Back to Journals » Cancer Management and Research » Volume 9
Rituximab in the treatment of follicular lymphoma: the future of biosimilars in the evolving therapeutic landscape
Authors Subramanian J, Cavenagh J, Desai B, Jacobs I
Received 25 August 2016
Accepted for publication 5 January 2017
Published 24 April 2017 Volume 2017:9 Pages 131—140
DOI https://doi.org/10.2147/CMAR.S120589
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kexin Xu
Janakiraman Subramanian,1 Jamie Cavenagh,2 Bhardwaj Desai,3 Ira Jacobs4
1Division of Medical Oncology, Saint Luke’s Cancer Institute, Kansas City, MO, USA; 2Department of Haematology, St. Bartholomew’s Hospital, London, UK; 3Prospect Heights, IL, 4Global Established Pharma, Pfizer Inc., New York, NY, USA
Abstract: Follicular lymphoma (FL) is the second most common type of non-Hodgkin’s lymphoma. FL is an incurable disease with treatment options ranging from a “watch-and-wait” approach to localized therapy with radiation or systemic therapy with rituximab in combination with chemotherapy regimens. This review summarizes the role of rituximab across the spectrum of FL treatment and the evolving therapeutic landscape with the emergence of novel agents currently in clinical development. Despite the prospect of new agents on the horizon, it is widely accepted that rituximab will remain as the cornerstone of therapy because of its established long-term efficacy. Many biologics, including rituximab, have lost exclusivity of composition-of-matter patent or will do so in the next few years, which is a concern for patients and physicians alike. Moreover, access to rituximab is challenging, particularly in countries with restricted resources. Together, these concerns have fueled the development of safe and effective biosimilars. The term “biosimilar” refers to a biologic product that is highly similar to an approved reference (or originator) product, notwithstanding minor differences in clinically inactive components, and for which there are no clinically meaningful differences in purity, potency, or safety. Biosimilars are developed to treat the same condition(s) using the same treatment regimens as an approved reference biologic, and have the potential to increase access to more affordable treatment of FL. Herein, we also discuss the potential benefits of eagerly awaited rituximab biosimilars, which may mitigate the impact of the lack of access to rituximab.
Keywords: biosimilar, follicular lymphoma, non-Hodgkin’s lymphoma
Introduction
Non-Hodgkin’s lymphoma (NHL) accounts for approximately 3%–4% of all cancers worldwide.1 NHL is a growing problem, with the global prevalence projected to rise to more than 0.4 million in 2016, and estimates suggest that approximately half of new NHL cases will result in death.2
Follicular lymphoma (FL) is the second most common type of NHL and accounts for 10%–20% of all lymphomas.3 The most ubiquitous indolent lymphoma, FL is typically diagnosed in individuals 55–60 years of age and is slightly more prevalent in females.3 FL is characterized by painless swelling in several lymph node sites, with bone marrow involvement in approximately 70% of cases.4 Approximately 19% of patients present with “B symptoms”, such as fever, weight loss, or night sweats, at the time of diagnosis.3,4 FL predominantly originates from B lymphocytes. CD20 is a B-cell-specific antigen expressed on both malignant B cells, including FL and healthy cells, and is involved in the proliferation and differentiation of normal B cells.5 As such, targeting CD20 is an optimal therapeutic strategy and plays a central role in the treatment of FL.
Rituximab (Rituxan®; Genentech, South San Francisco, CA, USA/MabThera®; Roche, Basel, Switzerland) is a chimeric anti-CD20 monoclonal antibody that binds specifically to CD20.6,7 In addition to its role in the treatment of diffuse large B-cell lymphoma (DLBCL) in the first-line or relapsed/refractory settings, rituximab is also approved for use in FL as a single agent or in combination with first-line chemotherapy.6,7 Given the importance of rituximab across the spectrum of FL treatment and the evolving therapeutic landscape with the emergence of novel agents, the role of rituximab in the future management of FL is described. The role of rituximab biosimilars in the future of FL treatment and how the introduction of biosimilars can help to relieve this lack of access are also discussed.
