicenter, double-blind, randomized, and placebo-controlled, Phase III trials have been carried out by Castro et al76 with the primary end point of evaluating the effects of reslizumab on asthma exacerbations in patients with poorly controlled disease and blood eosinophilia. Inclusion criteria were based on the occurrence of one or more exacerbations treated with systemic corticosteroids during the previous year, associated with blood eosinophil counts ≥400 cells/μL, and with an inadequate asthma control despite the use of medium-to-high doses of inhaled corticosteroids, with the eventual addition of other drugs including long-acting β2 adrenergic agonists, leukotriene modifiers, cromolyn sodium, and even oral glucocorticoids. Recruited patients continued their usual asthma therapies at constant doses throughout both studies. Among 2,597 patients screened, 953 were enrolled and randomly assigned to receive intravenous infusions of either placebo or reslizumab, administered at a dosage of 3.0 mg/kg every 4 weeks for 52 weeks. When compared with placebo, reslizumab significantly lowered the annual rate of clinical asthma exacerbations by 50%–59%. Reslizumab also prolonged the time to first exacerbation. Moreover, in both trials reslizumab significantly decreased blood eosinophil numbers, improved asthma symptom control, and increased FEV1 values. These two parallel trials confirmed the good safety profile of reslizumab, which was found to be similar to that of placebo.76 In both studies, the most commonly reported adverse events, occurring in more than 5% of patients receiving reslizumab, were worsening of asthma symptoms, nasopharyngitis, upper respiratory tract infections, sinusitis, influenza, and headache. Overall, when compared with subjects treated with placebo, serious adverse events were less frequent in patients receiving reslizumab. Local reactions at injection sites were uncommon and not different between placebo and reslizumab arms. In study 2, two patients receiving reslizumab experienced anaphylactic reactions judged to be treatment related, which responded well to standard therapy at clinic site, but led to withdrawal from the trial;76 antidrug antibodies (ADA) were not detected in either subject. Transient, low-titer antireslizumab antibodies were found in eight patients (3%) in study 1, and in seven patients (2%) in study 2, respectively. However, in these subjects, the overall safety pattern of reslizumab was not different from that globally recorded in both study populations.
The positive effects on lung function, mainly referring to enlargement of proximal airways, can be integrated by further improvements also occurring at level of peripheral airways, as shown by another Phase III study conducted by Bjermer et al.77 In particular, the results of this trial (aimed to evaluate the effects of two different doses [0.3 and 3.0 mg/kg] of intravenous reslizumab) refer to 311 patients with persistent asthma, reversible airflow limitation, and high levels of blood eosinophils (≥400 cells/μL), not adequately controlled by inhaled corticosteroids. At the end of the first part of this investigation, 271 patients chosen among those receiving either drug (n=179) or placebo (n=92) were enrolled in an open-label extension study and received the 3.0 mg/kg dose of reslizumab. When compared to placebo after 16 weeks of treatment, both doses of reslizumab induced significant increases in mean FEV1 values (115 and 160 mL with 0.3 and 3 mg/kg, respectively). Furthermore, only when administered at the 3.0 mg/kg dose, reslizumab also elicited significant increases in mean values of both FVC (130 mL) and forced expiratory flow at 25%–75% of FVC (233 mL/s). At both doses (0.3 mg/kg and 3.0 mg/kg), reslizumab improved symptom control evaluated through asthma control questionnaire score, as well as decreased the use of inhaled rescue medications. Additionally, although both reslizumab doses significantly lowered blood eosinophil counts, a greater effect was observed when the 3 mg/kg dose was used. Treatment with reslizumab