Back to Journals » OncoTargets and Therapy » Volume 10
RNAi-mediated knockdown of CAIX enhances the radiosensitivity of nasopharyngeal carcinoma cell line, CNE-2
Authors Jiang L, Xu G, Li Z, Zeng X , Li Z, Liu J, Mei L, Li X
Received 24 June 2017
Accepted for publication 29 August 2017
Published 25 September 2017 Volume 2017:10 Pages 4701—4709
DOI https://doi.org/10.2147/OTT.S144756
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samir Farghaly
Liji Jiang,1 Gang Xu,1 Zihuang Li,1 Xiaowei Zeng,2 Zhuangling Li,1 Jingwen Liu,1 Lin Mei,2 Xianming Li1
1Department of Radiation Oncology, Second Clinical Medicine College of Jinan University, Shenzhen, Guangdong, People’s Republic of China; 2The Shenzhen Key Lab of Gene and Antibody Therapy, Division of Life and Health Sciences, Graduate School at Shenzhen, Tsinghua University, Shenzhen, People’s Republic of China
Abstract: Although radiotherapy remains the most powerful as well as the primary treatment modality for nasopharyngeal carcinoma (NPC), approximately 20% of NPC patients still have local recurrence. Carbonic anhydrase IX (CAIX)-related signaling pathways that mediate radioresistance have been found in various kinds of cancer. However, the role of CAIX in NPC radioresistance is still unknown. In this study, we investigated the effect of CAIX silencing on sensitization to ionizing radiation in NPC by using Lipofectamine 2000, which delivers small interfering ribonucleic acid (siRNA) that targets CAIX. Results showed that Lipofectamine 2000 effectively delivered siRNA into the CNE-2 cells, which resulted in the decrease of CAIX expression and cell viability, decrease in cell proliferation and colony formation, and increase in the number of CNE-2 cells stuck in the G2/M phase of the cell cycle upon induction of ionizing radiation. Increased sensitivity of radiotherapy in CNE-2 cells under hypoxic conditions was correlated with the suppression of CAIX. Cells treated with irradiation in addition to CAIX-siRNA1 demonstrated reduced radiobiological parameters (survival fraction at 2 Gy [SF2]) compared with those treated with irradiation only, with a sensitization-enhancing ratio of 1.47. These findings suggest that CAIX can be a promising therapeutic target for the treatment of radioresistant human NPC.
Keywords: carbonic anhydrase IX, small interference RNA, nasopharyngeal carcinoma, radioresistance, cell cycle
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common malignancies in Southeast Asia. The areas with highest incidence rate include Guangdong, Hong Kong, Indonesia, and Singapore, and NPC has an incidence of 15–50 per 100,000 individuals.1 Previous reports suggested that the incidence and mortality rates in Southern China, including Hong Kong, were 19.5 and 7.7 per 100,000 persons.2 Compared to other head and neck cancers, NPC tends to be more sensitive to ionizing radiation (IR).3 Therefore, radiotherapy remains the most powerful treatment modality for NPC, especially with the development of advanced imaging and radiation technologies.4 However, due to radioresistance, certain NPC patients present with local recurrences and distant metastases within 2 years after treatment.5 Thus, radioresistance still remains a major obstacle for treatment success in some NPC cases. Oxygenation is one of the most important parameters for radioresistance. Hypoxia was shown to induce metabolic and molecular changes in solid tumors including head- and -neck cancer, and so hypoxia has been suggested to be involved in the radioresistance.6 But the exact mechanisms underlying radioresistance of NPC still remain unknown.
Hypoxia as a consequence of low oxygenation caused by impaired and aberrant vascularization is a common feature of many malignant tumors.7 It has long been known to be associated with resistance to radiotherapy.8 Cells require oxygen to generate cytotoxic free radicals that damage DNA and stabilize DNA damage.9 Gatenby10 concludes that radioresistance occurs in breast cancer cells due to O2 concentrations falling below 1% in 30%–40% of tumors. At 0.1% O2, tumors can be 2~3 times more resistant to a given radiation dose because of fewer double-strand breaks. Hence, tumor hypoxia is increasingly being recognized as an important therapeutic target to improve the tumor radiosensitivity. In addition to hypoxia, increased acidification is also a characteristic of hypoxic tumors, and this has been suggested to play an indirect role in the poor radioresponse of hypoxic tumors.11 Moreover, lactate accumulation alone (in the absence of pH disruption) has also been suggested to reduce radiosensitivity of tumor cells.12 However, the effect of pH regulation on the efficacy of irradiation remains to be clarified.
