Back to Journals » International Journal of Nanomedicine » Volume 15
Role of Beetroot (Beta vulgaris) Juice on Chronic Nanotoxicity of Silver Nanoparticle-Induced Hepatotoxicity in Male Rats
Authors Albrahim T , Alonazi MA
Received 2 February 2020
Accepted for publication 1 May 2020
Published 15 May 2020 Volume 2020:15 Pages 3471—3482
DOI https://doi.org/10.2147/IJN.S248078
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Tarfa Albrahim,1 Mona A Alonazi2
1College of Health and Rehabilitation Sciences, Department of Health Sciences, Clinical Nutrition, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia; 2Department of Biochemistry, College of Science, King Saud University, Riyadh, Saudi Arabia
Correspondence: Tarfa Albrahim Email [email protected]
Introduction: Nanoparticles are at the forefront of rapidly developing nanotechnology and have gained much attention for their application as an effective drug delivery system and as a mediated therapeutic agent for cancer. However, the cytotoxicity of nanoparticles is still relatively unknown and, therefore, additional study is required in order to elucidate the potential toxicity of these nanoparticles on cells.
Materials and Methods: Thus, the following work aimed to investigate the capability of Beta vulgaris (beetroot) water extract (BWE; 200 mg/kg) to protect hepatic tissue following silver nanoparticles (AgNPs; 80 mg/kg; > 100 nm) intoxication in male rats.
Results: AgNPs-intoxication elevated the liver function markers – including serum transaminases and alkaline phosphatase activities – and decreased serum levels of albumin and total proteins, in addition to disturbing the oxidation homeostasis. This is evidenced by the increased lipid peroxidation, the depleted glutathione, and the suppressed activity of superoxide dismutase and catalase. In addition, an apoptotic reaction was observed following AgNPs treatment, as indicated by the up-regulation of p53 and down-regulating Bcl-2 expressions, examined by the immunohistochemistry method. Furthermore, AgNPs exhibited a marked elevation in liver DNA damage that was indicated by an increase in tail length, tail DNA% and tail movement. However, BWE eliminated the biochemical and histological alterations, reflecting its hepatoprotection effect in response to AgNPs.
Discussion: Collectively, the present data suggest that BWE could be used following AgNPs as a potential therapeutic intervention to minimize AgNPs-induced liver toxicity.
Keywords: silver nanoparticles, Beta vulgaris, oxidative stress, p53 and Bcl-2 expression, hepatotoxicity
Introduction
Nanotechnology, a facilitative technology that deals with nanometre-sized objects, is currently in the process of being developed at several levels. Due to their distinctive physicochemical and electrical properties, nano-sized materials have gained considerable traction in the fields of electronics, biotechnology, and aerospace engineering. In the field of medicine, NPs are being employed as a novel delivery system for drugs, proteins, DNA, and monoclonal antibodies.1,2 The small size of NPs means that they are able to easily enter the body and cross a variety of biological obstructions to reach the most vulnerable organs.3
Silver has long been known as an anti-bacterial substance thus the use of silver NPs (AgNPs) in a commercial and medical products – such as in wound dressings, the coating of surgical instruments, and prostheses4,5 - is extensive. Animal and human studies have demonstrated that, after inhalation and through oral exposure, AgNPs are distributed to the liver, heart, spleen, and brain, in addition to the lungs and gastrointestinal tract.6 Based on the special biokinetic characteristics of AgNPs discussed earlier, it is necessary to address the toxicity-related issues of AgNPs in appropriate experimental models, particularly in light of standard protocols with respect to the livers of experimental animals.
A wide range of plants are cultivated globally for food and for medicinal purposes, with several traditional medicinal plants displaying significant potential in the management and treatment of various modern health problems. These include beetroot (Beta vulgaris L), known in the Middle East as Shamandar, a plant of the family Amaranthaceae.7 The roots of beet have long been used in traditional medicine to treat a wide variety of diseases, and beetroot itself contains large quantities of pigments such as betaxanthins and betacyanin from the betalain family, a group of water-soluble nitrogen-containing pigments derived from betalamicacid. Studies indicate that the betalains in beetroot can act as protective molecules and, in addition, it is claimed that beetroot has a multitude of therapeutic uses due to its anti-depressant, hepatoprotective, anti-hypertensive, antioxidant, anti-hyperlipidaemic, radioprotective, and immunostimulatory effects. Moreover, they possess anti-cancer, immunomodulatory, anti-inflammatory, anti-mutagenic, anti-microbial and anti-fungal properties, and can be used as expectorants and carminatives.7–9
This study aims to investigate the relief of chronic nanotoxicity effects on the structure and function of the liver in male rats from the application of silver nanoparticles (AgNPs), by examining the role played by the administration of beetroot.