FL: the role of rituximab in the current standard of care
The National Comprehensive Cancer Network (NCCN)8 (Table 1) and the European Society for Medical Oncology (ESMO) guidelines9 (Figure 1) outline the recommended treatment regimens for patients with FL in the US and Europe, respectively. Owing to its established long-term efficacy, rituximab is an important component of FL treatment.10
  | Table 1 Current standard of care in Grade 1–2 follicular lymphoma: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) 20178 Notes: aSelection of patients requires adequate marrow cellularity >15% and <25% involvement of lymphoma in bone marrow, and platelets >100,000. In patients with prior autologous stem cell rescue, referral to a tertiary care center is highly recommended for radioimmunotherapy. bIf radioimmunotherapy is considered, bilateral cores are recommended and the pathologist should provide the % of overall cellular elements and the % of cellular elements involved in the bone marrow. Cytogenetics ± FISH for known MDS markers. cThe full impact of an induction regimen containing rituximab on RIT consolidation is unknown. dSpecial considerations for the use of small-molecule inhibitors (ibrutinib and idelalisib) available from NCCN.org. eFludarabine-containing regimens negatively impact stem cell mobilization for transplant. fRFND regimen may be associated with stem cell toxicity and secondary malignancies. *Based on the PRIMA study for patients with high tumor burden treatment with RCVP and RCHOP. No data following other regimens. **If initially treated with single agent rituximab. ***Consider for low tumor burden. ****Intention to proceed to high-dose therapy: DHAP (dexamethasone, cisplatin, cytarabine) ± rituximab; ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin) ± rituximab; GDP (gemcitabine, dexamethasone, cisplatin) ± rituximab or (gemcitabine, dexamethasone, carboplatin) ± rituximab; GemOx (gemcitabine, oxaliplatin) ± rituximab; ICE (ifosfamide carboplatin, etoposide) ± rituximab; MINE (mesna, ifosfamide, mitoxantrone, etoposide) ± rituximab. Non-candidates for high-dose therapy: Bendamustine ± rituximab; Brentuximab vedotin for CD30+ disease (category 2B); CEPP (cyclophosphamide, etoposide, prednisone, procarbazine) ± rituximab – PO and IV; CEOP (cyclophosphamide, etoposide, vincristine, prednisone) ± rituximab; DA-EPOCH ± rituximab; GDP ± rituximab or (gemcitabine, dexamethasone, carboplatin) ± rituximab; GemOx ± rituximab; Gemcitabine, vinorelbine ± rituximab (category 3B); Lenalidomide ± rituximab (non-GCB DLBCL); Rituximab. For second-line and subsequent therapy: i. Inclusion of any anthracycline or anthracenedione in patients with impaired cardiac functioning should have more frequent cardiac monitoring; ii. If additional anthracycline is administered after a full course of therapy, careful cardiac monitoring is essential. Dexrazoxane may be added as a cardioprotectant; iii. Rituximab should be included as second-line therapy if there is relapse after a reasonable remission (>6 months); however, rituximab should often be omitted in patients with primary refractory disease. All recommendations are category 2A unless otherwise indicated. Category 1: high-level evidence, there is uniform NCCN consensus that the intervention is appropriate, Category 2A: lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate, Category 2B: lower-level evidence, there is NCCN consensus that the intervention is appropriate, Category 3: any level of evidence, there is major NCCN disagreement that the intervention is appropriate. Adapted with permission from National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. B-cell Lymphomas. Version 1. 2017. Fort Washington, PA: NCCN; 2017 [updated 2017]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Accessed February 9, 2017.8 © 2017 National Comprehensive Cancer Network, Inc. Abbreviations: B, bendamustine, CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CVP, cyclophosphamide, vincristine, and prednisone; DLBCL, diffuse large B-cell lymphoma; L, lenalidomide; I, idelalisib; F, fludarabine; FISH, fluorescence in situ hybridization, FND, fludarabine, mitoxantrone, and dexamethasone; MDS, myelodysplastic syndrome, NCCN, National Comprehensive Cancer Network, O, obinutuzumab; R rituximab; RIT, radioimmunotherapy; PO, oral; IV, intravenous; DA-EPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; GCB, germinal center B-cell like. |
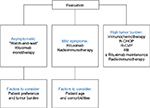  | Figure 1 Current standard of care in follicular lymphoma: ESMO guidelines 2014. Notes: Adapted from Dreyling M, Ghielmini M, Marcus R, Salles G, Vitolo U, Ladetto M; ESMO Guidelines Working Group. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii76–iii829 by permission of Oxford University Press and adapted from First-line treatment of follicular lymphoma: a patient-oriented algorithm, Feuerlein K, Zucca E, Ghielmini M, Leuk Lymphoma, 2009, Taylor & Francis, adapted by permission of the publisher Taylor & Francis Ltd, http://www.tandfonline.com. 69 Abbreviations: ESMO, European Society for Medical Oncology; RB, rituximab plus bendamustine; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab plus cyclophosphamide, vincristine, and prednisone. |