Carbonic anhydrase IX (CAIX), a member of the carbonic anhydrase family, is a zinc metalloenzyme that catalyzes the reversible hydration of carbon dioxide (H2O + CO2 = H+ + HCO3−).13 It has been clearly established as contributing to extracellular acidification.14 Current evidence indicates that CAIX expression is cell density-dependent and is strongly induced by hypoxia13 through hypoxia-inducible factor-1-mediated transcription.15 Overexpression of CAIX is commonly observed in several malignancies and has been found to be correlated with poor prognosis in breast, lung, liver, oral, and bladder cancers.16–21 Similarly, CAIX is also overexpressed and serves as an independent poor prognostic factor in NPC patients, as can be observed from the results of our previous study.22 In contrast, a large-scale clinical trial, the DAHANCA 5 study,23 indicated that CAIX has no prognostic or predictive potential in head and neck cancer patients treated with radiotherapy. However, multiple in vivo and in vitro24–26 studies demonstrated that the presence of CAIX was correlated with resistance to tumor radiotherapy. Unfortunately, there are no studies focusing on CAIX expression in NPC and its association with sensitivity to IR. Hence, the elucidation of the correlation between CAIX and radiosensitivity of NPC requires further research.
Consequently, in this study, we adopted an siRNA-mediated CAIX silencing strategy to investigate the effect of CAIX silencing on cell growth and to determine whether a combined therapy of irradiation with downregulation of CAIX would sensitize hypoxic human NPC cell line, CNE-2, to IR.
Materials and methods
Cell culture and hypoxic exposure
The human NPC cells, CNE-2 (American Type Culture Collection, Manassas, VA, USA) were maintained in Roswell Park Memorial Institute 1640 (pH 7.4) supplemented with 10 μg/mL streptomycin sulfate, 100 μg/mL penicillin G, and 10% (v/v) fetal bovine serum. Cells were incubated at 37°C in a 5% CO2 and 95% air incubator. Hypoxic condition was maintained by adding cobalt chloride (CoCl2, Aladdin, a hypoxia-mimicking agent) into the culture medium to a final concentration of 100 μmol/L for mimicking an intracellular hypoxic microenvironment.
siRNA
CAIX-targeting siRNA (CAIX-siRNA), negative control siRNA (NC-siRNA), and fluorescent carboxyfluorescein (FAM)-labeled siRNA (FAM-siRNA) were purchased from GenePharma Co, Ltd (Suzhou, Jiangsu, People’s Republic of China). The negative control for CAIX-siRNA was obtained with oligonucleotides: forward 5′–UUCUCCGAACGUGUCACGUTT–3 and reverse 5′–ACGUGACACGUUCGGAGAATT–3′. The sequences of CAIX-siRNA used were as follows: CAIX-siRNA1: forward 5′–GGAAGAAAUCGCUGAGGAATT–3′, reverse 5′–UUCCUCAGCGAUUUCUUCCTT–3′; CAIX-siRNA2: forward 5′–GCAACAAUGGCCACAGUGUTT–3′, reverse 5′–ACACUGUGGCCAUUGUUGCTT–3′; CAIX-siRNA3: forward 5′–CCAGUCCAGCUGAAUUCCUTT–3′, reverse 5′–AGGAAUUCAGCUGGACUGGTT–3′. RNase-free diethyl pyrocarbonate-treated Milli-Q water was used for all dilutions.
Cell transfection and transfection efficiency calculation
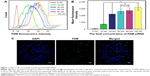
CNE-2 cells were seeded into six-well plates at a density of 4×105 cells per well. When the cells reached 30%–50% confluency, different concentrations of FAM-siRNA (final concentration, 0, 10, 30, 50, 80, and 100 nM) were transfected into the cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Flow cytometry (Beckman, Brea, CA, USA) was used to detect the intensity of fluorescence emitted by the intracellular FAM-siRNA to determine the optimal concentration for further experiments. The transfection efficiency was calculated using laser scanning confocal microscope (Olympus Corporation, Tokyo, Japan) after transfecting with FAM-siRNA for 6 hours and staining with DAPI. The FAM-siRNA was then successfully transfected into the CNE-2 cells using Lipofectamine 2000 in a transient manner. The intracellular uptake parameters of FAM-siRNA were supported by flow cytometry results (Figure 1A). Quantitative analysis (Figure 1B) indicated that siRNA at 100 nM yielded the highest uptake efficiency (P<0.01), while the cell viability was poorer compared with other groups under observation, and FAM-siRNA at 50 nM and 80 nM had no significant difference on the uptake efficiency (P=0.490). Furthermore, we detected the expression of the green fluorescence being emitted from the siRNA-labeled FAM at 50 nM using laser scanning confocal microscope (Figure 1C) and calculated the transfection efficiency (80%±5%), which was satisfied according to the experimental requirements. Therefore, we used the 50 nM concentration for all subsequent experiments.