Materials and Methods
Chemicals
Silver Nanoparticles
Silver nanopowder, with a particle size less than 100 nm and a 99.9% trace metals basis, was purchased from Sigma-Aldrich (St. Louis, MO, USA). According to the supplier, the prepared AgNPs is characterized using transmission electron microscopy (TEM), dynamic light scattering (DLS), Zeta potential measurements, and UV/Visible spectral analysis to guarantee consistent materials (monodisperse AgNPs free from agglomeration; refractive index n20/D 1.333; fluorescence—λem 388 nm) (http://www.sigmaaldrich.com/materials-science/nanomaterials/silver-nanoparticles.html). For an additional characterization of the size distribution of the particles, scanning electron microscope (SEM) was performed. Briefly, the AgNPs was dissolved in 0.5% aqueous carboxymethylcellulose (Sigma-Aldrich) and the prepared solution was then coated with carbon, mounted on an electron microscope grid (200 mesh), and visualized using a scanning electron microscope (SEM; type S-4700, JEOL Ltd., Tokyo, Japan) operating at 80kV. However, AgNPs in the injected doses should be distributed more uniformly by sonication for 10 minutes just before injection, to be taken by systemic circulation.10
Beetroot Preparation and Extraction
Red beetroot (B. vulgaris L.) was purchased from a local market in Riyadh, KSA. They were washed with tap water, chopped into small pieces, crushed with deionized water (1:2 w/v), and were then filtered and lyophilized to powder, according to the methods of Sana and Rahila.11
Experimental Design
Adult male Sprague-Dawley (SD) rats (n=40, 3 months old, 150–170 g weight). Animals were kept on basal diet and tap water, both of which were provided ad libitum. After two weeks of acclimation, animals were randomly divided into four equal groups (n=10): Group 1 (Control) received saline and served as control; group 2 (BWE) had beetroot water extract orally administered (200 mg/kg BWE/day); group 3 (AgNPs) received intraperitoneal injections of AgNPs (80 mg/kg; >100 nm) for 4 weeks; and group 4 (AgNPs+BWE) was treated intraperitoneally with AgNPs (80 mg/kg; >100 nm) for 4 weeks and then treated with beetroot water extract (200 mg/kg/day) for further 4 weeks. The dose of AgNPs was selected based on the study conducted by Singh et al12 unlike the dose of beetroot water extract, which was selected according to the work of El Gamal et al.13 The study protocols were approved by the Ethics Committee for Laboratory Animal Care of Princess Nourah bint Abdulrahman University in Riyadh (KSA; H-01-R-059; IRB long number: 19-0201) in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals, 8th edition (NIH Publication No. 85-23, revised 1985).
At the end of the experimental period, the rats were euthanized with intraperitoneal injection of sodium pentobarbital (200 mg/kg i.p.) and subjected to a complete necropsy. Blood samples were individually collected from the inferior vena cava of each rat and stored in anti-coagulant and non-heparinized glass tubes for hematological analysis and estimation of liver function biomarkers. Blood samples were incubated at room temperature for 10 minutes and left to clot then centrifuged at 3000 × g for 15 min. Following this, the sera was separated and kept in a clean stopper plastic vial at –80°C for analysis of serum parameters.
Hematological Analysis
The hematological parameters were determined on whole blood utilizing an automated analyzer (Abacus junior, Radim, Italy).
Liver Function Biomarkers
Serum liver parameters of alanine transaminase (ALT) and aspartate transaminase (AST), alkaline phosphatase (ALP), albumin (Alb) and total proteins were estimated in order to assess liver function, using commercial diagnostic kits (Randox/Laboratory, Crumlin, United Kingdom) and following the manufacture’s protocols.
