Back to Journals » International Journal of General Medicine » Volume 9
Role of a proprietary propolis-based product on the wait-and-see approach in acute otitis media and in preventing evolution to tracheitis, bronchitis, or rhinosinusitis from nonstreptococcal pharyngitis
Authors Di Pierro F , Zanvit A, Colombo M
Received 4 August 2016
Accepted for publication 30 September 2016
Published 11 November 2016 Volume 2016:9 Pages 409—414
DOI https://doi.org/10.2147/IJGM.S118967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Francesco Di Pierro,1 Alberto Zanvit,2 Maria Colombo3
1Scientific Department, Velleja Research, 2Biological Dentistry Department, Italian Stomatology Institute, 3ATS, District 5, Milan, Italy
Abstract: Antipyretics and/or anti-inflammatory drugs along with a wait-and-see approach are the only treatments recommended in early acute otitis media (AOM) or viral pharyngitis. Propolis has been widely investigated for its antibacterial, antiviral, and anti-inflammatory properties and could perhaps be administered as an add-on therapy during watchful waiting in AOM or for better control of symptoms in nonstreptococcal pharyngitis. However, propolis has well-known problems of poor solubility and low oral bioavailability. We therefore analyzed a proprietary propolis-based product (Propolisina®) developed to overcome these limitations, in a retrospective, open-label, controlled study of Streptococcus pyogenes-negative children with a diagnosis of AOM or pharyngitis. Our results show that the use of propolis supplement for 72 hours lessens the severity of AOM and viral pharyngitis, reduces the use of antipyretics and anti-inflammatory drugs, and decreases the rate of evolution to tracheitis, bronchitis, and rhinosinusitis. Our study shows that propolis could be used as a safe add-on therapy in case of AOM and/or viral pharyngitis.
Keywords: pediatric infections, cogrinding, bioavailability, propolis
Introduction
Increasing bacterial resistance to antibiotic therapy is a major concern for the medical community.1 As a result, innovative strategies have been proposed including the use of specific probiotic strains to hinder the growth of pathogens due to the release of bacteriocins,2 and the use of phytochemicals able to inhibit biofilms, interfere with bacterial quorum sensing signaling pathways, or act as chelating agents and/or efflux pump inhibitors.3 A less innovative, but highly pragmatic, approach is referred to as wait-and-see. A wait-and-see approach (where antibacterial therapy is deferred for 48–72 hours) has been advocated in the treatment of acute otitis media (AOM) in children, mainly because of the increased antimicrobial resistance of bacteria causing respiratory infections.4 A meta-analysis of 33 randomized trials involving 5,400 children between 6 months and 18 years of age, revealed a significant but modest impact of antibiotics on AOM.5 However, another randomized double-blind prospective trial carried out in 240 children aged from 6 months to 2 years, showed that only a few actually needed antibiotic treatment.6 According to guidelines, all episodes of AOM in children below 2 years of age require immediate administration of antibiotics.4 Similarly, in cases of severe bilateral otalgia, fever, extensive erythema and bulging tympanum, or otorrhea, antibiotic administration is mandatory in children above 2 years of age.7 However, the wait-and-see approach should be adopted in the absence of these signs – particularly if bulging is not present.8 Unfortunately, inappropriate antibiotic prescription is common9 and encourages continual increases in bacterial respiratory pathogens and resistance.10 The many causes of inappropriate prescribing include diagnostic uncertainty, lack of knowledge, sociocultural and economic pressures, fear of litigation, and parental expectations.11
Group A streptococcus accounts for about 30% of cases of pharyngitis in children, with the remaining cases caused by viruses.12 The use of antibiotics for viral pharyngitis is incorrect, expensive, and encourages antibiotic resistance, in addition to causing adverse reactions (ie, rash, abdominal pain, diarrhea, and vomiting), with no medical benefit.13 Nonstreptococcal pharyngitis normally follows a benign course but: 1) can have an unusually long and severe symptomatology, which is disabling and prevents the normal daily activities of the child such as eating, and is treated with repeated administration of drugs like acetaminophen or ibuprofen; and 2) can evolve to tracheitis, bronchitis, or rhinosinusitis.14