Cell irradiation
Cells were irradiated at 100 cm from the source with a bolus (placed at the top of the dishes) of 1.9 cm. High-energy photons were used (6 MeV), delivered by a linear accelerator (Siemens, Munich, Germany) with a 20×20 cm posterior field. The dose rate of the Siemens instrument was 576 MU/min. Cells were irradiated with a single dose at room temperature.
Quantitative real-time PCR
After 24 hours of transfection under hypoxia, cells were washed twice with ice-cold phosphate-buffered saline. The total RNA was then extracted using Trizol reagent (Invitrogen) and then reverse transcribed into cDNA using FastQuant RT Super Mix (TIANGEN, Beijing, People’s Republic of China). β-actin was selected as an internal reference gene. Subsequent polymerase chain reaction (PCR) amplification was carried out with CAIX (primer sequences – forward: 5′–GGATCTACCTACCTACTGTTGAGGCT–3′; reverse: 5′–CATAGCGCCAATGACTCTGGT–3′) and β-actin (primer sequences – forward: 5′–CCACACTGTGCCCATCTAC–3′; reverse: 5′–AGGATCTTCATGAGGTAGTCAGTC–3′). The quantitative PCR amplification was performed in a real-time (RT) fluorescent measurement instrument (ABI7300, Thermo Fisher Scientific, Waltham, MA, USA) using the 2× SYBR Green qPCR Master Mix (Biotool, Bee Line Hwy, FL, USA) according to the manufacturer’s instructions. The PCR conditions were as follows: 95°C for 5 minutes, followed by 40 cycles at 95°C for 15 seconds, and 60°C for 60 seconds.
Western blotting
Cells were harvested after 48 hours of transfection by incubating under hypoxic conditions. The cells were lysed with radioimmunoprecipitaion assay buffer (Beyotime, Haimen, People’s Republic of China) and then centrifuged at 12,000 g for 5 minutes. The protein concentration was determined using a BCA Protein Assay Kit (Beyotime). The total proteins (20 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane (Millipore Corp, Billerica, MA, USA). Then tris-buffered saline with 0.1% Tween-20 solution containing 5% nonfat dry milk was used to block the membranes. The membranes were washed three times with TBST and then incubated overnight at 4°C with primary monoclonal mouse anti-human CAIX (GenTex, Zeeland, MI, USA) and mouse anti-human β-actin antibodies. After washing with TBST thrice, the membranes were incubated with secondary antibody for 1 hour at room temperature. The immunoreactive signals were detected by chemiluminescence using an enhanced chemiluminescence detection kit. The band intensity was digitized and analyzed using Quantity One 1-D software.
Cell viability
Cell proliferation was assessed with using the MTT assay. Briefly, cells were divided into 4 groups: The CAIX-siRNA-treated group (CAIX-siRNA), the negative control group (NC-siRNA), the transfection reagent-treatment group (Mock), and the untreated control group (Control). After transfection and incubation under hypoxic conditions for 24 hours, the cells were trypsinized and seeded in 96-well plates (2,000 cells/well, sextuplicate) and incubated for 12, 24, 48, and 72 hours. 20 μL of MTT solution (5 mg/mL) was added to each well and incubated at 37°C for 4 hours. Sequentially, the culture medium was removed instead of adding 100 μL DMSO was added to each well to dissolve the MTT formazan crystals. The cell viability was evaluated by measuring the absorbance of formazan products at 490 nm using a microplate reader (BMG Biotech, Aylesbury, UK). Experiments were performed in triplicate.