Treatment options for patients with limited-stage disease (stage I/II) include radiation,11–13 “watch-and-wait” approach,14 or rituximab alone or in combination with chemotherapy (eg, bendamustine plus rituximab [BR]; rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP]; or rituximab plus cyclophosphamide, vincristine, and prednisone [R-CVP]).15 Despite the paucity of randomized clinical trials, radiation therapy is the preferred treatment for patients with non-bulky stage I FL. Patients with Grade 3b FL or those undergoing histologic transformation are best treated with chemoimmunotherapy regimens, such as R-CHOP. Patients with stage II FL are appropriate candidates for treatment with either rituximab alone or in combination with chemotherapy, depending on their clinical presentation. In general, the treatment approach for patients with stage II FL includes “watch-and-wait”, single-agent rituximab vs rituximab plus chemotherapy, or radiation for those with contiguous nodal involvement. Patients with bulky disease or those with an adverse prognosis presenting with B symptoms and/or clinically significant tumor load are candidates for treatment with rituximab plus chemotherapy.8,9
Treatment options for patients with advanced-stage (stage III/IV) FL are similar to those for stage II FL. For asymptomatic patients with non-bulky disease, the watch-and-wait approach with treatment upon disease progression may be appropriate.16 Results from a study by Ardeshna et al conducted in asymptomatic patients with advanced-stage low-tumor-burden FL showed that 88% of patients on maintenance treatment with rituximab did not require treatment at 3 years compared with 46% in the watch-and-wait group (P<0.0001).15 The rituximab induction arm was affected by early closure because of poor accrual. Moreover, there were 18 serious adverse events, possibly as a consequence of treatment with rituximab. However, this study highlights that rituximab monotherapy is a possible option for patients with asymptomatic, low-tumor-burden FL, particularly for those who may not tolerate chemotherapy or for those with slowly progressive disease.8
Patients with advanced-stage FL that is progressive, symptomatic, and/or has associated cytopenias are candidates for chemoimmunotherapy. The choice of chemotherapy agents depends on the drug availability and physician choice. Common treatment regimens include BR, R-CHOP, and R-CVP. Recent evidence suggests that BR is effective as a first-line regimen in patients with advanced disease. In a randomized Phase III study, treatment with BR resulted in non-inferior clinical response compared with standard rituximab chemotherapy (R-CHOP or R-CVP), with an acceptable toxicity profile.17 In a prospective, randomized, open-label study in patients with previously untreated indolent lymphoma, first-line therapy with BR resulted in greater progression-free survival and fewer toxic effects compared with R-CHOP.18
Patients who respond to induction treatment with R-CVP or R-CHOP are potential candidates for maintenance therapy with rituximab. Primary RItuximab and MAintenance (PRIMA) was a randomized open-label study to investigate the effect of 2 years of rituximab maintenance therapy (375 mg/m² every 8 weeks) after first-line treatment with rituximab plus chemotherapy.19 At a median follow-up of 3 years, progression-free survival was significantly improved in the rituximab maintenance group (75%) compared with the observation group (58%) (P<0.0001). Furthermore, a significantly greater proportion of patients achieved a complete response with rituximab maintenance therapy than those in the observation group (72% vs 52%, respectively; P=0.0001). However, there was no difference in the overall survival between the 2 groups in this study.19 Further investigation is needed to understand the long-term toxicities of rituximab maintenance therapy and to identify the patients most likely to benefit from this treatment regimen.
A recent study by Kahl et al20 failed to demonstrate a significant clinical benefit from continuing rituximab maintenance treatment in patients with advanced-stage, low-tumor-burden FL after induction with the single-agent rituximab. Although rituximab maintenance therapy should be considered after rituximab plus chemotherapy, it is not recommended after rituximab monotherapy (Table 1; Figure 1). Furthermore, rituximab maintenance therapy after induction with rituximab monotherapy is of less clinical benefit in patients with low-tumor-burden FL, compared with rituximab re-treatment.20
Rituximab is also effective in the re-treatment setting and is used in combination with chemotherapy regimens for the treatment of relapsed/refractory FL. Common chemotherapy regimens include first-line (BR, R-CHOP, and R-CVP) and second-line (fludarabine, cyclophosphamide plus mitoxantrone or fludarabine monotherapy) regimens (Table 1). In patients with early relapse (1–2 years), a non-cross-resistant regimen is preferable (eg, bendamustine before or after CHOP). If patients subsequently experience remission for 6 months, the addition of rituximab should be considered (Table 1).8
Novel agents on the horizon: the evolving treatment landscape of FL
Following the clinical success of rituximab in the treatment of FL, novel agents are emerging and currently under development. Ibrutinib, an oral, selective, and covalent Bruton’s tyrosine kinase inhibitor, has been approved by the US Food and Drug Administration (FDA) for the treatment of patients with mantle cell lymphoma who have received at least 1 prior therapy,21 chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma, and Waldenström’s macroglobulinemia.22 Ibrutinib has also been approved by the European Medicines Agency (EMA) for the treatment of patients with relapsed/refractory mantle cell lymphoma, in the first-line, refractory, and relapsed/refractory settings of CLL, for those with Waldenström’s macroglobulinemia who have received at least 1 prior therapy, and in the first-line setting for patients unsuitable for chemoimmunotherapy. 23 Ibrutinib has shown promising results in the treatment of FL. Ibrutinib is currently under investigation for use in combination with rituximab (ClinicalTrials.gov, NCT01980654) and as monotherapy for relapsed/refractory FL (ClinicalTrials.gov, NCT01849263).