at both dosages was well tolerated, and only mild-to-moderate and self-limiting adverse events related to the study drug, including headache, nasopharyngitis, upper respiratory tract infections, and sinusitis, were recorded. Low ADA titers were detected in 12% and 11% of patients treated with reslizumab 0.3 and 3 mg/kg, respectively. However, the majority of these patients were found to be ADA-positive only once during the 16-week treatment period. Moreover, the safety profile of ADA-positive patients was similar to that observed in the global study population, and ADA positivity did not have any impact on blood eosinophil suppression caused by reslizumab, thus suggesting that ADAs were not neutralizing.77
The aforementioned data were indirectly confirmed by a further Phase III study performed by Corren et al,78 who completed their analysis in 492 patients with poorly controlled asthma, not selected on the basis of their blood eosinophil counts, who over a period of 16 weeks received every 4 weeks placebo (n=97) or 3.0 mg/kg of intravenous reslizumab (n=395). The authors did not report significant changes in FEV1 in the overall study population and in the subgroup of patients with less than 400 blood eosinophils/μL. However, in the subgroup of patients with more than 400 blood eosinophils/μL, with respect to placebo, reslizumab produced a significant mean FEV1 increase (270 mL). Reslizumab was well tolerated, and patients receiving this drug experienced fewer overall adverse events when compared with subjects assigned to the placebo arm (55% versus 73%). Low and transient ADA titers were detected in 5% of patients treated with reslizumab, but ADA positivity did not affect either safety profile or eosinophil depletion pattern, thereby indicating a lack of neutralizing activity.78
Conclusion
The well-established awareness of the role of IL-5 as a key player in the pathobiology of eosinophilic asthma has promoted the development of effective therapeutic strategies aimed to neutralize this cytokine. Indeed, as a result of several randomized controlled trials, mepolizumab and reslizumab have been recently approved for biological treatment of asthma. In particular, the pharmacologic profile of reslizumab is very interesting because this drug is characterized by a high affinity for an IL-5 epitope including residues 89–92 of the amino acid sequence.73 This small region of IL-5 molecule is critically involved in cytokine binding to its receptor α subunit. Therefore, reslizumab strongly prevents IL-5/IL-5Rα interaction, thereby very effectively blocking IL-5 bioactivity.
Of course, a focused selection of eligible asthmatic patients for anti-IL-5 biotherapies requires a careful phenotypic stratification based on reliable clinical, functional, and biologic features. In particular, subjects who can take the best advantages from the use of biologic drugs targeting IL-5 are likely those suffering from uncontrolled eosinophilic, allergic or nonallergic asthma, also experiencing recurrent disease exacerbations despite the use of inhaled corticosteroids at relatively high doses. Of course, the most important biomarkers of eosinophilic airway inflammation are sputum eosinophils. However, because of the practical unfeasibility of induced sputum in many clinical settings of real-life routine medical activity, peripheral blood eosinophils represent a very useful and easily measurable parameter to characterize these patients. Indeed, the levels of circulating eosinophils approximately reflect, even better than fractioned exhaled nitric oxide, the state of ongoing bronchial inflammation.79–81 Therefore, it seems very reasonable that anti-IL-5 add-on treatments will soon contribute to satisfy the unmet needs of many patients with moderate-to-severe eosinophilic asthma, not adequately controlled by current standard therapies.
Disclosure
No external help was received in writing this manuscript, and no author has a financial interest related to reslizumab or its manufacturer. The authors report no conflicts of interest in this work.