Clonogenic assay
For clonogenic assay, the cells were plated into six-well culture plates at various densities (200, 400, 600, and 5,000 cells) under hypoxic conditions. The control group, negative control group, and CAIX-siRNA1-treated group were irradiated with doses of 0, 2, 4, and 6 Gy. Following irradiation, the cells were cultured for 10~14 days until visible colonies appeared. The cells were then stained with crystal violet (Beyotime) after being fixed with 4% paraformaldehyde (Sigma). Colonies with >50 cells only were counted and fit to the multitarget single-hit model using Sigmaplot 13.0 software (Systat Software Inc., San Jose, CA, USA).
Cell apoptosis and cell cycle analysis
Cell apoptosis was determined by Annexin V–fluorescein isothiocyanate and propidium iodide (PI) staining (Beyotime) according to the manufacturer’s instructions. The cells were harvested 48 hours after treatment under hypoxic conditions and then stained with Annexin V–fluorescein isothiocyanate and PI for 20 minutes in the dark at room temperature before flow cytometric analysis. For cell cycle analysis, 24 hours after transfection or transfection combined with 6 Gy radiation, the cells were collected and fixed with 70% precooled ethanol overnight. The cell cycle phases were examined by flow cytometry (Beckman) after staining with PI (Beyotime) in the dark for 30 minutes at 37°C. All the results were analyzed using Flowjo 7.6 software (FlowJo, LLC., Ashland, OR, USA).
Statistical analyses
The data are expressed as the mean ± standard deviation of the mean. Student’s t-test for two groups and one-way analysis of variance for multiple groups were performed using SPSS software version 16.0 (Chicago, IL, USA), in addition to the least-significant difference test. A P-value of <0.05 was considered to indicate a statistically significant difference.
Results
CAIX-siRNA treatment reduces CAIX mRNA and protein expressions
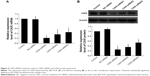
Compared to control and negative control groups (NC-siRNA), the expressions of CAIX mRNA (Figure 2A) and protein (Figure 2B) were significantly reduced after transfecting with three different siRNA chains (CAIX-siRNA1, CAIX-siRNA2, and CAIX-siRNA3). These results indicated that CAIX expression was effectively suppressed by CAIX-siRNA1, 2, and 3. Maximum reduction was achieved by CAIX-siRNA1, followed by CAIX-siRNA2. Therefore, CAIX-siRNA1 and CAIX-siRNA2 were used for subsequent experiments.
Knockdown of CAIX expression inhibits the growth of CEN-2 cells
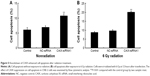
MTT assay was performed to determine the impact of CAIX knockdown on the growth of CNE-2 cells. Results revealed significant changes in the cell growth post RNAi knockdown (P<0.01), and cell viability in the CAIX-siRNA1 treatment group showed highest decrease at 72 hours posttransfection compared with the control group (Figure 3).
Knockdown of CAIX expression enhanced the sensitivity of CEN-2 cells to IR treatment
To examine whether the absence of CAIX could enhance radiosensitivity in NPC cells, we performed colony formation assay (Figure 4A). Compared with control group, the survival curve of CNE-2 cells in CAIX-siRNA1 group was significantly reduced following exposure to various doses of radiation (Figure 4B). The survival fraction at 2 Gy (SF2) was 0.47 for the control group cells, 0.48 for negative control group cells, and 0.32 for CAIX absence group cells. Our results demonstrated that downregulation of CAIX levels inhibited the proliferation of CNE-2 cells effectively and that combined treatment of CAIX-siRNA1 and IR more significantly reduced the survival fraction of CNE-2 cells compared to IR treatment alone.
Silencing of CAIX increased the apoptotic rate of NPC CNE-2 cells to radiation
We investigated whether the suppression of CAIX increased apoptosis in CNE-2 cells in response to radiation. For this, flow cytometry was used to detect the apoptotic rate of cells after treatment with a single dose of 6 Gy radiation. Results showed that the apoptotic rate of cells in the CAIX-siRNA1 treatment group was slightly higher than that in the control and negative control groups (Figure 5A), with an apoptotic rates of 6.13±0.73, 6.98±0.47, and 10.81%±0.62% (P<0.01), respectively. However, the apoptotic rate was obviously significantly increased in the CAIX-siRNA1 treatment group when combined with radiation (Figure 5B), with and thean apoptotic rates of were 8.87±0.38, 9.77±0.46, and 20.19%±0.47% (P<0.01) in the control, negative control, and CAIX-siRNA1 treatment groups. These results were consistent with the cell colony formation assay. Therefore, we hypothesized that knockdown of CAIX enhanced the cells’ sensitivity toward radiation, resulting in a higher apoptotic rate in the CNE-2 cells treated with CAIX-siRNA1.