Propolis is a well-known natural resinous mixture produced by honeybees from exudates from buds, plants, poplars, conifers, birch, pine, alder, willow, palm, Baccharis dracunculifolia, and Dalbergia ecastaphyllum.15 Raw propolis consists of about 50% resins, 30% waxes, 10% essential oils, 5% pollen, and 5% various organic compounds,16 including flavonoids, phenylpropanoids, terpenes, stilbenes, lignans, coumarins, and their prenylated derivatives, with >300 different substances identified.17 The precise chemical composition of propolis depends on geographical location, botanical origin, and bee species involved.18–21 The main chemical components in propolis, studied mostly in terms of pharmacological activity, are pinocembrin, pinobanksin, caffeic acid phenetyl ester, artepillin C, cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid, isoferulic acid, chrysin, galangin, kaempferol, and quercetin.22 Being the main constituents, flavonoids contribute greatly to the pharmacological activities of propolis. Flavonoids from propolis, almost exclusively aglycones, despite their antibacterial, antiviral, antifungal, and anti-inflammatory properties,23–27 are characterized by low solubility and poor bioavailability.28,29 The solubility and oral bioavailability of flavonoids have been reported to be increased by utilizing the phytosome forms and cogrinding technology.30–32 We therefore retrospectively investigated the role of a proprietary propolis-based product developed as a mixture of phytosome and propolis coground in a ratio 1:1 and administered during 72 hours of watchful waiting in children with initial signs of AOM and nonstreptococcal pharyngitis, to lessen the severity and length of symptomatology and possible evolution to tracheitis, bronchitis, and rhinosinusitis.
Materials and methods
Product
The investigated proprietary propolis is a mixture (ratio 1:1) of propolis–phytosome, obtained by complexing propolis in aprotic solvent with food grade phosphatidylcholine, and l-lysine cogrounded propolis. Manufacturing details are described in WO 2011/057686.33 The mixture, branded as Proposoma-lisclatrato®, was formulated in sachet form as a water-soluble oral-dissolving powder by Procemsa (Nichelino, Turin, Italy) and notified to the Italian Ministry of Health as Propolisina® by Omeopiacenza (Pontenure, Italy), according to the provisions of law No. 169 of 2004, on June 2014 (notification number: 70758). The propolis supplement used in this retrospective analysis contained 200 mg/sachet of Proposoma-lisclatrato® corresponding to 75 mg/sachet of pure propolis. According to the manufacturer’s specifications, the product is free of fluoroquinolones, Escherichia coli, Salmonella, Staphylococcus aureus, and lactose, and contains gluten (<20 ppm), lead (<0.4 ppm), cadmium (<0.1 ppm), and mercury (<0.005 ppm) below the limits established by European law.
Clinical analysis
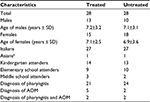
This open-label, retrospective, controlled clinical analysis was conducted in 56 children (23 males and 33 females) recruited from a single routine day-care center in the Milan area of Italy. The children were treated (N=28) or not treated (N=28) between September 2015 and June 2016 with the propolis supplement. This retrospective analysis followed international guidelines and was conducted in accordance with the Declaration of Helsinki and with the approval of the Milan Ethics Committee (Italy). The parents of participants were informed of the retrospective analysis and signed the appropriate consent and privacy policy documents.
Inclusion criteria
Children were included in the analysis if they had an initial diagnosis of AOM and/or pharyngitis free of streptococcal disease as established by a rapid throat swab test for group A streptococcus.
Exclusion criteria
Children were excluded from the analysis if they were below 2 years of age, immunocompromised, had undergone tonsillectomy, or had an indication for adenotonsillectomy. Other exclusion criteria included a history of rheumatic disorders, bronchospasm, a diagnosis of asthma and/or allergy, and a diagnosed systemic disorder. Children were also excluded if they were undergoing pharmacological therapy to prevent recurrent respiratory infections or if they presented with conditions that could favor the development of AOM, including severe atopy, acquired or congenital immunodeficiency, cleft palate, a chronically ruptured eardrum, craniofacial abnormalities or obstructive adenoids, sleep apnea syndrome, or placement of tympanostomy tubes.