Preparation of Liver Homogenates
The livers from all experimental groups were removed, weighed and stored at −20°C. Thereafter, a 10% w/v homogenate was prepared by grinding 0.3 g of tissue in 3 mL of 50 mM phosphate buffer saline, pH 7.4.
Oxidative Stress Status Assays
The liver homogenates were assayed for the purpose of analyzing their oxidant/antioxidant status, using a variety of biomarkers. The measurement of lipid peroxidation (LPO) was performed according to the parameters outlined in the protocol of Ohkawa et al and was based on determining the amount of formed malondialdehyde (MDA), a lipid peroxidation end-product marker.14 The content of glutathione (GSH) was determined according to the method described by Ellman15 and the hepatic activity of superoxide dismutase (SOD) was assayed based on the method described by Nishikimi et al.16 Finally, catalase (CAT) activity was estimated according to the method devised by Aebi, measuring the decomposition rate of hydrogen peroxide (H2O2) at 240 nm.17
Comet Assay (Determination of DNA Damage)
A comet assay (single cell gel electrophoresis) was employed to assess and quantify the levels of liver tissue DNA damage, based on the described method of Olive and Banáth.18
Histological Preparation
After necropsy, the liver was immediately removed and repaired by immersion in 10% neutral buffered formalin solution for 24–48 hours. The specimens were subsequently dehydrated, cleared and embedded in paraffin. Serial sections of 5 µm thick were cut by means of rotary microtome (Litz, Wetzlar; Germany) and were then stained with hematoxylin and eosin.19 For silver particle detection in the liver, semithin sections were prepared on glass slides through cutting at 1-um using an ultramicrotome (EM UC7 from Leica Microsystems). Following this preparation, the sections were stained with Toluidine blue for 25 s and examined by a light microscope.
Apoptotic (p53) and Anti-Apoptotic (Bcl-2) Markers Expression
The expression of apoptotic p53 proteins and anti-apoptotic Bcl-2 proteins in liver sections was detected using avidin Biotin Complex (ABC) (Elite–ABC, Vector Laboratories, CA, USA). Sections were incubated with anti-rabbit p53 or anti-rabbit Bcl-2 monoclonal antibody (dilution 1:80 and 1:2000; DAKO Japan Co, Ltd, Tokyo, Japan) according to the methodology of Harrison-Bernard et al.20
Statistical Analysis
Data were expressed as mean values ± SE (standard error). Significant differences among treatment groups were statistically analyzed by one way ANOVA. The criterion for statistical significance was set at p<0.05 for the biochemical data. All statistical analyses were performed using SPSS statistical version 21 software package (SPSS® Inc., USA).
Results
AgNPs Characterization
AgNPs used in the study were formed in spheroids of 80–90 nm in diameter, as seen by the SEM (Figure 1).
 |
Figure 1 Scanning electron micrograph of AgNPs. Bar=100 nm. |
Effect of Silver Nanoparticles and/or Beta vulgaris on Hematological Parameters
Compared with the data obtained from the control group of rats, the administration of AgNPs in the other groups led to a significant decrease in both the Hb content and RBC count, whilst there was a significant increase in WBCs and platelet counts (Table 1). However, the post-administration of BWE to AgNPs induced a significant amelioration in the AgNPs-mediated modulation in hematological parameters and restored those values towards the results observed in the control. Interestingly, BWE treatment alone substantially increased the Hb content compared with the control value.
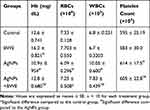 |
Table 1 Changes in Complete Blood Pictures in Different Groups Under Study |
Effect of Silver Nanoparticles and/or Beta vulgaris on Liver Functions
The rats treated with AgNPs exhibited a significant increase in the activity of serums ALT, AST and ALP, and a significant decrease in serum albumin (Alb) and total protein levels in comparison with the control and BWE groups (Figure 2). Nevertheless, treatment of rats chronically exposed to AgNPs with BWE (AgNPs+BWE) exhibited a considerable decrease in the activity of serums ALT, AST and ALP and a notable increase in albumin serum and total proteins level, compared to the AgNPs group.