Idelalisib, an oral phosphoinositide 3-kinase-delta inhibitor, has been approved by the FDA for the treatment of patients with relapsed/refractory CLL and relapsed/refractory FL.24 A Phase I/II study to evaluate the efficacy and safety of idelalisib in patients with previously treated low-grade FL is ongoing (ClinicalTrials.gov, NCT01306643). A Phase II study is planned to explore the efficacy and safety of idelalisib in combination with rituximab in patients with previously untreated FL and small lymphocytic lymphoma (ClinicalTrials.gov, NCT02258529).
Obinutuzumab is a humanized immunoglobulin G1 monoclonal antibody, with a novel glycoengineered Fc region. In combination with chemotherapy, obinutuzumab has been approved by the FDA for the treatment of CLL. Obinutuzumab is approved for use in combination with bendamustine followed by obinutuzumab monotherapy for the treatment of patients with FL who relapsed after, or are refractory to, a rituximab-containing regimen.25 Approval was based on an open-label, randomized, Phase III trial in patients with indolent NHL refractory to rituximab.26 In another study, obinutuzumab demonstrated clinical efficacy and an acceptable safety profile in the relapsed/refractory FL setting.27 In a Phase III study, obinutuzumab-based induction and maintenance therapy resulted in small improvements in progression-free survival in treatment-naïve patients with FL; however, a higher incidence of Grades 3–5 adverse events was observed compared with rituximab.28 A Phase I study in patients with NHL or CLL showed that induction and maintenance therapy with obinutuzumab offered clinical benefits, with a favorable safety profile.29 Similarly, obinutuzumab showed encouraging results in patients with relapsed/refractory NHL.30
Lenalidomide, an immunomodulatory agent, has been approved by the FDA for the treatment of patients with multiple myeloma, myelodysplastic syndromes, and relapsed/refractory mantle cell lymphoma.31 In a Phase II trial, lenalidomide in combination with rituximab was effective and well tolerated as initial treatment for patients with advanced indolent NHL.32 In light of these promising results, a Phase III study to evaluate the efficacy and safety of rituximab in combination with lenalidomide compared with R-CHOP, R-CVP, or BR in patients with previously untreated FL is ongoing (ClinicalTrials.gov, NCT01476787).
Rituximab: challenges for the future
Despite an array of novel agents emerging and in clinical development, it is likely that rituximab will remain at the forefront of FL treatment.33 However, patents for many biologics have expired or will lose exclusivity over the coming years (Figure 2). Importantly, the composition-of-matter patent covering rituximab in Europe (MabThera®) expired in 2013, and its counterpart in the US (Rituxan®) will expire in 2018. Moreover, rituximab is not widely available to physicians and patients in several countries, including the US, Mexico, Turkey, Russia, and Brazil. In a recent survey of 450 oncologists and hematologists, 31% considered rituximab an easily accessible option in Brazil. Similarly, 25% and 19% reported challenges in getting access to rituximab in Mexico and Russia, respectively. Issues related to insurance coverage, treatment guidelines, and patient comorbidities were the most common barriers to access or use of rituximab. Indeed, these barriers have the potential to compromise clinical outcomes and patient care across the spectrum of FL treatment, potentially resulting in a change or delay in treatment with rituximab, largely the result of payment issues or patient response. However, Baer et al found physicians who participated in the survey would increase use of rituximab if a more affordable version were available, without compromising efficacy, safety, or patient care.34
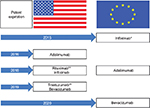  | Figure 2 Patent expiration of biologics in the US and Europe. Notes: *Loss of exclusivity in some European countries in 2014. **Loss of exclusivity in the European Union in 2014. |
Another concern in the biologics arena is that these agents are likely to be susceptible to drug shortages as a consequence of sparse active ingredients, coupled with obstacles in manufacture and supply. In addition, suboptimal compliance with current good manufacturing practice, changes in clinical standards, a greater demand for rituximab, and manufacturing delays are the most common factors that contribute to drug shortages.35 The supply of chemotherapeutics, such as 5-fluorouracil, liposomal doxorubicin, and fludarabine, as well as rituximab, may be adversely affected by looming drug shortages, with potentially pernicious outcomes for patients.36 Drug shortages can lead to delays in treatment, use of alternative, potentially less effective treatment, increased case management for health care professionals, and poor availability of more effective, well-tolerated combination therapies.35
What’s next? The rationale for developing biosimilars