Knockdown of CAIX enabled CNE-2 cells arrest in the G2/M phase induced by radiation
Cell cycle analysis using flow cytometry was performed to determine whether CAIX gene silencing or silencing combined with IR affected the cell cycle distribution of CNE-2 cells. As a result, CAIX knockdown alone in CNE-2 cells (CAIX-siRNA1) did not cause accumulation in G2/M phase population accumulation at 24 hours posttransfection compared with that of control and negative control (NC-siRNA) group cells (Figure 6A and C). But, the proportion of CAIX-siRNA1-treated cells in the G2/M phase was remarkably higher than that of NC-siRNA group cells and control group cells after exposure to 6 Gy radiation for 24 hours, and the proportionof cells in the G0/G1 phase was decreased compared with the control group cells (Figure 6B and C). These results suggest that the knockdown of CAIX and induction by IR enabled CNE-2 cells to escape from G0/G1 phase and get arrested in the G2/M phase.
Discussion
Although radiotherapy remains to be effective for the treatment of majority of NPC cases, there are still approximately 20% of patients whose tumors were radioresistant.2 Indeed, a common cause of local recurrence and poor survival in NPC was found to be due to radioresistance.27 Tumor hypoxia,8 acidosis,25 and DNA repair of tumor cells28 have been showed to be involved in the radioresistance. Hypoxia is one of the most common characteristics occurring in solid tumors including NPC and is responsible for the poor radioresponse of tumor cells, as mentioned previously.8 However, acidosis, arises from the hypoxia-induced metabolic shift from oxidative phosphorylation to glycolysis within the tumor microenvironment, which plays a role in tumor radioresistance.25 CAIX as a hypoxia-related protein is involved in pH regulation in hypoxic tumor cells, contributing to the acidification of microenvironment, which that then favors tumor growth, invasion, and development.14 Thus, CAIX is considered as an important target for anticancer drug design. A recent study25 revealed that increase in intracellular acidosis by silencing CAIX could radiosensitize tumor cells. Furthermore, evidences suggest that CAIX can promote NPC cell growth and colony formation while dramatically enhancing migration and invasion of NPC cells in vitro and metastasis in vivo.29 But the knowledge on mechanisms regarding how CAIX contributes to radioresistance in NPC is limited. Several clinical trials have revealed that overexpression of CAIX was correlated with poor prognosis in NPC.22,30 In our experiment, we adopted siRNA-mediated CAIX gene knockdown using Lipofectamine 2000 in hypoxic CNE-2 cells. To investigate the effect of CAIX silencing on sensitization to IR in NPC in vitro, the cells were incubated with 100 μmol/L CoCl2 for mimicking intracellular hypoxic microenvironment. The results demonstrated that suppression of CAIX expression effectively decreased cellular proliferation and colony formation, and significantly increased cell apoptosis after IR treatment. This suggests that the absence of CAIX expression enhances NPC cells’ sensitivity to radiotherapy in vitro.
The cell cycle progression and arrest in response to irradiation may determine the sensitivity of cells to irradiation.31 Jérome Doyen et al25 demonstrated that silencing of CAIX resulted in decreased number of cells in the radioresistant S phase and showed a 50% increase in cell death induced by radiation. This led to the conclusion that absence of CAIX was related with radiosensitivity, and the probable mechanism was thought to be by decreasing the proportion of cells in the S phase of cell cycle because of problems in the DNA double-strand break repair systems, such as homologous recombination, that usually occur in the S phase.32 However, in our study, the distribution of cells in the various phases of the cell cycle was unaltered after transfection. But, an increased number of cells were found to be arrested in the G2/M phase after exposure to the radiation, and an even more obvious result observed was the G2/M cell cycle arrest in the CAIX-siRNA1 treatment group. It has been reported that alterations in cell cycle progression post-IR are associated with IR sensitivity of tumor cells.33 Previous studies34–36 have demonstrated that radiation-induced G2/M phase cell cycle arrest was correlated with radioresistance in tumor cells. This might be due to a delay in the G2/M phase in tumor cells, which may provide time for the repair processes to operate that are critical for ensuring cell survival after sublethal DNA damage.37 On the contrary, a research38 study has shown that radiation induces a longer G2/M phase delay in the radiosensitive cell lines than in the matched normal or resistant cells. Moreover, several recent studies39–41 have demonstrated that radiation-induced G2/M phase cell cycle arrest was associated with increased radiosensitivity and not with radioresistance. Similarly, in our study, we observed a significant increase in the arrest of the number of cells in the G2/M phase, and we also observed that cell apoptosis was induced by IR was more obvious in CAIX absence group. Consequently, we provide the first evidence that downregulation of CAIX expression significantly promotes radiosensitivity in CNE-2 cells by intensifying the radiation-induced G2/M cell cycle arrest. These findings also provide uncertainty on the use of G2/M delay induced by IR as a predictor of radiosensitivity.