Effect of Silver Nanoparticles and/or Beta vulgaris on Liver Oxidative Stress
There was a substantial increase in liver LPO and a substantial decrease in GSH content in the rats treated with silver nanoparticles, as well as a decrease in the activity of SOD and CAT, in comparison with the control and BWE groups (Figure 3). On the other hand, the rats chronically treated with silver nanoparticles along with Beta vulgaris (AgNPs+BWE) exhibited a significant decrease in LPO level, whilst there was a significant increase in the liver content of GSH and activities of SOD and CAT, compared to the AgNPs-treated group.
Liver DNA Fragmentation in Rats Treated with Silver Nanoparticles and/or Beta vulgaris
Rats treated with silver nanoparticles exhibited a significant increase in liver DNA damage (P < 0.05) that was indicated by an increase in tail length, tail DNA% and tail movement, compared to the control and Beta vulgaris groups. A significant decrease in liver DNA damage was observed in the rats that were chronically treated with silver nanoparticles and Beta vulgaris (AgNPs+BWE), in comparison with the DNA damage observed in the livers of the group that were treated solely with AgNPs (Figure 4 and Table 2).
 |
Table 2 Liver DNA Damage (Comet Assay Parameters Obtained by Image Analysis in Cells) in Different Groups |
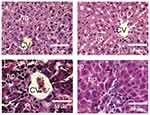
Effect of Silver Nanoparticles and/or Beta vulgaris on Liver Histopathology
The liver sections from both the control group and the group of rats treated with BWE revealed a normal hepatocyte structure, where the hepatocytes are polygonal in shape with eosinophilic granular cytoplasm and vesicular basophilic nuclei (Figure 5A and B). A variety of major histopathological lesions were observed in the liver sections of rats chronically treated with AgNPs, as well as the diffused infiltration of inflammatory cells, mainly eosinophils, atrophied and vacuolated hepatocytes, and degeneration with focal area necrosis (Figure 5C). Liver sections in rats treated with AgNPs and BWE (AgNPs+BWE) revealed a moderate degree of improvement in hepatocytes, where there were only a few atrophied and/or vacuolated hepatocytes and a mild infiltration of inflammatory cells (Figure 5D).
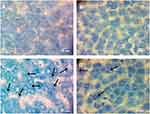
Furthermore, Toluidine blue staining revealed that the presence of AgNPs had formed within the cytoplasm of hepatocytes (Figure 6C). Nonetheless, the number of AgNPs within the hepatocytes decreased in the liver sections of rats post-treated with the BWE (AgNPs+BWE) (Figure 6D), suggesting the ability of beetroot to facilitate the removal of AgNPs from the liver. Both Figure 6A and B of the control and BWE-treated alone liver revealed no silver has appeared within the cells.
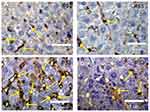
Effect of Silver Nanoparticles and/or Beta vulgaris on Liver p53 Expressions
Expression of cytoplasmic p53 and the incidence of apoptotic cells were faintly positive reactions in liver sections in both the control and BWE groups (Figure 7A and B). In contrast, (Figure 7C) demonstrated strong p53 expression in the liver sections in rats treated with AgNPs, while liver sections in AgNPs+BWE revealed mildly positive reactions for p53 expression (Figure 7D).
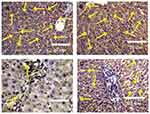
Effect of Silver Nanoparticles and/or Beta vulgaris on Liver Bcl-2 Expressions
The expression of cytoplasmic anti-apoptotic Bcl-2 and the incidence of apoptotic cells in the liver sections of rats in the control and BWE groups showed strong positive reactions (Figure 8A and B). In contrast, (Figure 8C) showed faint Bcl-2 expression in the liver sections of rats treated with AgNPs while liver sections in AgNPs+BWE revealed moderately positive reactions for Bcl-2 expression (Figure 8D).