Study pattern
In this retrospective analysis all enrolled individuals first underwent a general medical examination, pharyngeal swab (Test Strep-A; Gima, Gessate, Italy), and tympanic evaluation (Otoscope Eurolight C10; Asberg, KaWe, Germany). If the swab was negative for streptococcus, the child was administered with propolis in the form of sachets every 6 hours for a maximum of 72 hours. A control group who did not receive the treatment was retrospectively analyzed. The parents of the children in the treated group were instructed on how to use the product, which could be slowly dissolved in the mouth without water, or taken after dissolving it in a glass of water. It was requested that children be brought to the clinic for an immediate medical examination at the first sign of any worsening of ear and/or oropharyngeal symptoms during the trial period. If required, the parents could contact the physician for up to a week after taking part. In case of worsening AOM, with severe bilateral otalgia, persisting fever, extensive erythema and bulging tympanum, or otorrhea, antibiotic treatment was prescribed according to international guidelines.34 The clinical effect of the product was monitored using a visual analog scale where zero indicated no symptom; one, mild symptoms; two, moderate symptoms; and three, severe symptoms. The visual analog scale was used to score sore throat, fever, adenomegalia, pharyngeal erythema, pharyngeal exudate, nasal secretions in the case of pharyngitis; and otalgia, fever, and nasal secretions in the case of AOM. The visual analog scale was also used for fever where 0 indicates no fever, 1 indicates <38˚C, 2 indicates >38˚C, <39˚C, and 3 indicates >39˚C. The results obtained from otoscopy were categorized as: normal tympanic membrane (score=0); light to moderate tympanic membrane redness (score=1); redness and light bulging of the tympanic membrane (score=2); and severe redness and bulging of the tympanic membrane (score=3).
Study aims
The present analysis aimed to evaluate the following: 1) clinical effect of the product administered during 72 hours of watchful waiting, after a diagnosis of AOM or, in the case of nonstreptococcal pharyngitis, any reduction in symptom length and severity and rate of evolution to tracheitis, bronchitis, or rhinosinusitis; 2) doses of acetaminophen and/or ibuprofen administered for fever or pain control; 3) compliance, tolerability, side effects, and dropout during therapy; and 4) parental opinions on the product.
Statistical analysis
The equivalence of the two groups was determined using Fisher’s exact test and the two-tailed Wilcoxon–Mann–Whitney test. Differences in symptom severity and drug use were determined using the two-tailed Wilcoxon–Mann–Whitney test. JMP 10 statistical software for Mac OsX was used and statistical significance was set at P<0.05.
Results
The aim of our research was to investigate the role of a proprietary propolis-based product during 72 hours of watchful waiting in children with early AOM and, in the case of nonstreptococcal pharyngitis, to reduce the length and severity of symptomatology and rate of evolution to tracheitis, bronchitis, and rhinosinusitis. We retrospectively analyzed 56 streptococcal-negative children diagnosed with AOM or viral pharyngitis treated with the propolis-based product (N=28) or receiving no treatment (N=28). We then retrospectively analyzed the children for symptoms and disease evolution for the following 72 hours.
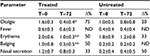
As shown in Table 1, the children in the two groups had similar characteristics with no significant differences. Table 2 shows symptom severity in children with AOM in the treated group (n=7) and in the untreated group (n=4) during watchful waiting. Otalgia, tympanic erythema and tympanic bulging were significantly reduced in children treated with the propolis-based product. In the untreated group, a nonsignificant decrease was observed for otalgia and tympanic erythema with no clear signs of improvement in the other symptoms. After 72 hours of watchful waiting, one in seven children in the treated group and one in four in the untreated group presented with worsening symptoms with persistent bilateral otalgia, fever >39˚C, and clear signs of tympanic erythema and bulging. These children were administered antibiotics and at the end of treatment had a positive outcome with resolution of pathology and no further consequences (data not shown).