The loss of exclusivity and limited access to rituximab has led to the development of well-characterized, safe, and effective biosimilars to rituximab. Biosimilars are defined as biologic products that are highly similar to the reference biologic product, notwithstanding minor differences in clinically inactive components.37 The availability of biosimilars may mitigate the impact of the limited access to rituximab. Biosimilars provide a greater spectrum of treatment choices and may offer efficiencies to the health care system. In addition, biosimilars increase access to biologics and foster greater use of biologic therapies, which may facilitate improved overall health outcomes at a time when a shortage of biologics may become a barrier to the treatment of patients with FL. Biosimilars have the potential to increase access to therapies by offering more affordable treatment options. Savings with biosimilars could facilitate the reallocation of expenditure to optimize treatment of patients with FL, resulting in patients receiving treatment earlier in therapy, with more patients able to access medicines such as rituximab.37
Understanding biosimilars: an overview of development and pathways to approval
Biosimilars are large, structurally complex molecules developed to be similar to and treat the same condition(s), using the same treatment regimens as an existing licensed or approved (originator or reference) biologic. Development of biosimilars involves a stepwise approach of physicochemical and biological evaluation, along with a series of comparative nonclinical and clinical studies. Several factors influence the extent of clinical data required for the approval of biosimilars, including the similarity to the originator biologic and strength of preclinical data as well as the molecular complexity. The ultimate aim of biosimilar development is to demonstrate that there are no clinically meaningful differences based on the “totality of evidence” encompassing all available analytical, nonclinical, and clinical data. The totality of evidence approach is specifically tailored for biosimilars and applies a different paradigm than the regulation of originator biologics, for which the aim is to establish de novo efficacy and safety. Furthermore, the biosimilar developer is not required to demonstrate efficacy to the reference biologic in each indication, as the extrapolation of clinical data across indications is permitted, and any differences may be considered in the totality of evidence, given appropriate scientific justification.38
The approval of biosimilars is a highly regulated and comprehensive process (Table 2). The EMA and the US FDA have published extensive guidance documents with their respective definitions of biosimilars, and the requirement of a series of detailed analytical and similarity assessments, nonclinical in vivo evaluation, and safety and efficacy studies, all in comparison with the reference biologic.38,39
  | Table 2 Regulatory definitions of biosimilars Abbreviations: EEA, European Economic Area; EMA, European Medicines Agency; FDA, US Food and Drug Administration. |
The biosimilars approval pathway was established in the European Union (EU), with 8 biosimilars (under 20 different trade names) authorized by the EMA over the past decade.40 The first biosimilar in the US (filgrastim-sndz [Zarxio®]), a biosimilar version of Neupogen®, was granted approval in 2015 under the US Biologics Price Competition and Innovation Act of 2009.41,42 Although complex, the regulatory approval process for biosimilars in Europe,39 the US,38 and elsewhere43 has been tailored and is often shorter than that required for originator biologics.
The advent of rituximab biosimilars: where are we now?
A biosimilar version of rituximab (TruximaTM) has recently been granted approval for the treatment of NHL, CLL, and rheumatoid arthritis (RA) in South Korea.44 Several potential rituximab biosimilars are currently in development, each in comparison with the originator (reference) rituximab (Table 3). A study in patients with newly diagnosed advanced FL showed pharmacokinetic similarity between a potential biosimilar to rituximab, CT-P10, and rituximab (each administered with CVP), with similar B-cell kinetics and immunogenicity.45 In another study, CT-P10 and rituximab showed equivalent pharmacokinetics, and comparable efficacy, pharmacodynamics, immunogenicity, and safety in patients with RA.46 A clinical study in patients with previously untreated FL showed therapeutic equivalence in overall response rate between another proposed biosimilar to rituximab, GP-2013, and rituximab sourced from the EU. In this trial, similarity was demonstrated for efficacy, pharmacokinetic, and pharmacodynamic parameters, with similar safety findings.47 In addition, the efficacy and safety of another potential biosimilar, BI-695500, in patients with low-tumor-burden lymphoma is under clinical investigation (ClinicalTrials.gov, NCT01950273). ABP 798, a potential biosimilar to rituximab, is in clinical development in patients with CD20-positive B-cell NHL (ClinicalTrials.gov, NCT02747043). Another potential biosimilar to rituximab, MabionCD20, is in development in patients with CD20-positive DLBCL (ClinicalTrials.gov, NCT02617485).