Discussion
Despite the growing beneficial biomedical purposes of AgNPs, there is a limit to their uses due to the scarcity of knowledge about the interaction between AgNPs and different cellular processes. Nonetheless, recent studies suggest that AgNPs have a range of cellular targets, among which is the stimulation of the apoptosis pathway, as well as oxidative stress due to the overproduction of reactive oxygen species (ROS) leading to liver injury.21,22 Hence, in this study, the potential role of beetroot was examined in relation to the stimulation of the apoptotic pathway and oxidative stress caused by AgNPs in rats. After exposure, AgNPs can be absorbed, reach the blood and then deposited predominantly in the liver, leading to liver damage.23 In the present study, AgNPs administrated at 80 mg/kg caused liver damage, evidenced by the distribution in serum liver markers, apoptosis and DNA damage. These findings are in accordance with previous studies performed by Patlolla et al24 and Heydrnejad et al.23 The results obtained in previous studies demonstrated that the oral administration of AgNPs in rats (50–100 mg/kg) could significantly increase liver markers and oxidative stress, causing DNA damage.
In toxicological studies, the size of AgNPs is important. Gliga et al25 found that AgNPs measuring 10 nm in diameter were toxic, whereas 40–75 nm AgNPs were not. In the same manner, Park et al26 found that 4 nm AgNPs considerably elevated the production of ROS and pro-inflammatory cytokines released from macrophage immune cells, unlike those AgNPs measuring 20–70 nm. Additionally, Cho et al27 discovered that the single intraperitoneal injection of AgNPs measuring 10 nm in diameter at a dose of 0.2 mg per mouse, induced hepatotoxicity characterized by congestion, vacuolation, single cell necrosis, and focal necrosis. This is a direct contrast with the results obtained with 60 and 100 nm of AgNPs. Indeed, the previous studies demonstrated that the sizes of the nanoparticles may be the foremost factor influencing the toxicity of AgNPs.
In the current study, the RBC count and hemoglobin content decreased in the AgNPs-treatment group. Similar findings were also reported in Carassius auratus gibelio larval exposed to 1, 2, and 3 ppm AgNPs (5 nm), which may be indicators of acute anemia, erythropoiesis disorder leading to deformation of RBCs and inhibition of heme synthesis.28 Adragna et al29 suggested that silver particles cause stress, leading to loss of RBCs. Increases in WBCs and platelet counts after exposure to AgNPs are considered a normal reaction in rats, due to the stimulatory effect on their immune system.30,31 However, Forouhar Vajargah et al28 found that long‐term exposure to AgNPs in Carassius auratus gibelio larval caused stressful conditions, leading to a decrease in WBC count due to cortisol secreted during stress reaction. This could encourage lymphocytes apoptosis and thus shorten their life span, and also reduce their proliferation.32
Consistent with the previous studies, AgNPs measuring less than 100 nm in diameter in the present study caused liver damage. Elevated levels of hepatic enzymes (ALT, AST and ALP) as well as decreased serum levels of albumin and total proteins are crucial indicators of liver injury. Moreover, Hassanen et al31 reported that 25 and 50 mg/kg of chitosan-coated AgNPs produced histopathological alterations and disturbances in liver markers in rats. The possible mechanism for liver damage could be due to the accumulation of Ag+ hepatocytes, which generate excessive amounts of ROS, leading to the loss of the functional integrity of hepatocyte membranes. This then leads to cellular enzyme leakage into the blood and, subsequently, liver dysfunction. Nonetheless, post-administration of beetroot to AgNPs-treated rats decreased serum levels of ALT, AST, and ALP, and increased albumin and total protein levels to those of the control group. The obtained results indicate the ability of BWE to protect against AgNPs-induced hepatotoxicity, which is in accordance with the earlier work of Krajka-Kuzniak et al,33 who demonstrated that beetroot protected liver from N-nitrosodiethylamine and carbon tetrachloride-induced liver damage. Similarly, Iahtisham-Ul-Haq et al34 reported the beneficial effect of beetroot‐based beverages on serum levels of these enzymes. The hepatoprotective effect of beetroot, however, could be attributed to the antiradical scavenging activity of the extract.