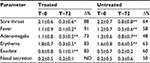
Table 3 shows symptom evolution in children with nonstreptococcal pharyngitis. The treated group (n=23) showed a significant positive trend in symptoms, with a noticeable reduction in sore throat, fever, adenomegalia, pharyngeal erythema, and exudate with only nasal secretion showing no clear signs of improvement. The untreated group (n=26) demonstrated a similar trend but symptom severity was less significantly reduced than in the control group. Also, symptom length appeared to be shorter in the treated compared with the control group. In four of 23 children (~18%), symptoms totally disappeared after 48 hours of treatment with propolis, while symptoms in all the untreated children persisted after 48 hours (data not shown). One of 23 treated children stopped treatment after the first two doses of propolis due to vomiting. Consequently, in Table 3, the scores in column T=0 for the treated group refer to 23 children, but to 22 in the T=72 column (Table 3).
The remaining 22 children were then monitored for possible disease evolution (Table 4). Pharyngitis can evolve in the lower respiratory tract to tracheitis, bronchitis, or rhinosinusitis. None of 22 children in the treated group developed other expected respiratory pathologies, but one child whose pharyngitis clearly worsened, was found to be positive for mycoplasma. After standard antibiotic treatment, the child recovered completely from this infection (data not shown). However, some of the children diagnosed with nonstreptococcal pharyngitis developed tracheitis (three children), bronchitis (one child), and rhinosinusitis (two children). Overall, 21 of 22 children in the treated group versus 20 of 26 children in the untreated group did not experience disease evolution following their initial nonstreptococcal pharyngitis.
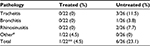  | Table 4 Evolution to tracheitis, bronchitis, or rhinosinusitis 72 hours after a diagnosis of nonstreptococcal pharyngitis Notes: #Mycoplasma infection; **P<0.05 vs untreated. |
All enrolled children were prescribed drugs to control symptom severity and fever. As shown in Table 5, during the 72 hours of monitoring, the treated children were administered eight doses of acetaminophen, 14 doses of ibuprofen, and six nasal washes. In contrast, the untreated group were administered 88 doses of acetaminophen, 82 doses of ibuprofen, 12 doses of N-acetylcysteine, six doses of local antibiotic plus anesthetic (neomycin plus lidocaine), nine doses of hypertonic aerosol, and 12 nasal washes.
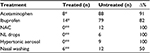  | Table 5 Use of drugs, aerosols, and nasal washing in all children (total doses in 72 hours) Notes: *P<0.01; **P<0.05. Abbreviations: NAC, N-acetylcysteine; NL, neomycin plus lidocaine. |
In terms of acceptability of treatment (a very important factor given the young age of some participants), one child was administered only three sachets a day, instead of four, due to lack of acceptability of the product, while two children had trouble swallowing the product because of nausea and vomiting (Table 6). One of these two children dropped out on the first day of treatment. At the end of treatment, we asked all the parents in writing what they thought about the propolis-based product administered to their children and if they would repeat the treatment in case of a new episode of AOM or nonstreptococcal pharyngitis. Most responses (25 out of 28) declared they were very satisfied and would repeat the treatment if required.
Discussion
Our analysis examined the possible benefits of add-on therapy with propolis in children with nonsevere, initial signs of AOM or with a diagnosis of nonstreptococcal pharyngitis, as these two pathologies do not require treatment with antibiotics. We decided to investigate the role of propolis because it is widely described in scientific literature as having antibacterial, antiviral, and anti-inflammatory activity. It is generally perceived as beneficial and without side effects and therefore is well accepted, especially by parents and physicians seeking complementary and alternative therapies.