In nonclinical studies, PF-05280586, a proposed biosimilar to rituximab, showed the same primary amino acid sequence and similar physicochemical and in vitro functional properties as the licensed originator biologic.48 A study in patients with active RA demonstrated pharmacokinetic similarity of PF-05280586 to rituximab sourced from the EU (rituximab-EU) and the US (rituximab-US) and that of rituximab-EU to rituximab-US.49 In this trial, all 3 treatments were generally well tolerated, and the incidence of treatment-related adverse events was low. These encouraging findings support the ongoing development of PF-05280586 as a potential biosimilar to rituximab. A trial is ongoing to compare the efficacy, safety, pharmacokinetics, and immunogenicity of PF-05280586 to rituximab in patients with low-tumor-burden FL (ClinicalTrials.gov, NCT02213263).
Conclusion
FL accounts for up to 20% of all lymphomas. Debate continues over the optimal treatment strategy for untreated FL: the watch-and-wait approach vs therapeutic intervention. However, for patients who are candidates for systemic therapy, an anti-CD20 monoclonal antibody alone or in combination with chemotherapy remains at the forefront of the therapeutic landscape.
It is well recognized that rituximab will remain as the cornerstone of therapy in the treatment of patients with FL. The loss of exclusivity of composition-of-matter patents of biologics in recent and coming years, and the lack of access to rituximab in some countries are of particular concern for patients and physicians. Thus, it is relevant, appropriate, and necessary to develop a biosimilar to rituximab with the potential to generate cost savings and efficiencies for health care systems and to increase access to patients worldwide, which can help augment resources for other important aspects of health care.
It will be crucial to engage clinicians on the importance of biosimilars, and moreover, to increase understanding of data that underpin the development of biosimilars and how these data may translate into clinical practice. At present, several potential rituximab biosimilars are in development. As a result of extensive studies, the availability of safe and effective rituximab biosimilars is eagerly anticipated, potentially offering a greater range of therapeutic options and improved clinical benefits to patients with FL.
Acknowledgments
Medical writing support was provided by Neel Misra, MSc, of Engage Scientific Solutions, and funded by Pfizer Inc.
Author contributions
All the authors made substantial contributions to conception and design, execution, or analysis and interpretation of data; drafted the article or revised it critically; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
This review was supported by Pfizer Inc. JS has served on advisory boards for Pfizer, Boehringer Ingelheim, and Bristol-Myers Squibb, and on speakers bureaus for Celgene, Boehringer Ingelheim, and Eli Lilly. BD was a full-time employee of Pfizer at the time this manuscript was initiated. IJ is a full-time employee of Pfizer. JC has no conflicts of interests to declare in this work.
References
Cancer Research UK. Non-Hodgkin lymphoma incidence statistics. London: Cancer Research UK; 2011. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/nhl/incidence/uk-nonhodgkin-lymphoma-incidence-statistics#world. Accessed August 5, 2016. | ||
Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon: International Agency for Research on Cancer; 2013 [updated October 9, 2014]. Available from: http://globocan.iarc.fr. Accessed August 5, 2016. | ||
Union for International Cancer Control. Follicular lymphoma. Union for International Cancer Control, 2014 review of cancer medicines on the WHO list of essential medicines. Executive summary. Geneva: World Health Organization; 2014. Available from: http://www.who.int/selection_medicines/committees/expert/20/applications/FollicularLymphoma.pdf. Accessed August 5, 2016. | ||
Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–1265. | ||
Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980;125(4):1678–1685. | ||
Genentech Inc. Rituxan (rituximab) injection prescribing information. South San Francisco, CA: Genentech Inc.; 1997 [updated April 2016]. Available from: http://www.gene.com/download/pdf/rituxan_prescribing.pdf. Accessed August 5, 2016. | ||
Roche. MabThera 100 mg and 500 mg concentrate for solution for infusion. eMC+; 2014 [updated May 26, 2016]. Available from: http://www.medicines.org.uk/emc/medicine/2570/SPC/Mabthera+100mg+and+500mg+concentrate+for+solution+for+infusion. Accessed August 5, 2016. | ||
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. B-cell Lymphomas. Version 1. 2017. Fort Washington, PA: NCCN ; 2017 [updated 2017]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Accessed February 9, 2017. | ||
Dreyling M, Ghielmini M, Marcus R, Salles G, Vitolo U, Ladetto M; ESMO Guidelines Working Group. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii76–iii82. | ||
Czuczman MS, Gregory SA. The future of CD20 monoclonal antibody therapy in B-cell malignancies. Leuk Lymphoma. 2010;51(6):983–994. | ||
Mac Manus MP, Hoppe RT. Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. J Clin Oncol. 1996;14(4):1282–1290. | ||