In the current study, after 28 days exposure to AgNPs in rats, a significant increase in the LPO level and a significant depletion in reduced GSH content – as well as inhibition in SOD and CAT activities – were observed in liver tissue. This data demonstrates that AgNPs may cause oxidative injury through a ROS-mediated process. AgNPs toxicity appears to boost ROS generation, through the liberation of Ag+ ions inside the cells, as documented by Hassanen et al.31 Dabrowska-Bouta et al35 found that AgNPs altered the expression of oxidative stress-related genes, as well as biochemical markers in myelin membranes extracted from AgNPs-exposed rats. They also suggested that Ag may interact with metal–proteins, such as thiol-proteins, and causing conformational changes and dysfunction. GSH depletion has a marked effect on lipid and protein oxidation. Notably, these entire disturbances can be improved by BWE post-treatment. The free radical scavenging activity of beetroot has been documented previously.36,37 The antiradical activity of BWE may be due to its high source of polyphenols and flavonoids, such as betalains. Further evidence supports the ability of polyphenols to enhance detoxifying/antioxidant enzymes of Phase II, exerting hepatoprotective effect.38 Indeed, BWE post-administration in the present study maintained the endogenous antioxidant defense system at normal cellular concentrations following AgNPs.
Oxidative stress and ROS overproduction in hepatocytes following exposure to AgNPs mediated p53 overexpression. Our results are in accordance with Barcińska et al39 who found that induced apoptosis and cell cycle arrest in pancreatic ductal adenocarcinoma cells (PANC-1) through inducing up-regulation in p53. The ability of AgNPs to induce apoptosis was extensively studied and supported in its use in cancer therapy. AgNPs with a diameter of less than 10 nm can interact extracellularly with cellular membrane proteins and deactivate signaling pathways, causing inhibition of cell proliferation.40 AgNPs can enter cells through diffusion or endocytosis to cause production of ROS, resulting in apoptosis. Although p53 enhances the antioxidant defense system against ROS, after extensive and prolonged exposure to ROS, p53 instead promotes cellular senescence or apoptosis. p53 plays a vital role in distributing cellular homeostasis, ultimately activating apoptosis in severe or protracted protein damage.41 Furthermore, in oxidative damage, p53 mediates apoptosis by enhancing the expression of pro-apoptotic proteins and inhibiting anti-apoptotic proteins. In the present study, AgNPs also down-regulated Bcl-2 expression in liver tissue. The findings of the present study align with those recorded by Zielinska et al,42 who reported that AgNPs significantly down-regulated Bcl-2 expression in PANC-1 cells. Conversely, BWE post-administration augmented the increase in p53, and enhanced Bcl-2 in the liver tissue. In the same manner, El Gamal et al13 found that BWE administration up-regulated the anti-apoptotic proteins and down-regulated the pro-apoptotic proteins in response to toxicants. Thus, our findings suggest that BWE protects against AgNPs-induced hepatotoxicity in rats via the modulation of the apoptotic pathway.
In conclusion, AgNPs (>100 nm in diameter) were found to elicit toxicity in the liver tissue of rats through different mechanisms, including disturbing liver function, inducing oxidative stress and enhancing apoptotic signaling. Nevertheless, BWE augmented the changes provoked by AgNPs, reflecting its hepatoprotective activity.
Acknowledgments
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bahadar H, Maqbool F, Niaz K, Abdollahi M. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed J. 2016;20(1):1–11. doi:10.7508/ibj.2016.01.001
2. Othman MS, Hafez MM, Moneim AE. The potential role of zinc oxide nanoparticles in MicroRNAs dysregulation in STZ-induced type 2 diabetes in rats. Biol Trace Elem Res. 2019. doi:10.1007/s12011-019-02012-x
3. Pourmand A, Abdollahi M. Current opinion on nanotoxicology. Daru. 2012;20(1):95. doi:10.1186/2008-2231-20-95
4. Vazquez-Muñoz R, Borrego B, Juárez-Moreno K, et al. Toxicity of silver nanoparticles in biological systems: does the complexity of biological systems matter? Toxicol Lett. 2017;276:11–20. doi:10.1016/j.toxlet.2017.05.007
5. Alajmi RA, Al-Megrin WA, Metwally D, et al. Anti-Toxoplasma activity of silver nanoparticles green synthesized with Phoenix dactylifera and Ziziphus spina-christi extracts which inhibits inflammation through liver regulation of cytokines in Balb/c mice. Biosci Rep. 2019;39(5). doi:10.1042/BSR20190379