Conclusion
In conclusion, downregulation of CAIX expression significantly suppresses cell growth, increases cell apoptosis after IR treatment, and promotes radiosensitivity in CNE-2 cells by intensifying the radiation-induced G2/M cell cycle arrest.
Acknowledgment
This work was supported by Science and Technology Planning Project of Shenzhen, China (No JCYJ20150403102020229).
Disclosure
The authors report no conflicts of interest in this work.
References
Ho JH. An epidemiologic and clinical study of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1978;4(3–4):182–198. | ||
Chen ZT, Liang ZG, Zhu XD. A review: proteomics in nasopharyngeal carcinoma. Int J Mol Sci. 2015;16(7):15497–15530. | ||
Huang L, Liao L, Wan Y, et al. Downregulation of annexin A1 is correlated with radioresistance in nasopharyngeal carcinoma. Oncol Lett. 2016;12(6):5229–5234. | ||
Zhang L, Su B, Sun W, et al. Twist1 promotes radioresistance in nasopharyngeal carcinoma. Oncotarget. 2016;7(49):81332–81340. | ||
Qu S, Li XY, Liang ZG, et al. Protein expression of nucleophosmin, annexin A3 and nm23-H1 correlates with human nasopharyngeal carcinoma radioresistance in vivo. Oncol Lett. 2016;12(1):615–620. | ||
Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal. 2014;21(10):1516–1554. | ||
Simko V, Takacova M, Debreova M, et al. Dexamethasone downregulates expression of carbonic anhydrase IX via HIF-1alpha and NF-kappaB-dependent mechanisms. Int J Oncol. 2016;49(4):1277–1288. | ||
Gray LH, Conger AD, Ebert MB, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1954;26(312):638–648. | ||
Brown JM. Tumor hypoxia in cancer therapy. Methods Enzymol. 2007;435:297–321. | ||
Gatenby RA, Smallbone K, Maini PK, et al. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer. 2007;97(5):646–653. | ||
Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14(3):198–206. | ||
Quennet V, Yaromina A, Zips D, et al. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother Oncol. 2006;81(2):130–135. | ||
Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res. 2004;64(17):6160–6165. | ||
Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26(2):299–310. | ||
Ambrosio MR, Di Serio C, Danza G, et al. Carbonic anhydrase IX is a marker of hypoxia and correlates with higher Gleason scores and ISUP grading in prostate cancer. Diagnostic Pathol. 2016;11(1):45. | ||
Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71(9):3364–3376. | ||
Chu CY, Jin YT, Zhang W, et al. CA IX is upregulated in CoCl2-induced hypoxia and associated with cell invasive potential and a poor prognosis of breast cancer. Int J Oncol. 2016;48(1):271–280. | ||
Schweiger T, Kollmann D, Nikolowsky C, et al. Carbonic anhydrase IX is associated with early pulmonary spreading of primary colorectal carcinoma and tobacco smoking. Eur J Cardio-Thoracic Surg. 2014;46(1):92–99. | ||
Kang HJ, Kim IH, Sung CO, Shim JH, Yu E. Expression of carbonic anhydrase 9 is a novel prognostic marker in resectable hepatocellular carcinoma. Virchows Arch. 2015;466(4):403–413. | ||
Perez-Sayans M, Suarez-Penaranda JM, Pilar GD, et al. Expression of CA-IX is associated with advanced stage tumors and poor survival in oral squamous cell carcinoma patients. J Oral Pathol Med. 2012;41(9):667–674. | ||
Klatte T, Seligson DB, Rao JY, et al. Carbonic anhydrase IX in bladder cancer: a diagnostic, prognostic, and therapeutic molecular marker. Cancer. 2009;115(7):1448–1458. | ||
Chen Y, Li X, Wu S, et al. Expression of HIF-1alpha and CAIX in nasopharyngeal carcinoma and their correlation with patients’ prognosis. Med Oncol. 2014;31(12):304. | ||