Wilder RB, Jones D, Tucker SL, et al. Long-term results with radiotherapy for Stage I-II follicular lymphomas. Int J Radiat Oncol Biol Phys. 2001;51(5):1219–1227. | ||
Pugh TJ, Ballonoff A, Newman F, Rabinovitch R. Improved survival in patients with early stage low-grade follicular lymphoma treated with radiation: a Surveillance, Epidemiology, and End Results database analysis. Cancer. 2010;116(16):3843–3851. | ||
Ardeshna KM, Smith P, Norton A, et al; British National Lymphoma Investigation. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet. 2003;362(9383):516–522. | ||
Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol. 2014;15(4):424–435. | ||
Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 1997;15(3):1110–1117. | ||
Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944–2952. | ||
Rummel MJ, Niederle N, Maschmeyer G, et al; Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–1210. | ||
Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51. | ||
Kahl BS, Hong F, Williams ME, et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: Eastern Cooperative Oncology Group Protocol e4402. J Clin Oncol. 2014;32(28):3096–3102. | ||
Janssen Biotech, Inc. Imbruvica prescribing information. Horsham, PA: Janssen Biotech, Inc; 2016 [updated June 2016]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/205552Orig1s012lbl.pdf. Accessed December 5, 2016. | ||
National Cancer Institute. FDA approval for ibrutinib (Imbruvica): approved for chronic lymphocytic leukemia after previous treatment. Bethesda, MD: US Department of Health and Human Services; 2013 [updated April 8, 2015]. Available from: http://www.cancer.gov/about-cancer/treatment/drugs/fda-ibrutinib. Accessed August 5, 2016. | ||
Janssen-Cilag International NV. IMBRUVICA 140 mg hard capsules. eMC+; 2014 [updated October 21, 2014]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003791/WC500177775.pdf. Accessed December 5, 2016. | ||
US Food and Drug Administration. FDA approves Zydelig for three types of blood cancers [FDA news release]. Silver Spring, MD: US Department of Health and Human Services; 2014 [updated July 23, 2014]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm406387.htm. Accessed August 5, 2016. | ||
Genentech Inc. Gazyva prescribing information. South San Francisco, CA: Genentech Inc.; 2016 [updated February 2016]. Available from: https://www.gene.com/download/pdf/gazyva_prescribing.pdf. Accessed December 5, 2016. | ||
Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081–1093. | ||
Radford J, Davies A, Cartron G, et al. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000). Blood. 2013;122(7):1137–1143. | ||
Marcus RE, Davies AJ, Ando K, et al. Obinutuzumab-based induction and maintenance prolongs progression-free survival (PFS) in patients with previously untreated follicular lymphoma: primary results of the randomized phase 3 GALLIUM study. Poster presented at: American Society of Hematology (ASH); December 3–6, 2016; Washington, DC. | ||
Sehn LH, Assouline SE, Stewart DA, et al. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119(22):5118–5125. | ||
Salles G, Morschhauser F, Lamy T, et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119(22):5126–5132. | ||
Celgene. Revlimid prescribing information. Summit, NJ: Celgene; 2015 [updated February 2015]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021880s041lbl.pdf. Accessed December 5, 2016. | ||
Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol. 2014;15(12):1311–1318. | ||
Vital EM, Kay J, Emery P. Rituximab biosimilars. Expert Opin Biol Ther. 2013;13(7):1049–1062. | ||
Baer WH, Maini A, Jacobs I. Barriers to the access and use of rituximab in patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: a physician survey. Pharmaceuticals (Basel). 2014;7(5):530–544. | ||
Li E, Subramanian J, Anderson S, Thomas D, McKinley J, Jacobs IA. Development of biosimilars in an era of oncologic drug shortages. Drug Des Devel Ther. 2015;9:3247–3255. | ||
Gogineni K, Shuman KL, Emanuel EJ. Survey of oncologists about shortages of cancer drugs. N Engl J Med. 2013;369(25):2463–2464. | ||
Weise M, Bielsky MC, De Smet K, et al. Biosimilars: what clinicians should know. Blood. 2012;120(26):5111–5117. | ||
US Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. Silver Spring, MD: U.S. Department of Health and Human Services, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER); 2015 [updated April 2015]. Available from: http://www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed August 5, 2016. | ||
European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. London: European Medicines Agency; 2015 [updated December 18, 2014]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf. Accessed August 5, 2016. | ||
European Medicines Agency. European public assessment reports (EPAR) for human medicines: biosimilars. London: European Medicines Agency; 2016. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d125. Accessed June 21, 2016. | ||
Sandoz, Inc. Zarxio (filgrastim-sndz) injection prescribing information. Princeton, NJ: Sandoz, Inc.; 2015 [updated March 20, 2015]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125553lbl.pdf. Accessed August 5, 2016. | ||