References
Holgate ST, Arshad HS, Roberts GC, Howarth PH, Thurner P, Davies DE. A new look at the pathogenesis of asthma. Clin Sci. 2013;118(7):439–450. | ||
Pelaia G, Vatrella A, Busceti MT, et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm. 2015;879783. | ||
Masoli M, Fabian D, Holt S, Beasley R; Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy. 2004;59(5):469–478. | ||
Chanez P, Humbert M. Asthma: still a promising future? Eur Respir Rev. 2014;23(134):405–407. | ||
Ray A, Oriss TB, Wenzel SE. Emerging molecular phenotypes of asthma. Am J Physiol Lung Cell Mol Physiol. 2015;308(2):L130–L140. | ||
Gauthier M, Ray A, Wenzel SE. Evolving concepts of asthma. Am J Respir Crit Care Med. 2015;192(6):660–668. | ||
Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL (GOAL) study. Am J Respir Crit Care Med. 2004;170(8):836–844. | ||
Fanta CH. Drug therapy: asthma. N Engl J Med. 2009;360(10):1002–1014. | ||
Global Initiative for Asthma (GINA) [homepage on the Internet]. Global strategy for asthma management and prevention. Available from: http://www.ginasthma.org. Accessed June 27, 2016. | ||
Boulet LP. Influence of comorbid conditions on asthma. Eur Respir J. 2009;33(4):897–906. | ||
Serra-Batlles J, Plaza V, Morejon E, Comella A, Brugués J. Costs of asthma according to the degree of severity. Eur Respir J. 1998;12(6):1322–1326. | ||
Antonicelli L, Bucca C, Neri M, et al. Asthma severity and medical resource utilisation. Eur Respir J. 2004;23(5):723–729. | ||
Accordini S, Corsico AG, Braggion M, et al. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013;160(1):93–101. | ||
Heaney LG, Conway E, Kelly C, et al. Predictors of therapy resistant asthma: outcome of a systematic evaluation protocol. Thorax. 2003;58(7):561–566. | ||
Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46(3):622–639. | ||
Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nat Rev Drug Discov. 2012;11(12):958–972. | ||
Fajt ML, Wenzel SE. Biologic therapy in asthma: entering the new age of personalized medicine. J Asthma. 2014;51(7):669–676. | ||
Darveaux J, Busse WW. Biologics in asthma – the next step toward personalized medicine. J Allergy Clin Immunol Pract. 2015;3(2):152–160. | ||
Mitchell PD, El-Gammal AI, O’Byrne PM. Emerging monoclonal antibodies as targeted innovative therapeutic approaches to asthma. Clin Pharmacol Ther. 2016;99(1):38–48. | ||
Szefler SJ, Wenzel S, Brown R, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol. 2012;129(3 Suppl):S9–S23. | ||
Izuhara K, Conway SJ, Moore BB, et al. Roles of periostin in respiratory disorders. Am J Respir Crit Care Med. 2016;193(9):949–956. | ||
Wenzel SE. Complex phenotypes in asthma: current definitions. Pulm Pharmacol Ther. 2013;26(6):710–715. | ||
Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–54. | ||
Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthma. Nat Med. 2013;19(8):977–979. | ||
Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol. 2014;205(5):621–631. | ||
Yu S, Kim HY, Chang YJ, DeKruyff RH, Umetsu DT. Innate lymphoid cells and asthma. J Allergy Clin Immunol. 2014;133(4):943–950. | ||
Weltman JK, Karim AS. IL-5: biology and potential therapeutic applications. Expert Opin Investig Drugs. 2000;9(3):491–496. | ||
Stirling RG, van Rensen EI, Barnes PJ, Chung KF. Interleukin-5 induces CD34+ eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1403–1409. | ||
Molfino NA, Gossage D, Kolbeck R, Parker JM, Geba GP. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin Exp Allergy. 2012;42(5):712–737. | ||
Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov. 2013;12(2):117–129. | ||
Barnes PJ, Adcock IM. How do corticosteroids work in asthma? Ann Intern Med. 2003;139(5 Pt 1):359–370. | ||