Eriksen JG, Overgaard J, Danish H, Neck Cancer Study G. Lack of prognostic and predictive value of CA IX in radiotherapy of squamous cell carcinoma of the head and neck with known modifiable hypoxia: an evaluation of the DAHANCA 5 study. Radiother Oncol. 2007;83(3):383–388. | ||
Duivenvoorden WC, Hopmans SN, Gallino D, et al. Inhibition of carbonic anhydrase IX (CA9) sensitizes renal cell carcinoma to ionizing radiation. Oncol Rep. 2015;34(4):1968–1976. | ||
Doyen J, Parks SK, Marcie S, Pouyssegur J, Chiche J. Knock-down of hypoxia-induced carbonic anhydrases IX and XII radiosensitizes tumor cells by increasing intracellular acidosis. Front Oncol. 2012;2:199. | ||
Bache M, Munch C, Guttler A, et al. Betulinyl sulfamates as anticancer agents and radiosensitizers in human breast cancer cells. Int J Mol Sci. 2015;16(11):26249–26262. | ||
Luftig M. Heavy lifting: tumor promotion and radioresistance in NPC. J Clin Invest. 2013;123(12):4999–5001. | ||
Wada S, Kurahayashi H, Kobayashi Y, et al. The relationship between cellular radiosensitivity and radiation-induced DNA damage measured by the comet assay. J Vet Med Sci. 2003;65(4):471–477. | ||
Sang Y, Wang L, Tang JJ, et al. Oncogenic roles of carbonic anhydrase IX in human nasopharyngeal carcinoma. Int J Clin Exp Pathol. 2014;7(6):2942–2949. | ||
Koukourakis MI, Bentzen SM, Giatromanolaki A, et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol. 2006;24(5):727–735. | ||
Wang Q, Wang Y, Du L, et al. shRNA-mediated XRCC2 gene knockdown efficiently sensitizes colon tumor cells to X-ray irradiation in vitro and in vivo. Int J Mol Sci. 2014;15(2):2157–2171. | ||
Hwang HS, Davis TW, Houghton JA, Kinsella TJ. Radiosensitivity of thymidylate synthase-deficient human tumor cells is affected by progression through the G1 restriction point into S-phase: implications for fluoropyrimidine radiosensitization. Cancer Res. 2000;60(1):92–100. | ||
Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(4):928–942. | ||
Guo Y, Sun W, Gong T, et al. miR-30a radiosensitizes non-small cell lung cancer by targeting ATF1 that is involved in the phosphorylation of ATM. Oncol Rep. 2017;37(4):1980–1988. | ||
Chen Y, Li Z, Dong Z, et al. 14-3-3sigma contributes to radioresistance by regulating DNA repair and cell cycle via PARP1 and CHK2. Mol Cancer Res. 2017;15(4):418–428. | ||
Cui F, Hou J, Huang C, et al. C-Myc regulates radiation-induced G2/M cell cycle arrest and cell death in human cervical cancer cells. J Obstet Gynaecol Res. 2017;43(4):729–735. | ||
Fingert HJ, Chang JD, Pardee AB. Cytotoxic, cell cycle, and chromosomal effects of methylxanthines in human tumor cells treated with alkylating agents. Cancer Res. 1986;46(5):2463–2467. | ||
Nagasawa H, Keng P, Harley R, et al. Relationship between gamma-ray-induced G2/M delay and cellular radiosensitivity. Int J Radiat Biol. 1994;66(4):373. | ||
Wang X, Li Q, Jin H, et al. miR-424 acts as a tumor radiosensitizer by targeting aprataxin in cervical cancer. Oncotarget. 2016;7(47):77508–77515. | ||
Jiang X, Zhang QL, Tian YH, Huang JC, Ma GL. RNA interference-mediated gene silencing of cyclophilin A enhances the radiosensitivity of PAa human lung adenocarcinoma cells in vitro. Oncol Lett. 2017;13(3):1619–1624. | ||
Wang J, Wang Y, Mei H, et al. The BET bromodomain inhibitor JQ1 radiosensitizes non-small cell lung cancer cells by upregulating p21. Cancer Lett. 2017;391:141–151. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.