The results of our retrospective analysis clearly demonstrate that the use of a propolis-based product lessens the severity of symptoms in case of AOM and reduces the length (at least in a few cases) and severity of symptoms in case of nonstreptococcal pharyngitis and rate of evolution to tracheitis, bronchitis, and/or rhinosinusitis. Its use in AOM and pharyngitis significantly reduces the administration of antipyretics and anti-inflammatory drugs in children, thus reducing the risks of possible side effects to the liver and gastric mucosa. In similar trials, other authors demonstrated the effect of propolis in reducing recurrent upper respiratory tract viral infections and episodes of AOM in children.35,36
In this work, a proprietary propolis-based product developed to overcome the well-recognized problems of poor solubility and low oral bioavailability was retrospectively analyzed. The chemical and physical properties of propolis polyphenols, likely the most important propolis fraction, make them poorly water soluble and therefore unable to dissolve and spread over the throat mucosa to possibly exert a local effect. Moreover, the same fraction demonstrates poor gut absorption and consequently minimal systemic availability. To overcome these two main problems, the propolis used in this retrospective analysis was formulated as a mixture of two fractions. Propolis was first subjected to a mechanical/chemical activation process, also known as cogrinding, in off-axis mills using l-lysine as carrier. Propolis completely loses its crystalline structure after cogrinding with l-lysine, shifting toward an amorphous state along with the carrier. Its characteristics change remarkably after cogrinding, resulting in a product with enhanced water-dissolution kinetics. Propolis was then linked to a phospholipidic carrier that makes it more lipophilic so it can be emulsified by bile salts and then absorbed and distributed systemically. Some authors have tried other methods to solve the solubility and bioavailability problems of propolis37,38 by focusing mainly on the use of liposomes and microemulsions but without, as far as we know, clinically testing the possible impact of these new formulations.
Our analysis has some limitations: it was performed with a small number of participants (especially with AOM), it was not performed under blinded conditions, and the control group did not receive a placebo or a different treatment. However, the results for the treated group compared with the control group are significant, confirming this type of propolis could be used as a short add-on therapy in children during watchful waiting in case of AOM or for better control of symptoms, and to avoid further disease evolution in case of nonstreptococcal pharyngitis.
Disclosure
FDP is a member of the Scientific Council of Omeopiacenza® which is the proprietor of the tested product and reports no other conflicts of interest in this work. The other authors report no conflicts of interest in this work.
References
Falagas ME, Mavroudis AD, Vardakas KZ. The antibiotic pipeline for multi-drug resistant gram negative bacteria: what can we expect? Expert Rev Anti Infect Ther. 2016;14(8):747–763. | ||
Wescombe PA, Hale JD, Heng NC, Tagg JR. Developing oral probiotics from Streptococcus salivarius. Future Microbiol. 2012;7(12):1355–1371. | ||
Borges A, Abreu AC, Dias C, Saavedra MJ, Borges F, Simões M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules. 2016;21(7):pii:E877. | ||
Corbeel L. The ‘wait-and-see’ approach of acute otitis media. Eur J Pediatr. 2005;164(1):1–2. | ||
Rosenfeld RM, Vertrees JE, Carr J, Cipolle RJ, Uden DL, Giebink GS, Canafax DM. Clinical efficacy of antimicrobial drugs for acute otitis media: meta-analysis of 5400 children from 33 randomized trials. J Pediatr. 1994;124(3):355–367. | ||
Damoiseaux RA, van Balen FA, Hoes AW, Verheij TJ, de Melker RA. Primary care based randomised, double blind trial of amoxicillin versus placebo for acute otitis media in children aged under 2 years. BMJ. 2000;320(7231):350–354. | ||
Leach AJ, Morris PS. Antibiotics for the prevention of acute and chronic suppurative otitis media in children. Cochrane Database Syst Rev. 2006;(4):CD004401. | ||
Corbeel L. What is new in otitis media? Eur J Pediatr. 2007;166(6):511–519. | ||
Yagupsky P. Selection of antibiotic-resistant pathogens in the community. Pediatr Infect Dis J. 2006;25(10):974–976. | ||