Zhang X, Moilanen E, Kankaanranta H. Enhancement of human eosinophil apoptosis by fluticasone propionate, budesonide, and beclomethasone. Eur J Pharmacol. 2000;406(3):325–332. | ||
Zhang X, Moilanen E, Adcock IM, Lindsay MA, Kankaanranta H. Divergent effect of mometasone on human eosinophil and neutrophil apoptosis. Life Sci. 2002;71(13):1523–1534. | ||
Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131(3):636–645. | ||
Kato T, Takeda Y, Nakada T, Sendo F. Inhibition by dexamethasone of human neutrophil apoptosis in vitro. Nat Immun. 1995;14(4):198–208. | ||
Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major sub-phenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–395. | ||
Sakuishi K, Oki S, Araki M, Porcelli SA, Miyake S, Yamamura T. Invariant NKT cells biased for IL-5 production act as crucial regulators of inflammation. J Immunol. 2007;179(6):3452–3462. | ||
Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G. The role of human mast cell-derived cytokines in eosinophil biology. J Interferon Cytokine Res. 2004;24(5):271–281. | ||
Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38(5):709–750. | ||
Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39(6):798–806. | ||
Wood LJ, Sehmi R, Dorman S, et al. Allergen-induced increases in bone marrow T lymphocytes and interleukin-5 expression in subjects with asthma. Am J Respir Crit Care Med. 2002;166(6):883–889. | ||
Dorman SC, Efthimiadis A, Babirad I, et al. Sputum CD34+ IL-5Rα+ cells increase after allergen: evidence for in situ eosinophilopoiesis. Am J Respir Crit Care Med. 2004;169(5):573–577. | ||
Park SW, Kim DJ, Chang HS, et al. Association of interleukin-5 and eotaxin with acute exacerbation of asthma. Int Arch Allergy Immunol. 2003;131(4):283–290. | ||
Xu J, Jiang F, Nayeri F, Zetterstrom O. Apoptotic eosinophils in sputum from asthmatic patients correlate negatively with levels of IL-5 and eotaxin. Respir Med. 2007;101(7):1447–1454. | ||
Ilmarinen P, Moilanen E, Kankaanranta H. Regulation of spontaneous eosinophil apoptosis – a neglected area of importance. J Cell Death. 2014;7:1–9. | ||
Walker JA, Barlow JL, McKenzie AM. Innate lymphoid cells: how did we miss them? Nat Rev Immunol. 2013;13(2):75–87. | ||
Rossjohn J, McKinstry WJ, Woodcock JM, et al. Structure of the activation domain of the GM-CSF/IL-3/IL-5 receptor common β-chain bound to an antagonist. Blood. 2000;95(8):2491–2498. | ||
Murphy JM, Young IG. IL-3, IL-5, and GM-CSF signaling: crystal structure of the human β-common receptor. Vitam Horm. 2006;74:1–30. | ||
Johanson K, Appelbaum E, Doyle M, et al. Binding interactions of human interleukin 5 with its receptor α subunit. Large scale production, structural, and functional studies of Drosophila-expressed recombinant proteins. J Biol Chem. 1995;270(16):9459–9471. | ||
Ishino T, Harrington AE, Gopi H, Chaiken I. Structure-based rationale for interleukin 5 receptor antagonism. Curr Pharm Des. 2008;14(12):1231–1239. | ||
Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21(12):1303–1309. | ||
Pazdrak K, Stafford S, Alam R. The activation of the JAK-STAT 1 signalling pathway by IL-5 in eosinophils. J Immunol. 1995;155(1):397–402. | ||
Stout BA, Bates ME, Liu LY, et al. IL-5 and granulocyte-macrophage colony-stimulating factor activate STAT3 and STAT5 and promote Pim-1 and cyclin D3 protein expression in human eosinophils. J Immunol. 2004;173(10):6409–6417. | ||
Pazdrak K, Olszewska-Pazdrak B, Stafford S, et al. Lyn, Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of interleukin-5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulation. J Exp Med. 1998;188(3):421–429. | ||
Adachi T, Alam R. The mechanism of IL-5 signal transduction. Am J Physiol. 1998;275(3 Pt 1):C623–C633. | ||
Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20(3):288–294. | ||
Bates ME, Green VL, Bertics PJ. ERK1 and ERK2 activation by chemotactic factors in human eosinophils is interleukin 5-dependent and contributes to leukotriene C4 biosynthesis. J Biol Chem. 2000;275(15):10968–10975. | ||
Pelaia G, Cuda G, Vatrella A, et al. Mitogen-activated protein kinases and asthma. J Cell Physiol. 2005;202(3):642–653. | ||
Adachi T, Choudhuri BK, Stafford S, et al. The differential role of extracellular signal-regulated kinases and p38 mitogen-activated protein kinase in eosinophil functions. J Immunol. 2000;165(4):2198–2204. | ||
Ip WK, Wong CK, Wang CB, et al. Interleukin-3, -5, and granulocyte macrophage colony-stimulating factor induce adhesion and chemotaxis of human eosinophils via p38 mitogen-activated protein kinase and nuclear factor-κB. Immunopharmacol Immunotoxicol. 2005;27(3):371–393. | ||
Sano M, Leff AR, Myou S, et al. Regulation of interleukin-5-induced β2-integrin adhesion of human eosinophils by phosphoinositide 3-kinase. Am J Respir Cell Mol Biol. 2005;33(1):65–70. | ||
Gallelli L, Busceti MT, Vatrella A, et al. Update on anticytokine treatment for asthma. BioMed Res Int. 2013;104315. | ||
Garlisi CG, Kung TT, Wang P, et al. Effects of chronic anti-interleukin-5 monoclonal antibody treatment in a murine model of pulmonary inflammation. Am J Respir Cell Mol Biol. 1999;20(2):248–255. | ||
Mauser PJ, Pitman AM, Fernandez X, et al. Effects of an antibody to interleukin-5 in a monkey model of asthma. Am J Respir Crit Care Med. 1995;152(2):467–472. | ||
Walsh GM. Therapeutic potential of targeting interleukin-5 in asthma. BioDrugs. 2013;27(6):559–563. | ||
Patterson MF, Borish L, Kennedy JL. The past, present, and future of monoclonal antibodies to IL-5 and eosinophilic asthma. J Asthma Allergy. 2015;8:125–134. | ||
Varricchi G, Bagnasco D, Borriello F, et al. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol. 2016;16(2):186–200. | ||
Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386(9998):1086–1096. | ||
Walsh GM. Profile of reslizumab in eosinophilic disease and its potential in the treatment of poorly controlled eosinophilic asthma. Biologics. 2013;7:7–11. | ||
Egan RW, Athwal D, Bodmer MW, et al. Effect of Sch 55700, a humanized monoclonal antibody to human interleukin-5, on eosinophilic responses and bronchial hyperreactivity. Arzneimittelforschung. 1999;49(9):779–790. | ||
Cardet JC, Israel E. Update on reslizumab for eosinophilic asthma. Expert Opin Biol Ther. 2015;15(10):1531–1539. | ||
Lim H, Nair P. Efficacy and safety of reslizumab in patients with moderate to severe eosinophilic asthma. Expert Rev Respir Med. 2015;9(2):135–142. | ||
Zhang J, Kuvelkar R, Murgolo NJ, et al. Mapping and characterization of the epitope(s) of Sch 55700, a humanized mAb, that inhibits human IL-5. Int Immunol. 1999;11(12):1935–1944. | ||
Kips JC, O’Connor BJ, Langley SJ, et al. Effects of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med. 2003;167(12):1655–1659. | ||
Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125–1132. | ||
Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. | ||
Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;S0012-3692(16):47551–47553. | ||
Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;S0012-3692(16):45715–45716. | ||
Wagener AH, de Nijs SB, Lutter R. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70(2):115–120. | ||
Pavord ID, Agusti A. Blood eosinophil count: a biomarker of an important treatable trait in patients with airway disease. Eur Respir J. 2016;47(5):1299–1303. | ||
George L, Brightling CE. Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther Adv Chronic Dis. 2016;7(1):34–51. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.