6. El-Khadragy M, Alolayan EM, Metwally DM, et al. Clinical efficacy associated with enhanced antioxidant enzyme activities of silver nanoparticles biosynthesized using moringa oleifera leaf extract, against cutaneous leishmaniasis in a murine model of leishmania major. Int J Environ Res Public Health. 2018;15(5):1037. doi:10.3390/ijerph15051037
7. Albasher G, Albrahim T, Alsultan N, et al. Red beetroot extract mitigates chlorpyrifos-induced reprotoxicity associated with oxidative stress, inflammation, and apoptosis in rats. Environ Sci Pollut Res Int. 2020;27(4):3979–3991. doi:10.1007/s11356-019-07009-6
8. Ul Kabir A, Samad MB, Ahmed A, et al. Aqueous fraction of Beta vulgaris ameliorates hyperglycemia in diabetic mice due to enhanced glucose stimulated insulin secretion, mediated by acetylcholine and GLP-1, and elevated glucose uptake via increased membrane bound GLUT4 transporters. PLoS One. 2015;10(2):e0116546. doi:10.1371/journal.pone.0116546
9. Nade VS, Kawale LA, Zambre SS, Kapure AB. Neuroprotective potential of Beta vulgaris L. in Parkinson’s disease. Indian J Pharmacol. 2015;47(4):403–408. doi:10.4103/0253-7613.161263
10. Kim YS, Song MY, Park JD, et al. Subchronic oral toxicity of silver nanoparticles. Part Fibre Toxicol. 2010;7:20. doi:10.1186/1743-8977-7-20
11. Sana S, Rahila N. Evaluation of anti-inflammatory effect of natural dietary supplement Beta vulgaris (Beet root) in animal models of inflammation. RMJ. 2017;42(3):385.
12. Singh A, Dar MY, Joshi B, Sharma B, Shrivastava S, Shukla S. Phytofabrication of silver nanoparticles: novel drug to overcome hepatocellular ailments. Toxicol Rep. 2018;5:333–342. doi:10.1016/j.toxrep.2018.02.013
13. El Gamal AA, AlSaid MS, Raish M, et al. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediators Inflamm. 2014;2014:12. doi:10.1155/2014/983952
14. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi:10.1016/0003-2697(79)90738-3
15. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi:10.1016/0003-9861(59)90090-6
16. Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46(2):849–854. doi:10.1016/S0006-291X(72)80218-3
17. Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126.
18. Olive PL, Banáth JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1(1):23–29. doi:10.1038/nprot.2006.5
19. Drury RAD, Wallington EA. Carleton’s Histological Technique. New York: Oxford University Press; 1981.
20. Harrison-Bernard LM, Navar LG, Ho M, Vinson G, El-Dahr S. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol Renal Physiol. 1997;273(1):F170–F177. doi:10.1152/ajprenal.1997.273.1.F170
21. Adeyemi OS, Adewumi I, Faniyan TO. Silver nanoparticles influenced rat serum metabolites and tissue morphology. J Basic Clin Physiol Pharmacol. 2015;26(4):355–361. doi:10.1515/jbcpp-2013-0092
22. Sun X, Wang Z, Zhai S, Cheng Y, Liu J, Liu B. In vitro cytotoxicity of silver nanoparticles in primary rat hepatic stellate cells. Mol Med Rep. 2013;8(5):1365–1372. doi:10.3892/mmr.2013.1683
23. Heydrnejad MS, Samani RJ, Aghaeivanda S. Toxic effects of silver nanoparticles on liver and some hematological parameters in male and female mice (Mus musculus). Biol Trace Elem Res. 2015;165(2):153–158. doi:10.1007/s12011-015-0247-1
24. Patlolla AK, Hackett D, Tchounwou PB. Silver nanoparticle-induced oxidative stress-dependent toxicity in Sprague-Dawley rats. Mol Cell Biochem. 2015;399(1–2):257–268. doi:10.1007/s11010-014-2252-7
25. Gliga AR, Skoglund S, Wallinder IO, Fadeel B, Karlsson HL. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol. 2014;11:11. doi:10.1186/1743-8977-11-11
26. Park J, Lim D-H, Lim H-J, et al. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun. 2011;47(15):4382–4384. doi:10.1039/c1cc10357a
27. Cho YM, Mizuta Y, Akagi JI, Toyoda T, Sone M, Ogawa K. Size-dependent acute toxicity of silver nanoparticles in mice. J Toxicol Pathol. 2018;31(1):73–80. doi:10.1293/tox.2017-0043
28. Forouhar Vajargah M, Imanpoor MR, Shabani A, Hedayati A, Faggio C. Effect of long-term exposure of silver nanoparticles on growth indices, hematological and biochemical parameters and gonad histology of male goldfish (Carassius auratus gibelio). Microsc Res Tech. 2019;82(7):1224–1230. doi:10.1002/jemt.23271
29. Adragna NC, Alla PK, Pavel-Sizmore IE, Paluri ASL, Yaklic J, Lauf PK. Assessment of silver-nanoparticles-induced erythrocyte cytotoxicity through ion transport studies. Cell Physiol Biochem. 2019;53(3):532–549.