Jacobs MR, Felmingham D, Appelbaum PC, Gruneberg RN; Alexander Project Group. The Alexander Project 1998–2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother. 2003;52(2):229–246. | ||
Pichichero ME. Understanding antibiotic overuse for respiratory tract infections in children. Pediatrics. 1999;104(6):1384–1388. | ||
Cohen JF, Bertille N, Cohen R, Chalumeau M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database Syst Rev. 2016;7:CD010502. | ||
Spurling GK, Del Mar CB, Dooley L, Foxlee R. Delayed antibiotics for symptoms and complications of respiratory infections. Cochrane Database Syst Rev. 2004;(4):CD004417. | ||
National Institute for Health and Clinical Excellence. Respiratory Tract Infections – Antibiotic Prescribing: Prescribing of Antibiotics for Self-Limiting Respiratory Tract Infections in Adults and Children in Primary Care. London, UK: Centre for Clinical Practice at NICE; 2008. | ||
Bankova V, de Castro SL, Marcucci MC. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31(1):3–15. | ||
Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol. 1998;36(4):347–363. | ||
Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26(2):83–99. | ||
Salatino A, Fernandes-Silva CC, Righi AA, Salatino ML. Propolis research and the chemistry of plant products. Nat Prod Rep. 2011;28(5):925–936. | ||
Toreti VC, Sato HH, Pastore GM, Park YK. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid Based Complement Alternat Med. 2013;2013:697390. | ||
Bankova V. Recent trends and important developments in propolis research. Evid Based Complement Alternat Med. 2005;2(1):29–32. | ||
Silici S, Kutluca S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J Ethnopharmacol. 2005;99(1):69–73. | ||
Huang S, Zhang CP, Wang K, Li GQ, Hu FL. Recent advances in the chemical composition of propolis. Molecules. 2014;19(12):19610–19632. | ||
Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74(4):418–425. | ||
Dalben-Dota KF, Faria MG, Bruschi ML, Pelloso SM, Lopes-Consolaro ME, Svidzinski TI. Antifungal activity of propolis extract against yeasts isolated from vaginal exudates. J Altern Complement Med. 2010;16(3):285–290. | ||
Grange JM, Davey RW. Antibacterial properties of propolis (bee glue). J R Soc Med. 1990;83(3):159–160. | ||
Nolkemper S, Reichling J, Sensch KH, Schnitzler P. Mechanism of herpes simplex virus type 2 suppression by propolis extracts. Phytomedicine. 2010;17(2):132–138. | ||
Kujumgiev A, Tsvetkova I, Serkedjieva Y, Bankova V, Christov R, Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64(3):235–240. | ||
Konishi Y, Hitomi Y, Yoshida M, Yoshioka E. Absorption and bioavailability of artepillin C in rats after oral administration. J Agric Food Chem. 2005;53(26):9928–9933. | ||
Metzner J, Bekemeier H, Schneidewind EM, Wenzel U. [Pharmacokinetic studies of the propolis constituent pinocembrin in the rat (author’s transl)]. Pharmazie. 1979;34(3):185–187. German. | ||
Kaur H, Kaur G. A critical appraisal of solubility enhancement techniques of polyphenols. J Pharm (Cairo). 2014;2014:180845. | ||
Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009;14(3):226–246. | ||
Voinovich D, Perissutti B, Grassi M, Passerini N, Bigotto A. Solid state mechanochemical activation of Silybum marianum dry extract with betacyclodextrins: characterization and bioavailability of the co-ground systems. J Pharm Sci. 2009;98(11):4119–4129. | ||
Alvarez Favela MG. Oral formulations with high oral bioavailability consisting of mixture of lipophilic and hydrophilic fractions obtained from propolis. May 19, 2011. PCT/EP2010/003843. | ||
American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113(3):1451–1465. | ||
Crişan I, Zaharia CN, Popovici F, et al. Natural propolis extract NIVCRISOL in the treatment of acute and chronic rhinopharyngitis in children. Rom J Virol. 1995;46(3–4):115–133. | ||
Marchisio P, Esposito S, Bianchini S, et al. Effectiveness of a propolis and zinc solution in preventing acute otitis media in children with a history of recurrent acute otitis media. Int J Immunopathol Pharmacol. 2010;23(2):567–575. | ||
Ambardekar RV, Mahadik KR, Paradkar AR, Harsulkar AM. Enhancement of hepatoprotective efficacy of propolis by fabrication of liposomes, as a platform nano-formulation for multi-component natural medicine. Curr Drug Deliv. 2012;9(5):477–486. | ||
Fan Y, Ma L, Zhang W, et al. Microemulsion can improve the immune-enhancing activity of propolis flavonoid on immunosuppression and immune response. Int J Biol Macromol. 2014;63:126–132. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.