US Food and Drug Administration. Filgrastim-sndz. Silver Spring, MD: US Department of Health and Human Services, US Food and Drug Administration; 2015 [updated March 6, 2015]. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm436953.htm. Accessed August 5, 2016. | ||
Expert Committee on Biological Standardization. Guidelines on evaluation of similar biotherapeutic products (SBPs). Geneva: World Health Organization; 2009 [updated October 19–21, 2009]. Available from: http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf. Accessed August 5, 2016. | ||
Generics and Biosimilars Initiative (GaBI). Biosimilar rituximab approved in South Korea. GaBI online; 2016 [updated December 2, 2016]. Available from: http://gabionline.net/Biosimilars/News/Biosimilar-rituximab-approved-in-South-Korea. Accessed December 5, 2016. | ||
Coiffier B, Sancho JM, Jurczak W, et al. Pharmacokinetic and safety of CT-P10, a biosimilar candidate to the rituximab reference product, in patients with newly diagnosed advanced stage follicular lymphoma (AFL). Poster presented at: American Society of Hematology (ASH); December 3–6, 2016; Washington, DC. | ||
Yoo DH, Suh CH, Shim SC, et al. A multicentre randomised controlled trial to compare the pharmacokinetics, efficacy and safety of CT-P10 and innovator rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76(3):566–570. | ||
Jurczak W, Ilidia M, Govindbabu KS, et al. A phase III efficacy and safety study of the proposed rituximab biosimilar GP2013 versus rituximab in patients with previously untreated advanced follicular lymphoma. Poster presented at: American Society of Hematology (ASH); December 3–6, 2016; Washington, DC. | ||
Karnik S, Thompson MS, DeGruttola H, et al. Characterization and comparison of PF-05280586—a proposed rituximab biosimilar to the licensed product. Poster presented at: American Association of Pharmaceutical Scientists-National Biotechnology Conference (AAPS-NBC 2013); May 20–22, 2013; San Diego, CA. | ||
Becker JC, Melia LA, Gumbiner B, Thomas D, Spencer-Green G, Meng X. A phase I trial comparing PF-05280586 (a potential biosimilar) and rituximab in subjects with active rheumatoid arthritis. Poster presented at: 2014 American College of Rheumatology/Association of Rheumatology Health Professionals (ACR/ARHP) Annual Scientific Meeting; November 15–19, 2014; Boston, MA. | ||
Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly × 4 schedule. Blood. 2004;103(12):4416–4423. | ||
van Oers MH, Van Glabbeke M, Giurgea L, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28(17):2853–2858. | ||
Forstpointner R, Unterhalt M, Dreyling M, et al; German Low Grade Lymphoma Study Group (GLSG). Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood. 2006;108(13):4003–4008. | ||
Czuczman MS, Weaver R, Alkuzweny B, Berlfein J, Grillo-López AJ. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin’s lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol. 2004;22(23):4711–4716. | ||
Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–3732. | ||
McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–2833. | ||
Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–4586. | ||
Morschhauser F, Radford J, Van Hoof A, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol. 2008;26(32):5156–5164. | ||
Morschhauser F, Radford J, Van Hoof A, et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the International, Randomized, Phase III First-Line Indolent trial. J Clin Oncol. 2013;31(16):1977–1983. | ||
Hagenbeck A, Radford J, Van Hoof A, et al. 90Y-Ibritumomab tiuxetan (Zevalin®) consolidation of first remission in advanced-stage follicular non-Hodgkin’s lymphoma: updated results after a median follow-up of 66.2 months from the International, Randomized, Phase III First-Line Indolent Trial (FIT) in 414 patients. Blood. 2010;116 Suppl:abstr 594. | ||
Leonard J, Jung SH, Johnson JL, et al. CALGB 50401: a randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma. J Clin Oncol. 2012;30 Suppl:abstr 8000. | ||
Witzig TE, Flinn IW, Gordon LI, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20(15):3262–3269. | ||
Hainsworth JD, Litchy S, Burris HA 3rd, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20(20):4261–4267. | ||
Colombat P, Salles G, Brousse N, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood. 2001;97(1):101–106. | ||
Martinelli G, Schmitz SF, Utiger U, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol. 2010;28(29):4480–4484. | ||
Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018. | ||
Martin P, Jung SH, Johnson JL, et al. CALGB 50803 (Alliance): a phase II trial of lenalidomide plus rituximab in patients with previously untreated follicular lymphoma. J Clin Oncol. 2014;32 Suppl 5:abstr 8521. | ||
Czuczman MS, Koryzna A, Mohr A, et al. Rituximab in combination with fludarabine chemotherapy in low-grade or follicular lymphoma. J Clin Oncol. 2005;23(4):694–704. | ||
McLaughlin P, Hagemeister FB, Rodriguez MA, et al. Safety of fludarabine, mitoxantrone, and dexamethasone combined with rituximab in the treatment of stage IV indolent lymphoma. Semin Oncol. 2000;27(6 Suppl 12):37–41. | ||
Feuerlein K, Zucca E, Ghielmini M. First-line treatment of follicular lymphoma: a patient-oriented algorithm. Leuk Lymphoma. 2009;50(3):325–334. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