30. Li WT, Chang HW, Yang WC, et al. Immunotoxicity of silver nanoparticles (AgNPs) on the leukocytes of common bottlenose dolphins (Tursiops truncatus). Sci Rep. 2018;8(1):5593. doi:10.1038/s41598-018-23737-0
31. Hassanen EI, Khalaf AA, Tohamy AF, Mohammed ER, Farroh KY. Toxicopathological and immunological studies on different concentrations of chitosan-coated silver nanoparticles in rats. Int J Nanomedicine. 2019;14:4723–4739. doi:10.2147/IJN.S207644
32. Espelid S, LØKken GB, Steiro K, BØGwald J. Effects of cortisol and stress on the immune system in Atlantic Salmon (Salmo salarL.). Fish Shellfish Immunol. 1996;6(2):95–110. doi:10.1006/fsim.1996.0011
33. Krajka-Kuzniak V, Szaefer H, Ignatowicz E, Adamska T, Baer-Dubowska W. Beetroot juice protects against N-nitrosodiethylamine-induced liver injury in rats. Food Chem Toxicol. 2012;50(6):2027–2033. doi:10.1016/j.fct.2012.03.062
34. Iahtisham Ul H, Butt MS, Randhawa MA, Shahid M. Hepatoprotective effects of red beetroot-based beverages against CCl4-induced hepatic stress in Sprague Dawley rats. J Food Biochem. 2019;43(12):e13057.
35. Dabrowska-Bouta B, Sulkowski G, Struzynski W, Struzynska L. Prolonged exposure to silver nanoparticles results in oxidative stress in cerebral myelin. Neurotox Res. 2019;35(3):495–504. doi:10.1007/s12640-018-9977-0
36. Lorizola IM, Furlan CPB, Portovedo M, et al. Beet stalks and leaves (Beta vulgaris L.) protect against high-fat diet-induced oxidative damage in the liver in mice. Nutrients. 2018;10(7):872. doi:10.3390/nu10070872
37. Amaral AL, Mariano IM, Carrijo VHV, et al. A single dose of beetroot juice does not change blood pressure response mediated by acute aerobic exercise in hypertensive postmenopausal women. Nutrients. 2019;11(6):1327. doi:10.3390/nu11061327
38. Vieira Teixeira da Silva D, Dos Santos Baiao D, de Oliveira Silva F, et al. Betanin, a natural food additive: stability, bioavailability, antioxidant and preservative ability assessments. Molecules. 2019;24(3):458. doi:10.3390/molecules24030458
39. Barcinska E, Wierzbicka J, Zauszkiewicz-Pawlak A, et al. Role of oxidative and nitro-oxidative damage in silver nanoparticles cytotoxic effect against human pancreatic ductal adenocarcinoma cells. Oxid Med Cell Longev. 2018;2018:15. doi:10.1155/2018/8251961
40. Kang SJ, Ryoo IG, Lee YJ, Kwak MK. Role of the Nrf2-heme oxygenase-1 pathway in silver nanoparticle-mediated cytotoxicity. Toxicol Appl Pharmacol. 2012;258(1):89–98. doi:10.1016/j.taap.2011.10.011
41. Beyfuss K, Hood DA. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Rep. 2018;23(1):100–117. doi:10.1080/13510002.2017.1416773
42. Zielinska E, Zauszkiewicz-Pawlak A, Wojcik M, Inkielewicz-Stepniak I. Silver nanoparticles of different sizes induce a mixed type of programmed cell death in human pancreatic ductal adenocarcinoma. Oncotarget. 2018;9(4):4675–4697. doi:10.18632/oncotarget.22563
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.